Abstract
Chemoresistance is a prevalent issue in cancer, resulting in a poor prognosis. The transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2), a key regulator in cellular antioxidant responses, is implicated in cell survival, proliferation, and chemoresistance. It represents a promising target for treating Hepatocellular carcinoma (HCC). The NRF2 activity has been recently revealed to be controlled by the ubiquitination process mediated by the KEAP1-CUL3 E3 ligase, highlighting the importance of deubiquitination regulation. However, the specific deubiquitinase (DUB) responsible for NRF2 in liver cancer remains unclear. In this study, we demonstrate that Ubiquitin-Specific Protease 37 (USP37) acts as a novel regulator of NRF2 protein. Mechanistically, USP37 modulates the stability of NRF2 through enzymatic activity-dependent deubiquitination. Additionally, USP37 interacts with NRF2 and facilitates its deubiquitination. Elevated USP37 levels were associated with higher levels of NRF2 protein in samples from human patients. Importantly, the knockdown of USP37 results in increased NRF2 degradation and enhances cellular sensitivity to chemotherapy. Overall, our findings manifested the significant involvement of the USP37-NRF2 axis in regulating therapeutic interventions for HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-01913-9.
Keywords: NRF2, USP37, Deubiquitylation, Chemoresistance, Hepatocellular carcinoma
Introduction
Liver cancer is the fourth most common cancer worldwide [1, 2]. Primary liver malignancies are associated with significant clinical, economic, and psychological challenges, representing a substantial burden in both developed and developing countries [3]. Notably, conventional cytotoxic agents, including 5-fluorouracil (5-FU), adriamycin (ADM), cisplatin (DDP), and docetaxel, have been recommended as chemotherapeutic treatments for hepatocellular carcinoma (HCC). Moreover, molecular target inhibitors such as sorafenib have been engineered to selectively target vascular endothelial growth factor receptors (VEGFR), thereby obstructing the progression and angiogenesis of hepatocellular carcinoma (HCC). Numerous chemotherapeutic approaches have been formulated to combat HCC. The expression of nuclear factor erythroid 2-related factor 2 (Nrf2) is markedly elevated in HCC cells and is associated with chemoresistance [4]. Mutations in Nfe2l2 and Keap1 lead to the activation of the Nrf2/ARE pathway, which subsequently results in the upregulation of cytoprotective protein transcription and the overexpression of nuclear Nrf2. This process facilitates tumorigenesis and enhances the survival of tumor cells [5, 6]. Furthermore, hepatocellular carcinoma (HCC) cells exhibiting chemoresistance demonstrate an overexpression of Nrf2, contributing to the development of chemoresistance [7–9].
The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) constitutes a key regulator of cellular antioxidant responses. Additional functions of NRF2 have been recently unveiled, expanding its role beyond redox regulation. Over the past two decades, NRF2 has emerged as a primary target for cancer prevention and treatment research, revealing a more significant role than initially anticipated. This presents both new challenges and prospects to utilize NRF2 in cancer therapy. NRF2 belongs to the Cap’n’collar (CNC) leucine zipper (bZIP) transcription factor family. It is composed of seven Neh domains, each with a specific role [10]. Through heterodimerization with small MAF proteins, NRF2 governs the constitutive and inducible expression of more than 200 genes harboring antioxidant response elements (AREs) within their regulatory regions [11]. These NRF2 target genes play critical roles in regulating drug metabolism and excretion, maintaining redox homeostasis, controlling iron metabolism, managing energetic metabolism, regulating amino acid metabolism, facilitating autophagy, influencing proliferation and survival, facilitating DNA repair, maintaining mitochondrial physiology, and governing proteasomal degradation [10, 12, 13].
The ubiquitin–proteasome system (UPS) is crucial in governing various cellular processes, including cell growth, apoptosis, proliferation, and DNA repair [14, 15]. Recent research has shed light on the pivotal UPS implications in human disorders [16, 17]. Ubiquitination is a reversible and significant post-translational modification that modulates modified protein stability and activity. It is involved in the regulation of nearly all biological processes besides having a close association with tumorigenesis [18–20]. Ubiquitination is catalyzed via the synergistic E1 activating, E2 conjugating, and E3 ligating enzyme actions by covalently attaching ubiquitin to target proteins, resulting in diverse biological outcomes, particularly proteasomal degradation [21–23]. Despite NRF2 expression in all cell types, its basal protein levels are often low in the absence of stress. The regulation of NRF2 ubiquitylation and proteasomal degradation is governed by three distinct E3 ubiquitin ligase complexes: KEAP1-CUL3-RBX1[24–28], β-TrCP-SKP1-CUL1-RBX1[29, 30], and HRD1[31]. Although the E3 ubiquitin ligases have been extensively studied, the deubiquitinating enzymes (DUBs) involved in NRF2 regulation have yet to be fully explored. Identifying the DUB(s) responsible for NRF2 will facilitate a more comprehensive understanding of the regulatory network governing NRF2-ARE signaling, making it easier and more effective to investigate.
In our study, we conducted a screening of an expression library containing 42 DUBs to determine the specific DUB involved in regulating the stability of NRF2. Our findings revealed that ubiquitin-specific protease 37 (USP37) is responsible for modulating NRF2 protein stability in an activity-dependent manner. USP37 was found to interact with NRF2 and facilitate the deubiquitination of NRF2. Elevated USP37 levels were associated with higher levels of NRF2 protein in samples from human patients. Importantly, when USP37 was knocked down, it enhanced cell sensitivity to chemotherapy. Our results highlight the USP37 significance as a novel DUB in determining cell sensitivity to chemotherapy. The findings provide a promising avenue for clinical liver cancer management by targeting USP37. This research offers valuable insights into the regulatory mechanisms of NRF2 and opens up new possibilities for improving cancer treatment strategies.
Materials and methods
Cell culture
The HEK293T and HepG2 cell lines used in this study were obtained from the Cell Bank of the Chinese Academy of Sciences in Shanghai, China. The SMMC-7721 and Huh7 cell lines were purchased from iCell Bioscience. L02, Bel-7402, Hep3B and MHCC97 cell lines were provided with LASKERBIO company. All cell lines were subjected to culture in DMEM (HyClone) containing 10% fetal bovine serum, 100 mg/ml streptomycin, 100 IU penicillin, and 4 mM L-glutamine at 37 °C in a humidified incubator with 5% CO2.
Reagents
A total of seven antibodies were used in this study: Rabbit anti-Caspase-3 (#9662), Rabbit anti-Tubulin(11224-1-AP), Rabbit anti-NRE2L2 (NRF2) (16396-1-AP), Rabbit anti-HA (51064-2-AP), Rabbit anti-MYC (16286-1-AP), Rabbit anti-USP37 (18465-1-AP), Rabbit anti-Flag (20543-1-AP), mouse anti-GAPDH (60004-1-Ig) and mouse anti-β-actin (66009-1-Ig). Moreover, both CellTiter-Glo Luminescent Cell Viability Assay and Caspase-Glo 3/7 Assay Kits were procured from Promega Company. Cycloheximide (CHX) as well as MG-132 and sorafenib were purchased from Selleck Company as well as MCE Company, respectively. TRIzol reagent and qPCR kit (SYBR Green) were acquired from Beyotime Company and TIANGEN Company, respectively. Additionally, the EL Transfection Reagent and Exonuclease III for gene cloning were procured from Transgene Company and Takara Company, respectively.
Plasmids
This study used Lentivirus-based shRNA expression vectors. The designed DNA oligonucleotides containing specific shRNA sequences went through cloning into the pLV-H1-EF1α-puro expression vector. The used shRNA sequences targeting USP37 were as follows: USP37-sh1: CCGGATTTGCAGAAGATGATA USP37-sh3: CCCTAACTTCTCTGGCCTATT. These sequences were designed to specifically target and knock down the expression of USP37.
Transfection and DUB screening
In the DUB screening process, each individual DUB expression plasmid was co-transfected with NRF2 into 293 T cells through the EL Transfection Reagent from Transgene Company. This method allows for efficient transfection of the plasmids into the cells. After transfection, the cells were allowed to express the DUB proteins and NRF2. Cell lysis was then performed to harvest the cellular contents. The lysates were subsequently analyzed using specific antibodies, including an anti-HA antibody for NRF2 detection, a Flag antibody for DUBs detection, and an ACTIN antibody as a loading control. The analysis of the lysates using these antibodies helps in determining NRF2 and individual DUB protein expression levels in the transfected cells.
Analyses of real-time PCR and RNA extraction
As previously described [32, 33], RNA was extracted and real-time PCR was performed. TRIzol reagent was utilized for total RNA isolation from cells per the protocols. The FastKing gRNA Dispelling RT SuperMix (TIANGEN) was employed to perform cDNA synthesis using each RNA sample. The RNA expression levels were standardized based on the internal control GAPDH. The FastFire qPCR PreMix SYBR Green (TIANGEN) was utilized to conduct real-time PCR.
Ubiquitination assays
For the NRF2 ubiquitylation determination, 293 T cells were subjected to cotransfection with NRF2–HA and Flag-Ub, either with or without USP37 and its enzymatic mutant plasmids. Subsequently, the cells were treated for 6 h with MG132 (10 µM), collected, and lysis with RIPA buffer. Thereafter, NRF2 went through immunoprecipitating with anti-HA beads and was then subjected to SDS-PAGE. Eventually, an anti-Flag antibody was deployed to determine NRF2 ubiquitylation.
Immunoprecipitation
In brief, proteins were lysed in cold lysis buffer containing protease inhibitors and then immunoprecipitated to determine the interaction between proteins. Using anti-labeled M2 beads or anti-HA beads, the supernatant of the cell lysate was separated by centrifugation at 12,000 g for 30 min at 4 °C [32, 33].
Cell viability assay
Cell viability was evaluated following the kit procedures. In brief, nearly 2.5 × 104 cells were subjected to seeding into a 96-well plate and overnight incubation. Subsequently, we treated the cells for a suitable duration, regardless of the presence or lack of associated compounds. Subsequently, the 96-well plate and its contents went through 30-min incubation at room temperature. To the cell culture medium, add equal amounts of CellTiter-Glo reagent and shake for 2 min. After incubating the 96-well plate for an additional 15 min at room temperature, the luminescence values were recorded.
Statistical analyses
Statistical analyses were conducted utilizing SPSS software, with data presented as the mean ± standard deviation (SD). Comparisons of statistical differences among groups exceeding two in number were executed via a one-way analysis of variance (ANOVA), subsequently followed by Bonferroni’s post hoc test, assuming equal variances, or Tamhane’s T2 post hoc test when variances were unequal. For comparisons between two groups, Student's t-test was employed. A p-value of less than 0.05 was deemed indicative of statistical significance.
Tissue samples
Liver cancer specimens and adjacent non-cancerous tissues were procured from Luoyang Central Hospital, affiliated with Zhengzhou University (Luoyang, China). Each sample underwent rigorous histological evaluation by experienced pathologists to confirm a definitive diagnosis of liver cancer. Importantly, all specimens were collected from patients who had not undergone chemotherapy or radiotherapy prior to surgical intervention. The study protocols adhered to the ethical guidelines established by the Institutional Research Medical Ethics Committee of Luoyang Central Hospital, affiliated with Zhengzhou University. Informed consent was obtained from each participant through individually signed consent forms.
Results
USP37 governs NRF2 protein stability
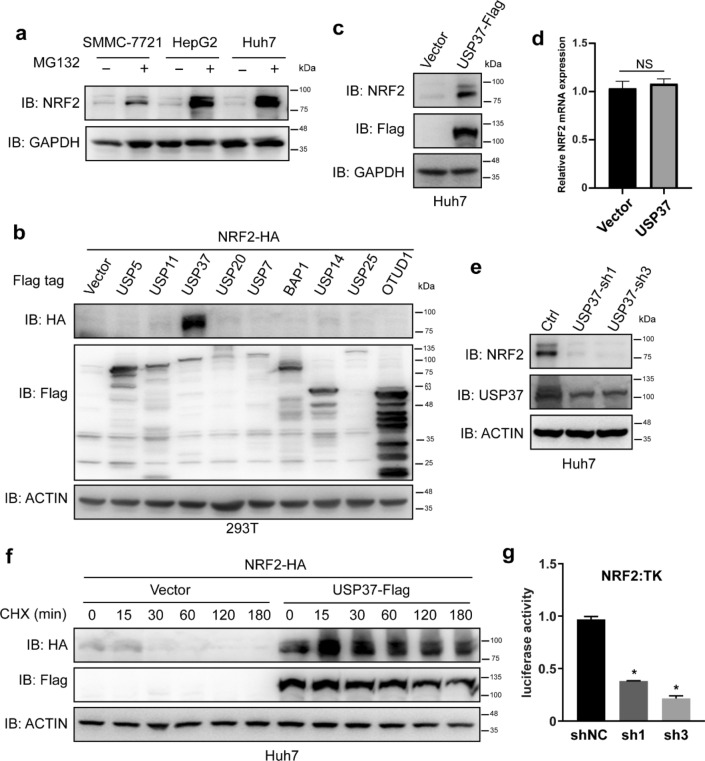
For facilitating the screening process of DUBs that regulate NRF2 stability, experiments were conducted to determine if exogenously expressed NRF2 protein undergoes degradation via a ubiquitination/proteasome-dependent mechanism. The proteasome inhibitor MG-132 significantly elevated NRF2 protein levels in liver cancer cell lines (Fig. 1a), indicating that exogenous NRF2 protein degradation is dependent on ubiquitination and the proteasome. Aiming at determining the DUBs responsible for regulating NRF2 protein degradation, NRF2 was co-transfected with expression plasmids harboring genes encoding individual DUBs into 293 T cells. Subsequently, these DUBs’ effects on NRF2 protein levels were evaluated. We discovered that the expression of one particular DUB, USP37, significantly upregulated the NRF2 protein level (Fig. 1b). Furthermore, USP37 expression raised endogenous NRF2 protein levels without affecting NRF2 RNA expression (Fig. 1c, d). Additionally, knocking down USP37 caused NRF2 protein level downregulation (Fig. 1e). To provide additional evidence about the involvement of USP37 in the regulation of NRF2 protein stability, Huh7 cells were subjected to co-transfection with USP37 and NRF2, followed by treatment with the eukaryotic protein synthesis inhibitor CHX. Figure 1f depicts that USP37 expression significantly promoted NRF2 protein stability. The stabilization of NRF2, a master transcriptional factor, is facilitated by USP37, prompting a need for further investigation into the impact of USP37 on NRF2 transcriptional activity. This study employed an ARE-dependent firefly luciferase reporter gene assay to assess the effects of USP37-shRNAs on NRF2 transcriptional activity, revealing a significant inhibition compared to the control group (Fig. 1g). In addition, the mRNA levels of NRF2 downstream genes HO-1, TXN1 and G6PD changed along with the amount of USP37 expression (Figure S1a).
Fig. 1.
USP37 governs NRF2 protein stability. a The treatment of SMMC-7721, HepG2, and Huh7 cell lines with the proteasome inhibitor MG132 (10 μM) for 6 h. Performing SDS-PAGE and Western blot analysis on the cells through relevant antibodies. b Co-transfecting a plasmid expressing NRF2-HA with individual DUB expression plasmids into 293 T cells. Collecting the cells after 36 h and analyzing them with the appropriate antibodies. c Huh7 cell infection with lentivirus expressing either an empty vector or USP37-Flag. Harvesting the cells and subjecting them to protein analysis using relevant antibodies. d Determining NRF2 RNA levels in Huh7 cells infected with lentivirus expressing either an empty vector or USP37-Flag. e Infecting Huh7 cells with lentivirus expressing either scramble or USP37-specific shRNAs. Harvesting and analyzing the cells with the relevant antibodies. f Co-transfecting a plasmid expressing NRF2-HA with either an empty vector or USP37-Flag expression plasmids into Huh7 cells. Cell treatment with cycloheximide (CHX, 40 µg/ml) after 24 h for the indicated time periods. Harvesting and analyzing the cells with the indicated antibodies. g Reduced USP37 inhibits NRF2 transcriptional activity. Huh7 cells were co-transfected with an ARE reporter firefly luciferase plasmid (100 ng), pRL-TK plasmid (10 ng), and varying amounts of shNC or shRNAs plasmid. Reporter assays were conducted 24 h post-transfection, with the results reported as the ratio of NRF2/TK luciferase activity
USP37 enzymatic activity is significant in regulating NRF2
To determine if USP37 enzymatic activity contributes to its regulation of NRF2 protein stability, an enzymatically inactive mutant of USP37, called USP37-C350S, was generated by mutating the cysteine at amino acid position 350 to a serine. Herein, the wild-type USP37, but not the enzymatically inactive mutant USP37-C350S, increased NRF2 protein levels (Fig. 2a). Furthermore, the researchers investigated which domain of the USP37 protein is responsible for its regulation of NRF2 protein stability. Consistent with the previous finding, only the USP37 construct containing its enzymatic domain (amino acids 341–979), but not the construct containing amino acids 1–340, significantly increased NRF2 protein levels (Fig. 2b). Additionally, USP37, rather than its enzymatically inactive mutant, promoted NRF2 protein stability (Fig. 2c and Figure S2a). These findings collectively suggest that USP37 regulates NRF2 protein stability through an enzymatic-dependent manner. Collectively, USP37 enzymatic activity is crucial for its ability to increase NRF2 protein levels and enhance its stability.
Fig. 2.
The significance of USP37 enzymatic activity in its NRF2 regulation. a Infecting Huh7 cells with lentivirus expressing either a vector, USP37-Flag, or USP37-C350S-Flag. Harvesting and analyzing the cells with the relevant antibodies. b The co-transfection of a plasmid expressing NRF2-HA with plasmids expressing an empty vector, USP37, or its related constructs into Huh7 cells. Harvesting and analyzing the cells with the appropriate antibodies after 36 h. c Co-transfecting a plasmid expressing NRF2-HA with plasmids expressing an empty vector, USP37-Flag, or USP37-C350S-Flag, into Huh7 cells. The cell treatment with cycloheximide (CHX, 40 µg/ml) after 24 h for the indicated time periods. Harvesting and analyzing the cells with the indicated antibodies
USP37 interacts with NRF2 and enhances NRF2 deubiquitination
To gain further insight into the mechanism underlying NRF2 protein stability regulation by USP37, we conducted experiments to examine the interaction between USP37 and NRF2. The results demonstrated reciprocal interactions between USP37 and NRF2 (Fig. 3a, b). We then investigated the protein domains involved in this interaction. The findings revealed that NRF2 is capable of interacting with the enzymatic domain of USP37 (amino acids 341–979) (Fig. 3c). Considering that many DUBs govern protein stability through the removal of ubiquitin chains from their substrates, we examined whether USP37 affects NRF2 ubiquitination. The results showed that USP37, rather than its enzymatically inactive mutant, was able to remove the ubiquitin chain from NRF2 (Fig. 3d). Collectively, these findings indicate that USP37 regulates NRF2 protein stability through the interaction with NRF2 and deubiquitination promotion. The interaction between USP37 and NRF2 facilitates the removal of ubiquitin chains from NRF2, thereby stabilizing the NRF2 protein.
Fig. 3.
USP37 interacted with and enhanced NRF2 deubiquitination. a Co-transfection of plasmids expressing USP37-Flag and empty vector or NRF2-HA into 293 T cells. Treating the cells with MG132 (10 µM) for 6 h prior to harvesting. Cell lysate immunoprecipitation using the indicated antibodies. Analyzing the total cell lysates (TCL) and the immunoprecipitates by immunoblotting using anti-HA or Flag antibodies. b Consistent with (a), but in this case, the co-transfection of the plasmid expressing NRF2-HA was with an empty vector or USP37-Flag into 293 T cells. Treating the cells with MG132 (10 µM) for 6 h prior to harvesting. Immunoprecipitating the cell lysates employing the indicated antibodies while immunoblotting TCL and immunoprecipitates using anti-HA or Flag antibodies. c Similar to (A), but in this case, co-transfecting the plasmid expressing NRF2-HA into the 293 T cells was conducted with an empty vector, USP37-Flag, or the enzymatic domain (aa 341–979). The cell treatment with MG132 (10 µM) for 6 h. Harvesting the cells and subjected them to immunoprecipitation with the indicated antibodies. Analyzing the TCL and immunoprecipitates by immunoblotting using anti-HA or Flag antibodies. d Co-transfecting empty vector, plasmids expressing USP37-MYC, or USP37 enzymatic mutant C350S-MYC into 293 T cells with plasmids expressing NRF2-HA and Flag-ubiquitin (Flag-Ub). Treating the cells with MG132 (10 µM) for 6 h prior to harvesting. Cell lysate immunoprecipitation using anti-HA antibody. Immunoblotting the TCL and immunoprecipitates utilizing antibodies against Flag (Flag-Ub), HA (NRF2-HA), and Myc (USP37-Myc)
USP37 triggers chemoresistance through NRF2 protein stabilizing in Hepatocellular carcinoma (HCC) and correlates with the NRF2 protein
Chemoresistance is a significant challenge in cancer treatment, and the UPS is crucial in governing protein levels and activities, including cell survival, proliferation, and apoptosis. Inhibition of the UPS has emerged as a potential strategy to overcome drug resistance in various cancers. In this study, the focus was on addressing chemoresistance in HCC. Previous studies have demonstrated that NRF2 protein level is closely correlated with cancer cell sensitivity to chemotherapy [34]. Since USP37 regulates NRF2 protein levels, the researchers investigated whether USP37 impacts Sorafenib toxicity, a chemotherapeutic drug, in Huh7, SMMC-7721 and HepG2 cell lines. The results showed that USP37 knockdown significantly enhanced Sorafenib-triggered apoptosis in Huh7, SMMC-7721 and HepG2 cell lines (Fig. 4a and Figure S3 b,c). Furthermore, the expression of caspase-3 was assessed using Western blot analysis, revealing a significant increase in the protein level of caspase-3 upon the knockdown of USP37(Figure S3 a). In addition, the researchers examined whether USP37 overexpression could reverse NRF2 downregulation triggered by Sorafenib. The findings demonstrated that USP37 attenuated Sorafenib-induced NRF2 downregulation in Huh7, SMMC-7721 and HepG2 cell lines (Fig. 4b and Figure S3 d,e). Sorafenib is a multi-targeted antineoplastic agent frequently employed in clinical settings, demonstrating dual antitumor activity by concurrently targeting neoplastic cells and angiogenesis. It is frequently utilized as a supplementary treatment for advanced liver cancer. Sorafenib has been shown to trigger the degradation of NRF2 protein, with the proteasome inhibitor MG132 able to counteract this effect. Additionally, Sorafenib induces the degradation of USP37 in a proteasome-dependent manner, as illustrated in Fig. 4c. Subsequently, our investigation focused on determining if the overexpression of USP37 could counteract the down-regulation of NRF2 caused by Sorafenib. The results depicted in Fig. 4d demonstrate that USP37 mitigated the decrease in NRF2 levels induced by Sorafenib in the Huh7 cell line. Given the pivotal role of NRF2 in human cancer development, it is plausible that USP37 may facilitate the deubiquitination and stabilization of NRF2 in human cancers. Consequently, we sought to explore the potential correlation between the expression levels of USP37 and NRF2 in liver cancer tissue samples. As illustrated in Fig. 4e and Figure S4 a,b, a heightened USP37 protein expression was associated with an elevated NRF2 expression level. Furthermore, protein levels of NRF2 and USP37 were assessed in the human embryonic kidney cell line HEK293T, the normal human liver cell line L02, and six liver cancer cell lines (SMMC-7721, HepG2, Bel-7402, Hep3B, Huh7, and MHCC97). The findings confirmed the overexpression of both NRF2 and USP37 in hepatocellular carcinoma (HCC) cell lines, with Huh7, SMMC-7721, and HepG2 exhibiting relatively higher expression levels (Fig. 4f). These results collectively suggest a correlation between USP37 and NRF2, leading to the stabilization of NRF2 and the induction of chemoresistance in liver carcinoma.
Fig. 4.
USP37 triggers chemoresistance via NRF2 protein stabilizing in Hepatocellular carcinoma and correlates with the NRF2 protein. a Ttransducing Huh7 cell lines with lentiviral vectors expressing either scrambled (control) or USP37 shRNA to establish stable cell lines. Treating these stable cell lines with Sorafenib at 15 μM concentration. Assessing cell viability through CellTiter-Glo Luminescent Cell Viability Assay. Caspase 3/7 activity, which are key enzymes involved in apoptosis, was evaluated through the Caspase-Glo 3/7 assay that provides insights into the impact of USP37 knockdown on Sorafenib-induced apoptosis in Huh7 cells. b Similarly, Ttransducing Huh7 cell lines with either empty or lentiviral vectors expressing USP37 or USP37-C350S for stable cell line generation. The treatment of these stable cell lines with Sorafenib at 15 μM concentration. Cell viability assessment via the CellTiter-Glo Luminescent Cell Viability Assay. c Huh7 cells were subjected to a 6-h stimulation with or without Sorafenib (15 mM), followed by Western blot analysis to assess protein levels of NRF2, USP37, and ACTIN. d A stable Huh7 cell line expressing either an empty vector or USP37 was treated with or without Sorafenib (15 mM) for 6 h, after which Western blot analysis was conducted using antibodies against NRF2, Flag, and ACTIN. e The protein expression levels of NRF2 and USP37 were analyzed in 18 representative pairs of liver tumor (T) and adjacent non-tumor tissues (N). f The protein expression of NRF2 and USP37 was examined in the human embryonic kidney cell line HEK293, the normal human liver cell line L02 and six liver cancer cell lines (SMMC-7721, HepG2, Bel-7402, Hep3B, Huh7, and MHCC97)
Discussion
Nrf2 is a transcription factor endowed with cytoprotective properties, exerting both advantageous and adverse effects on cancer [35, 36]. It plays a crucial role in inhibiting malignant transformation by safeguarding both healthy and cancerous cells from oxidative damage induced by free radicals. However, once cancer is established, Nrf2 contributes to therapeutic resistance [37]. Furthermore, Nrf2 is a pivotal element in the development of chemotherapy resistance, as it enhances drug metabolism and efflux [38]. The NRF2-ARE signaling pathway is critical in multiple pathophysiological processes, including metabolism, oxidative stress, and multidrug resistance [12, 13]. The ubiquitination-proteasome degradation system and the deubiquitinase (DUB) system play vital roles in various cellular processes, including cell cycle and transcriptional regulation, signal transduction, DNA repair, and protein degradation, alongside human disease development, including cancer [14, 15]. NRF2 overexpression has been revealed to be less responsive to chemotherapy drugs, indicating a link between NRF2 and chemoresistance [34, 39–42]. Prior investigations have substantiated the intricate interplay among NRF2 and DUB3, as well as between KEAP1 and DUB3, suggesting the formation of a substantial functional complex involving NRF2, DUB3, and KEAP1. Notably, the overexpression of DUB3 has been shown to induce NRF2-mediated resistance to chemotherapy in colon cancer cell lines [34]. USP37, a newly identified deubiquitinating enzyme (DUB), has been recognized as a potential factor associated with tumor progression and exhibits a significant correlation with sensitivity to chemotherapy drugs [43]. It is likely that a variety of DUBs are important in multiple types of cancer, suggesting that distinct deubiquitinating enzymes could be functioning in different tumors to control the NRF2 protein's stability. In this study, the researchers focused on investigating the molecular mechanism behind the NRF2-ARE signaling pathway regulation in hepatoma cells, with a specific focus on USP37 as a potential candidate.
In this study, the researchers aimed to establish a connection between USP37 and chemoresistance by investigating its role in the regulation of NRF2, a key protein involved in chemoresistance.The findings revealed that knockdown of USP37 significantly enhanced Sorafenib-induced cell death in hepatoma cell line. This suggests that USP37 plays a crucial role in promoting chemoresistance in hepatoma cells. The researchers also proposed that the mechanism underlying USP37-mediated chemoresistance involves the deubiquitination and stabilization of NRF2. By deubiquitinating NRF2, USP37 prevents its degradation, leading to increased NRF2 protein levels. This, in turn, activates the NRF2-ARE signaling pathway, which has been associated with chemotherapy resistance.
Our findings suggest that the NRF2-ARE signaling pathway activation, regulated by USP37-mediated NRF2 stabilization, may be responsible for the observed chemotherapy resistance in hepatoma cells. These results provide insights into the molecular mechanisms behind chemoresistance and highlight the potential significance of targeting USP37 as a therapeutic approach to overcome chemotherapy resistance in HCC. Further investigation is warranted to fully understand the role of USP37 in cancer chemotherapy and its potential as a therapeutic target. Overall, this study represents a significant discovery in the field, highlighting the role of USP37 in inducing chemotherapy resistance through NRF2 deubiquitination and stabilization. The findings provide a strong foundation for future investigations and suggest that USP37 could be a promising candidate for overcoming chemoresistance in liver cancer associated with NRF2 activation.
Supplementary Information
Acknowledgements
This work was supported by Henan Provincial Medical Science and Technology Research Program Provincial and Ministerial Joint Construction Project (Grant No.SB201901023) and Henan Provincial Medical Science and Technology Research Joint Construction Project (Grant No. LHGJ20210857).
Author contributions
SZ,FD and FJ performed the majority of the biochemical and cellular experiments. XL and SZ conducted the research design and authored the paper. The final manuscript was read and approved by all authors.
Data availability
The data are available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
IRB approval was obtained for this study at Luoyang Central Hospital Affiliated to Zhengzhou University, according to protocol code LWLL-2022-10-10. All subjects involved in the study provided informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deldar Abad Paskeh M, Mirzaei S, Ashrafizadeh M, Zarrabi A, Sethi G. Wnt/β-catenin signaling as a driver of hepatocellular carcinoma progression: an emphasis on molecular pathways. J Hepatocell Carcinoma. 2021;8:1415–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui F, Shen L, Li L, Wang H, Wang F, Bi S, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy. China Emerg Infect Dis. 2017;23(5):765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Zhang C, Zhang L, Yang Q, Zhou S, Wen Q, et al. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer. 2015;15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nault JC, Rebouissou S, Rossi JZ. NRF2/KEAP1 and Wnt//KEAP1, la in the multistep process of liver carcinogenesis in humans and rats. Hepatology. 2015;62(3):677–9. [DOI] [PubMed] [Google Scholar]
- 6.Yoo NJ, Kim HR, Kim YR, An CH, Lee SH. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60(6):943–52. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Ye W, Zhang Y, Yu D, Shao Q, Liang J, et al. miR-144 reverses chemoresistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Am J Trans Res. 2016;8(7):2992. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Zhang T, Du J. Ursolic acid sensitizes cisplatin-resistant HepG2/DDP cells to cisplatin via inhibiting Nrf2/ARE pathway. Drug Design Dev Ther. 2016;10:3471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao A-M, Ke Z-P, Wang J-N, Yang J-Y, Chen S-Y, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34(8):1806–14. [DOI] [PubMed] [Google Scholar]
- 10.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34(1):21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu M, Fahl WE. Functional characterization of transcription regulators that interact with the electrophile response element. Biochem Biophys Res Commun. 2001;289(1):212–9. [DOI] [PubMed] [Google Scholar]
- 12.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. [DOI] [PubMed] [Google Scholar]
- 13.Lee SB, Sellers BN, DeNicola GM. The regulation of NRF2 by nutrient-responsive signaling and its role in anabolic cancer metabolism. Antioxid Redox Signal. 2018;29(17):1774–91. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Chen ZJ. The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol. 2011;12(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witt A, Vucic D. Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling. Cell Death Differ. 2017;24(7):1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseau A, Bertolotti A. Regulation of proteasome assembly and activity in health and disease. Nat Rev Mol Cell Biol. 2018;19(11):697–712. [DOI] [PubMed] [Google Scholar]
- 17.Rape M. Ubiquitylation at the crossroads of development and disease. Nat Rev Mol Cell Biol. 2018;19(1):59–70. [DOI] [PubMed] [Google Scholar]
- 18.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18(2):69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458(7237):438–44. [DOI] [PubMed] [Google Scholar]
- 20.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20(11):1242–53. [DOI] [PubMed] [Google Scholar]
- 21.Heng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. 2017;86:129–57. [DOI] [PubMed] [Google Scholar]
- 22.Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18(6):579–86. [DOI] [PubMed] [Google Scholar]
- 23.Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016;26(4):399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281(34):24756–68. [DOI] [PubMed] [Google Scholar]
- 25.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao S, Liu P, Luo G, Rojo de la Vega M, Chen H, Wu T, et al. p97 negatively regulates NRF2 by extracting ubiquitylated NRF2 from the KEAP1-CUL3 E3 complex. Mol Cell Biol. 2017;37(8):e00660-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23(22):8137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32(32):3765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu T, Zhao F, Gao B, Tan C, Yagishita N, Nakajima T, et al. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28(7):708–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou Z, Shi W, Feng J, Wang W, Zheng E, Lin H, et al. Self-stabilizing regulation of deubiquitinating enzymes in an enzymatic activity-dependent manner. Int J Biol Macromol. 2021;181:1081–91. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Lin Y, Feng J, Qi Y, Wang X, Lin Q, et al. The deubiquitinating enzyme OTUD1 antagonizes BH3-mimetic inhibitor induced cell death through regulating the stability of the MCL1 protein. Cancer Cell Int. 2019;19:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Zhang ZY, Du H, Li SZ, Tu R, Jia YF, et al. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26(11):2300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimta A-A, Cenariu D, Irimie A, Magdo L, Nabavi SM, Atanasov AG, et al. The role of Nrf2 activity in cancer development and progression. Cancers. 2019;11(11):1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang M, Namani A, Wu S, Wang X. Nrf2: bane or blessing in cancer? J Cancer Res Clin Oncol. 2014;140(8):1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai X, Chen Y, Hou X, Huang M, Jin J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab Rev. 2016;48(4):541–67. [DOI] [PubMed] [Google Scholar]
- 39.Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–32. [DOI] [PubMed] [Google Scholar]
- 40.Sadagopan N, He AR. Recent progress in systemic therapy for advanced hepatocellular carcinoma. Int J Mol Sci. 2024;25(2):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim GS, Manandhar S, Shin DH, Kim TH, Kwak MK. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic Biol & Med. 2009;47:1619–31. [DOI] [PubMed] [Google Scholar]
- 42.Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, et al. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol Carcinog. 2013;52:824–34. [DOI] [PubMed] [Google Scholar]
- 43.Qin T, Li B, Feng X, Fan S, Liu L, Liu D, Mao J, Lu Y, Yang J, Yu X, Zhang Q, Zhang J, Song B, Li M, Li L. Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity. J Exp Clin Cancer Res. 2018;37(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request from the corresponding author.