Abstract
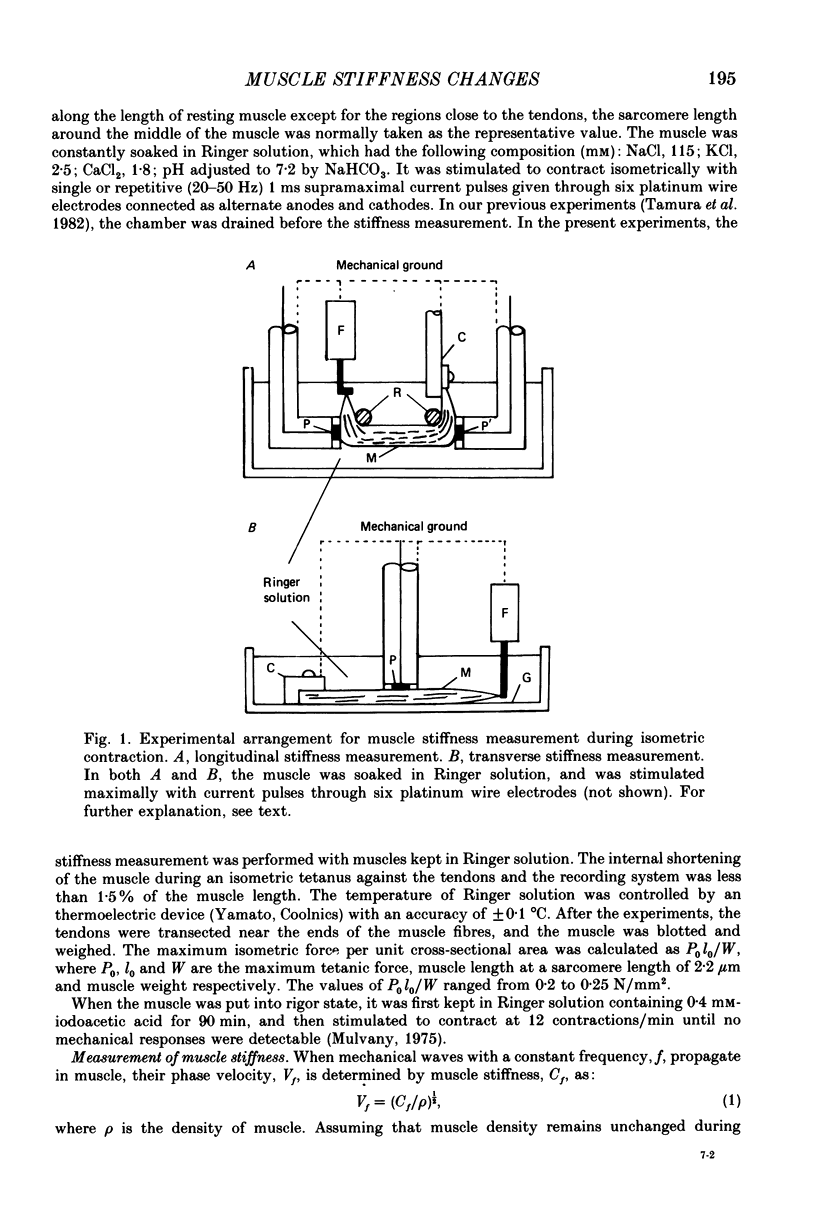
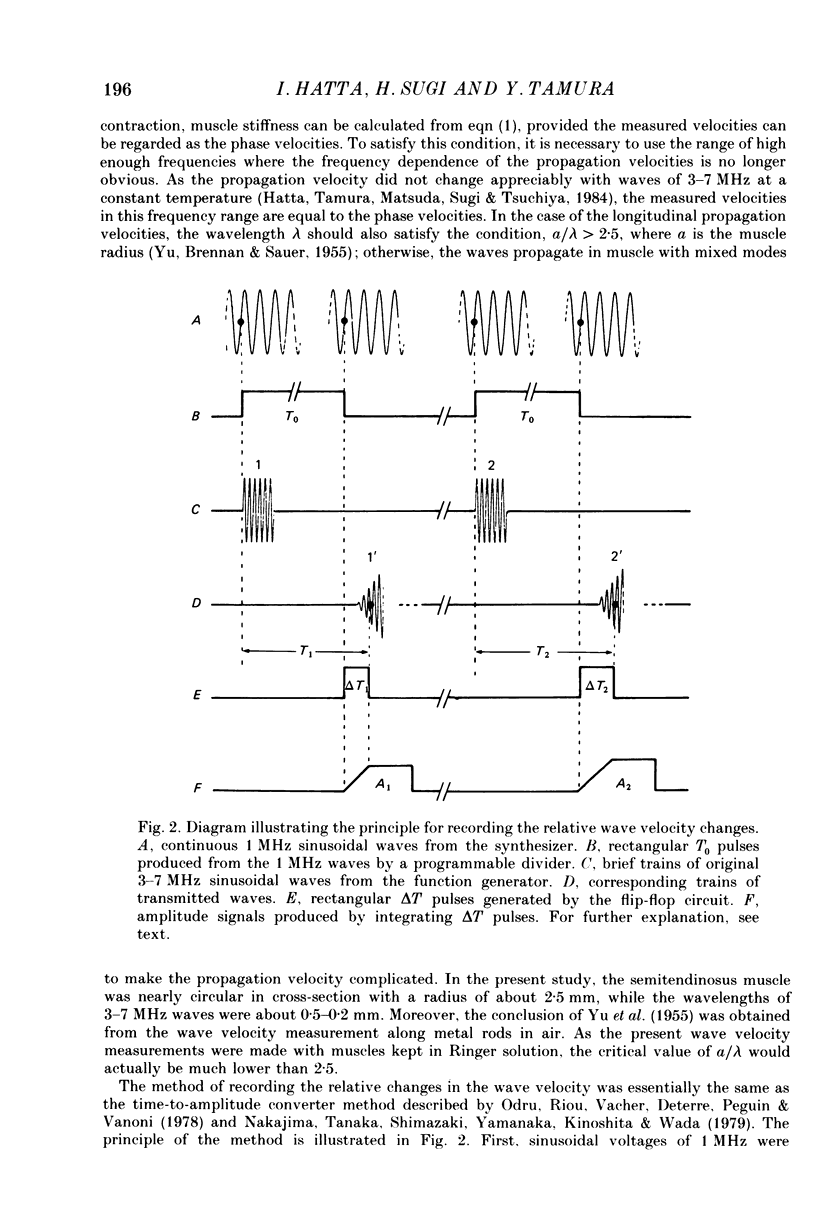
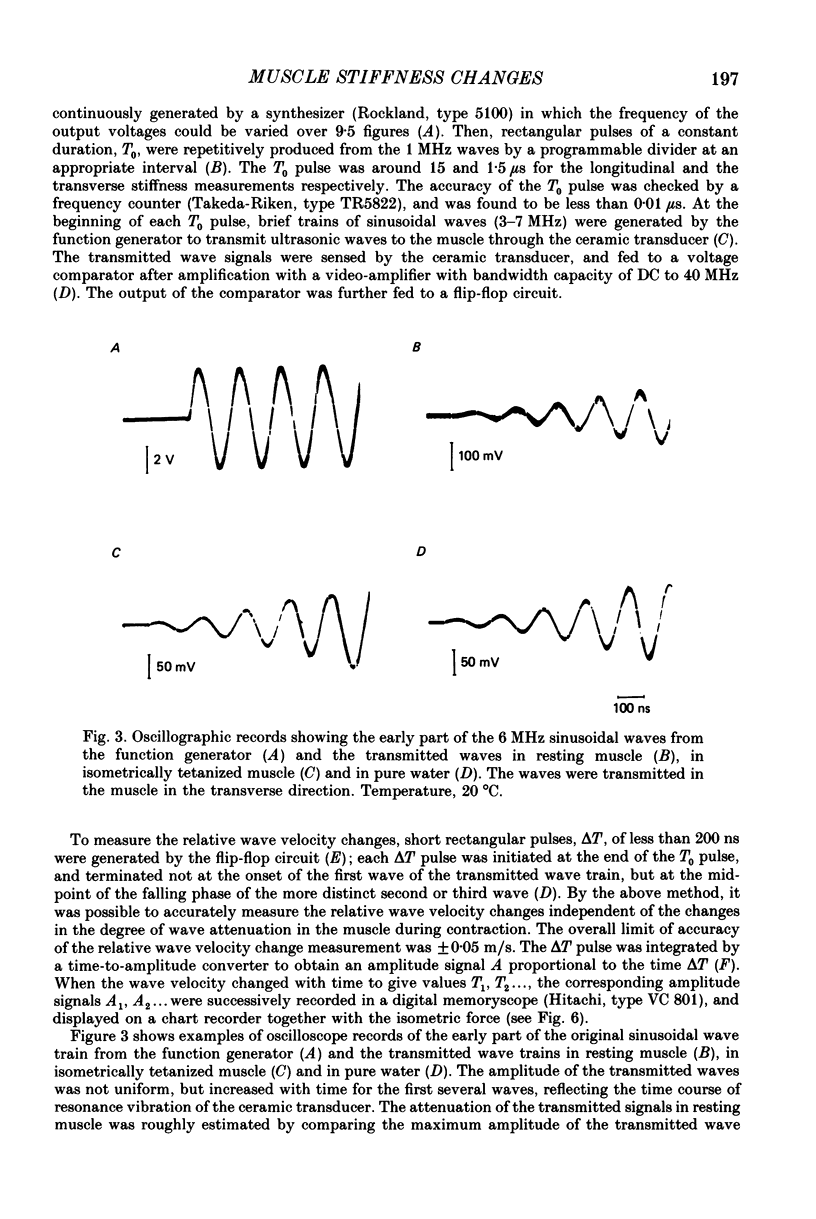
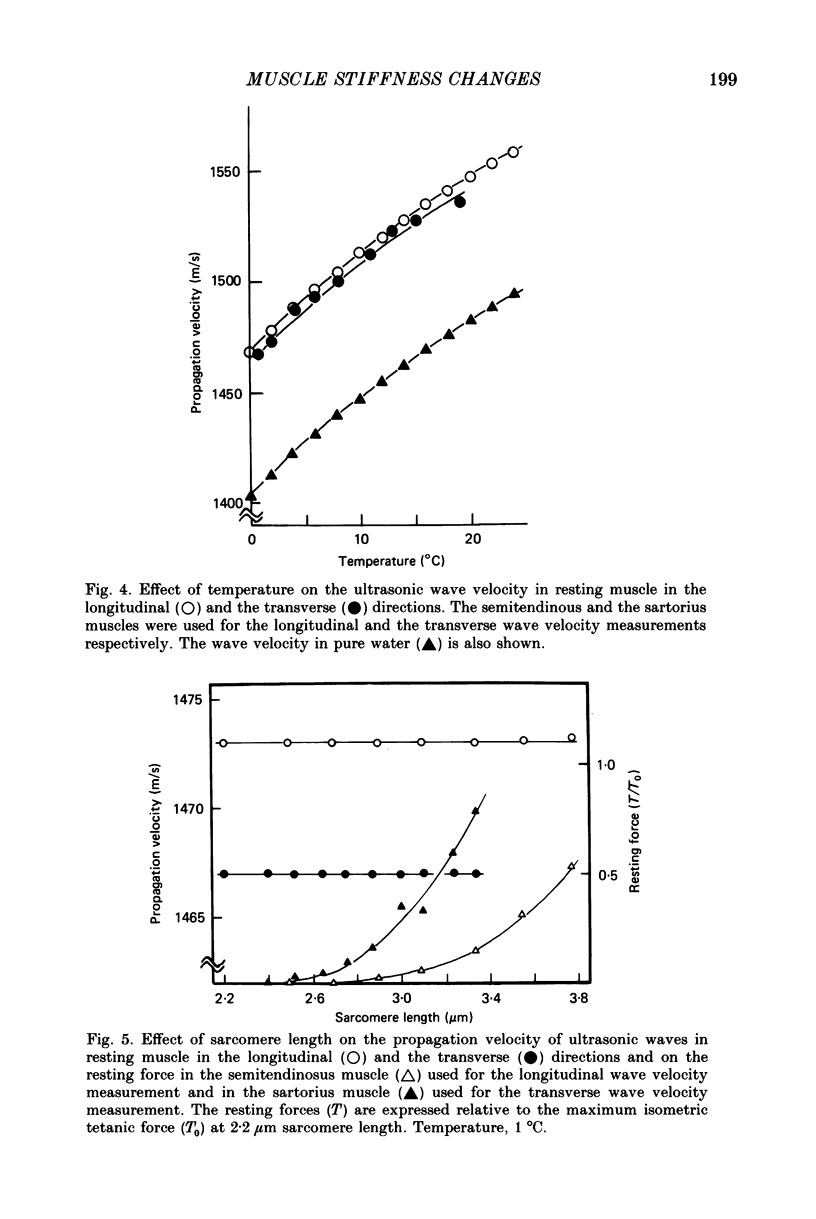
1. A technique has been developed with which the stiffness changes in frog skeletal muscle can be continuously recorded by measuring the propagation velocity of ultrasonic waves (3-7 MHz) with negligibly small perturbations to the contractile system. 2. The resting muscle stiffness was 2.256 +/- 0.002 x 10(9) N/m2 (S.D.) at 1-2 degrees C (n = 10) and 2.480 +/- 0.007 x 10(9) N/m2 at 19-20 degrees C (n = 12) in the longitudinal direction, and 2.223 +/- 0.008 x 10(9) N/m2 at 1-2 degrees C (n = 8) and 2.437 +/- 0.007 x 10(9) N/m2 at 19-20 degrees C (n = 9) in the transverse direction. 3. The resting muscle stiffness measured with ultrasonic waves was virtually insensitive to the resting force development, i.e. the extension of the parallel elastic component. 4. The longitudinal muscle stiffness increased during isometric contraction at a rate faster than the force development. The amount of increase of the longitudinal stiffness in an isometric tetanus at 2.2 microns sarcomere length was 2.4 +/- 0.1 x 10(7) N/m2 at 1-2 degrees C (n = 10) and 6.5 +/- 1.3 x 10(7) N/m2 at 19-20 degrees C (n = 12). 5. On the other hand, the transverse muscle stiffness decreased during isometric contraction at a rate faster than the force development. The amount of decrease of the transverse stiffness in an isometric tetanus at 2.2 microns sarcomere length was 5.6 +/- 0.1 x 10(7) N/m2 at 1-2 degrees C (n = 8) and 6.4 +/- 0.3 x 10(7) N/m2 at 19-20 degrees C (n = 9). 6. The amount of both the longitudinal and the transverse stiffness changes during an isometric tetanus decreased linearly with increasing sarcomere length, indicating that the stiffness changes during contraction reflect the formation of cross-links between the myofilaments. 7. Both the longitudinal and the transverse stiffness increased when resting muscle was put into rigor state. The rigor muscle stiffness was insensitive to small stretches, i.e. the strain of the rigor cross-links. 8. These results are discussed in connection with the behaviour of cross-bridges during isometric contraction and in rigor.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cecchi G., Griffiths P. J., Taylor S. The kinetics of cross-bridge attachment and detachment studied by high frequency stiffness measurements. Adv Exp Med Biol. 1984;170:641–655. doi: 10.1007/978-1-4684-4703-3_60. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during the rise of tetanic tension in frog muscle fibres. J Physiol. 1986 Mar;372:595–609. doi: 10.1113/jphysiol.1986.sp016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hasan H., Mason P. Pulse propagation in muscle. Phys Med Biol. 1978 Sep;23(5):917–927. doi: 10.1088/0031-9155/23/5/008. [DOI] [PubMed] [Google Scholar]
- Hatta I., Tamura Y., Matsuda T., Sugi H., Tsuchiya T. Muscle stiffness changes during isometric contraction in frog skeletal muscle as studied by the use of ultrasonic waves. Adv Exp Med Biol. 1984;170:673–686. doi: 10.1007/978-1-4684-4703-3_62. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Mason P. Dynamic stiffness and crossbridge action in muscle. Biophys Struct Mech. 1977 Dec 27;4(1):15–25. doi: 10.1007/BF00538837. [DOI] [PubMed] [Google Scholar]
- Mason P., Hasan H. Muscle crossbridge action in excitation and relaxation. Experientia. 1980 Aug 15;36(8):949–950. doi: 10.1007/BF01953810. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Yagi N. A time-resolved X-ray diffraction study of muscle during twitch. J Physiol. 1978 May;278:297–307. doi: 10.1113/jphysiol.1978.sp012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J. Mechanical properties of frog skeletal muscles in iodoacetic acid rigor. J Physiol. 1975 Nov;252(2):319–334. doi: 10.1113/jphysiol.1975.sp011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassiri D. K., Nicholas D., Hill C. R. Attenuation of ultrasound in skeletal muscle. Ultrasonics. 1979 Sep;17(5):230–232. doi: 10.1016/0041-624x(79)90054-4. [DOI] [PubMed] [Google Scholar]
- Odru R., Riou C., Vacher J., Deterre P., Peguin P., Vanoni F. New instrument for continuous and simultaneous recording of changes in ultrasonic attenuation and velocity. Rev Sci Instrum. 1978 Feb;49(2):238–238. doi: 10.1063/1.1135375. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Wells J. B., Podolsky R. J. Muscle compliance and the longitudinal transmission of mechanical impulses. J Gen Physiol. 1974 Dec;64(6):623–642. doi: 10.1085/jgp.64.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y., Hatta I., Matsuda T., Sugi H., Tsuchiya T. Changes in muscle stiffness during contraction recorded using ultrasonic waves. Nature. 1982 Oct 14;299(5884):631–633. doi: 10.1038/299631a0. [DOI] [PubMed] [Google Scholar]
- Truong X. T. Viscoelastic wave propagation and rheologic properties of skeletal muscle. Am J Physiol. 1974 Feb;226(2):256–264. doi: 10.1152/ajplegacy.1974.226.2.256. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Amemiya Y., Fujishima A., Kobayashi T., Hamanaka T., Sugi H., Mitsui T. Time-resolved x-ray diffraction studies on the intensity changes of the 5.9 and 5.1 nm actin layer lines from frog skeletal muscle during an isometric tetanus using synchrotron radiation. Biophys J. 1985 Jun;47(6):847–850. doi: 10.1016/S0006-3495(85)83989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



