Abstract
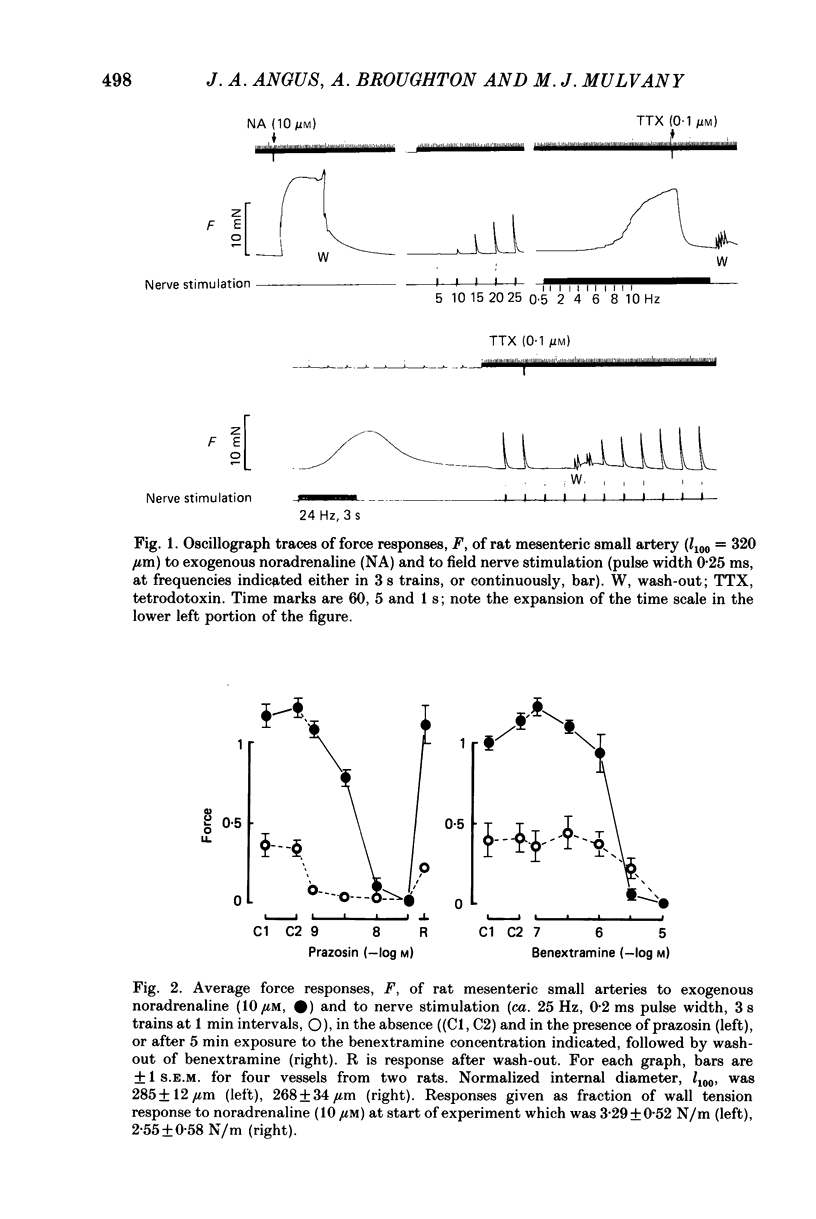
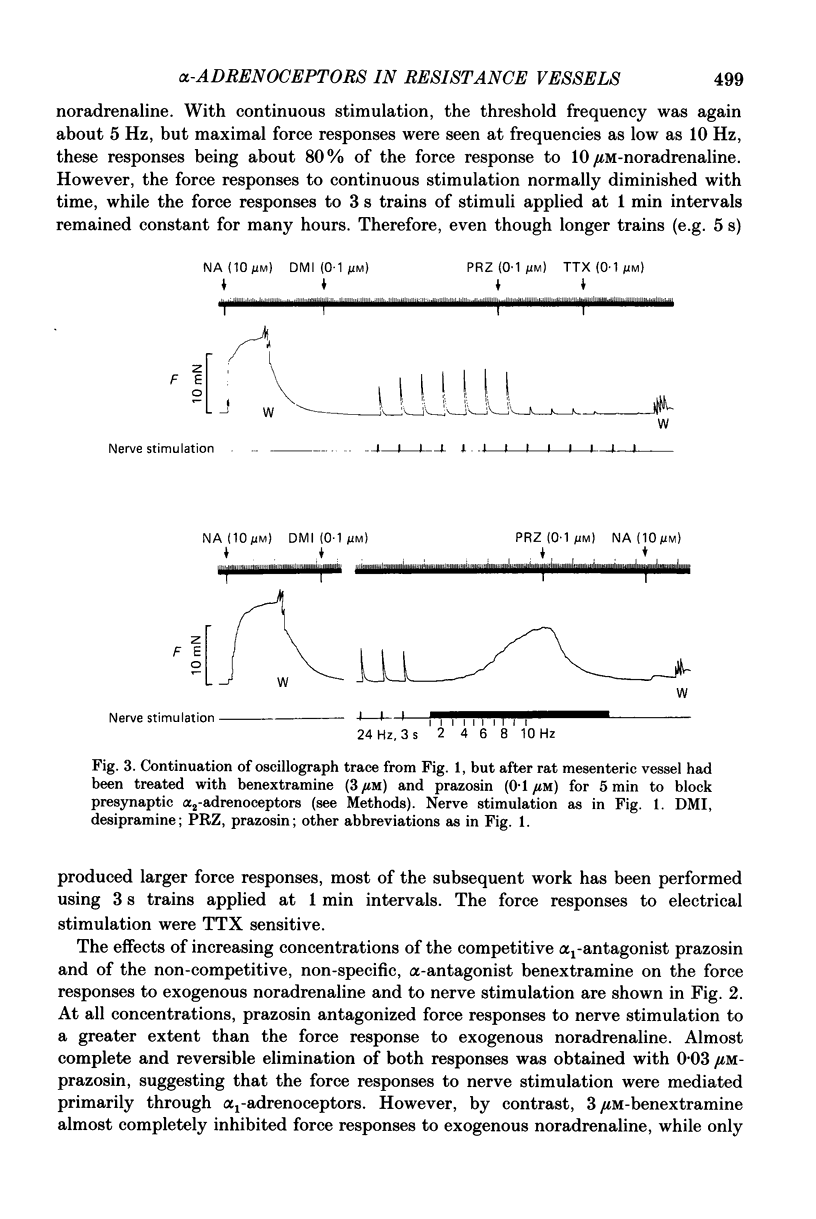
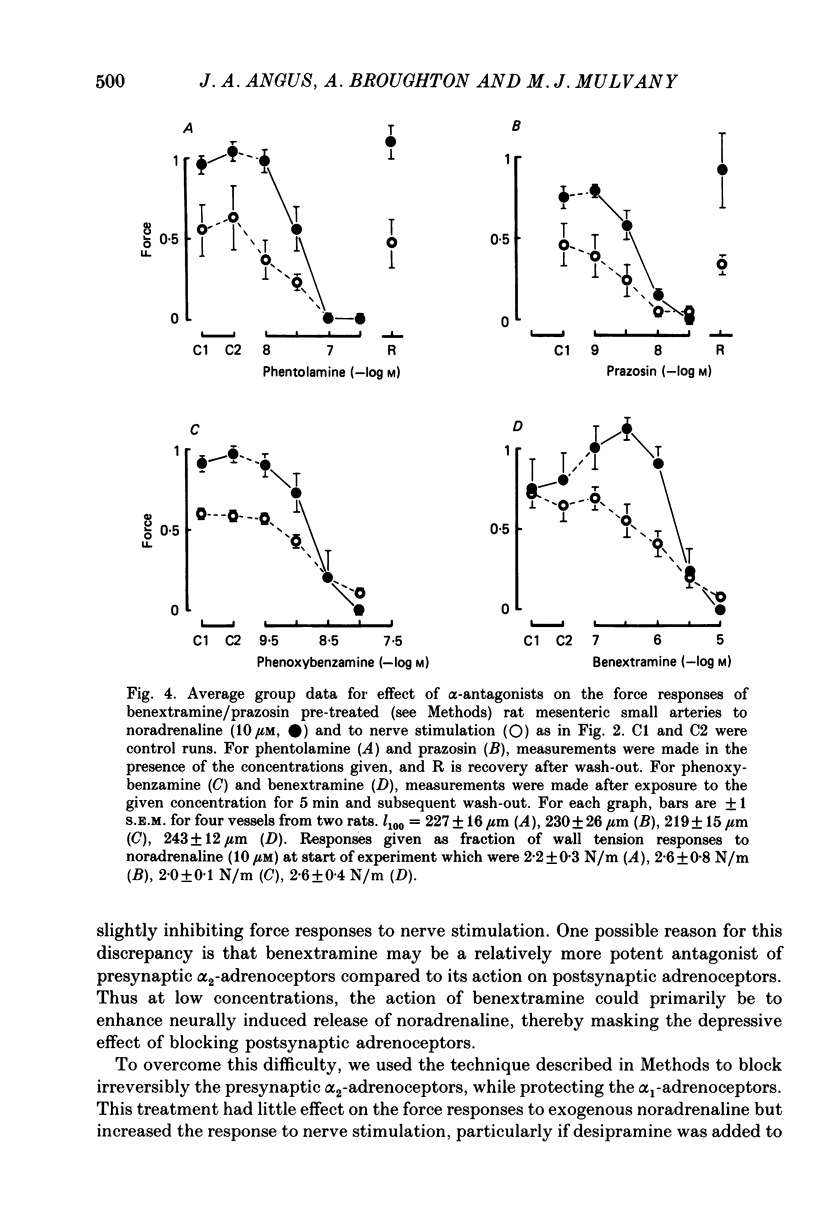
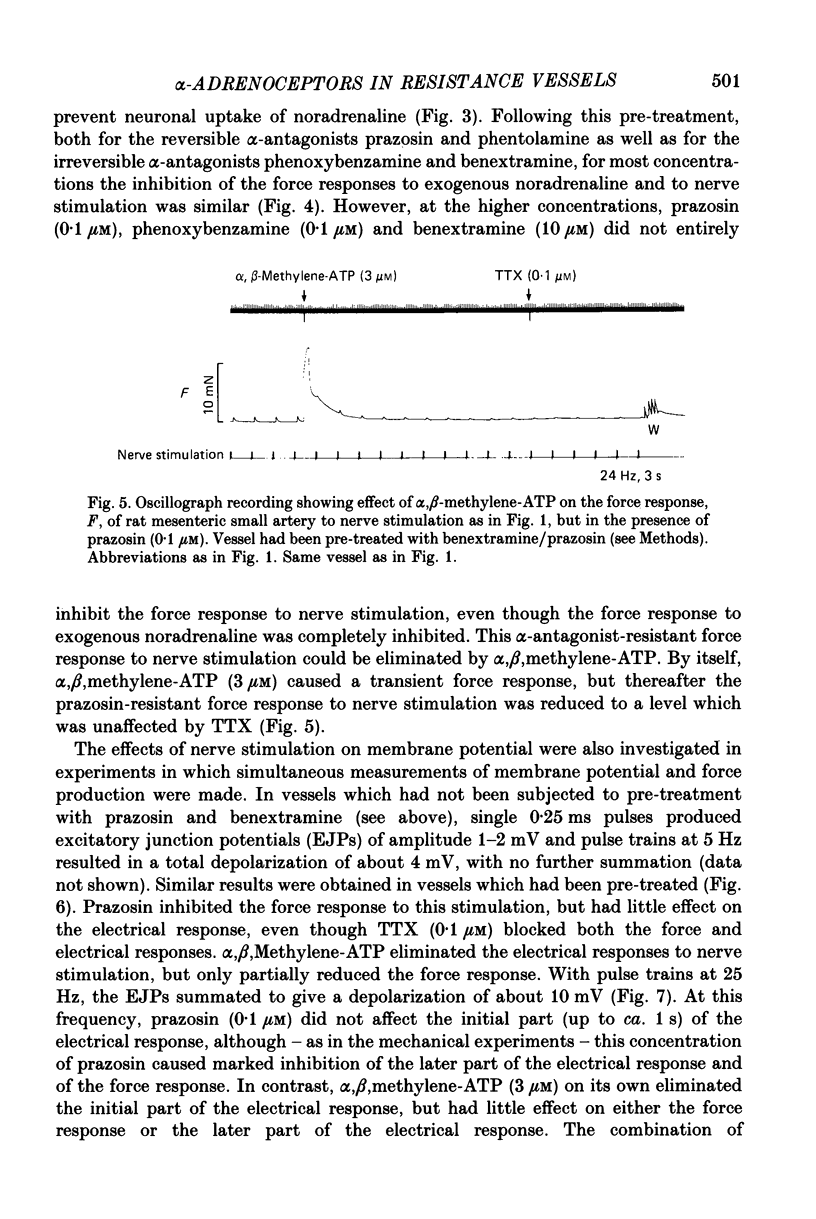
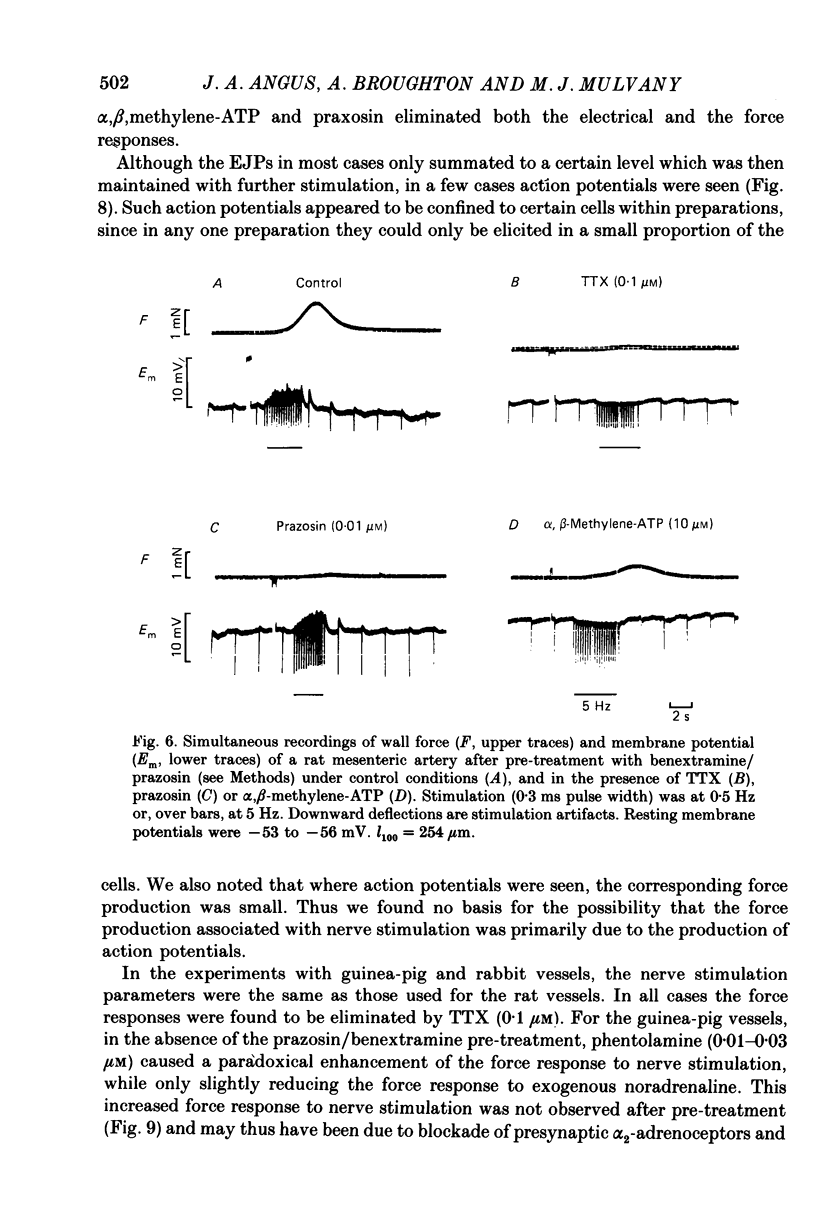
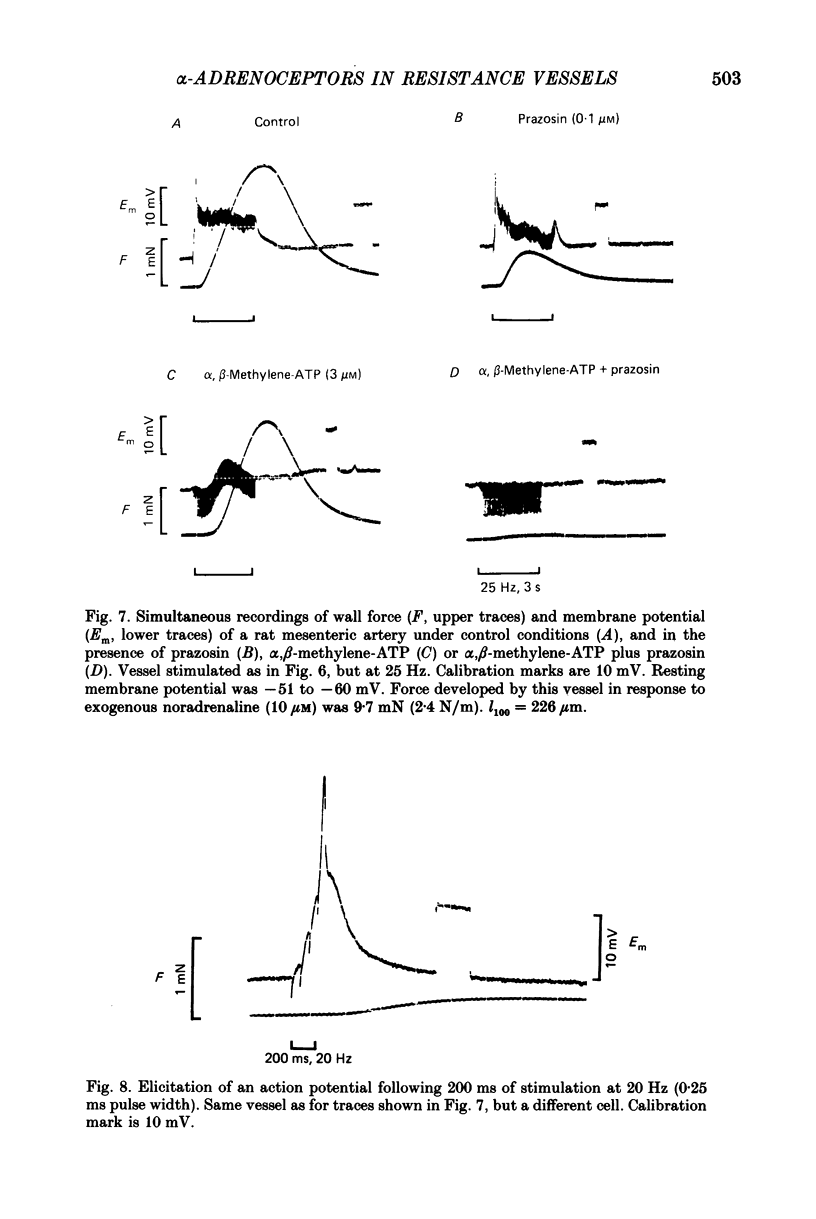
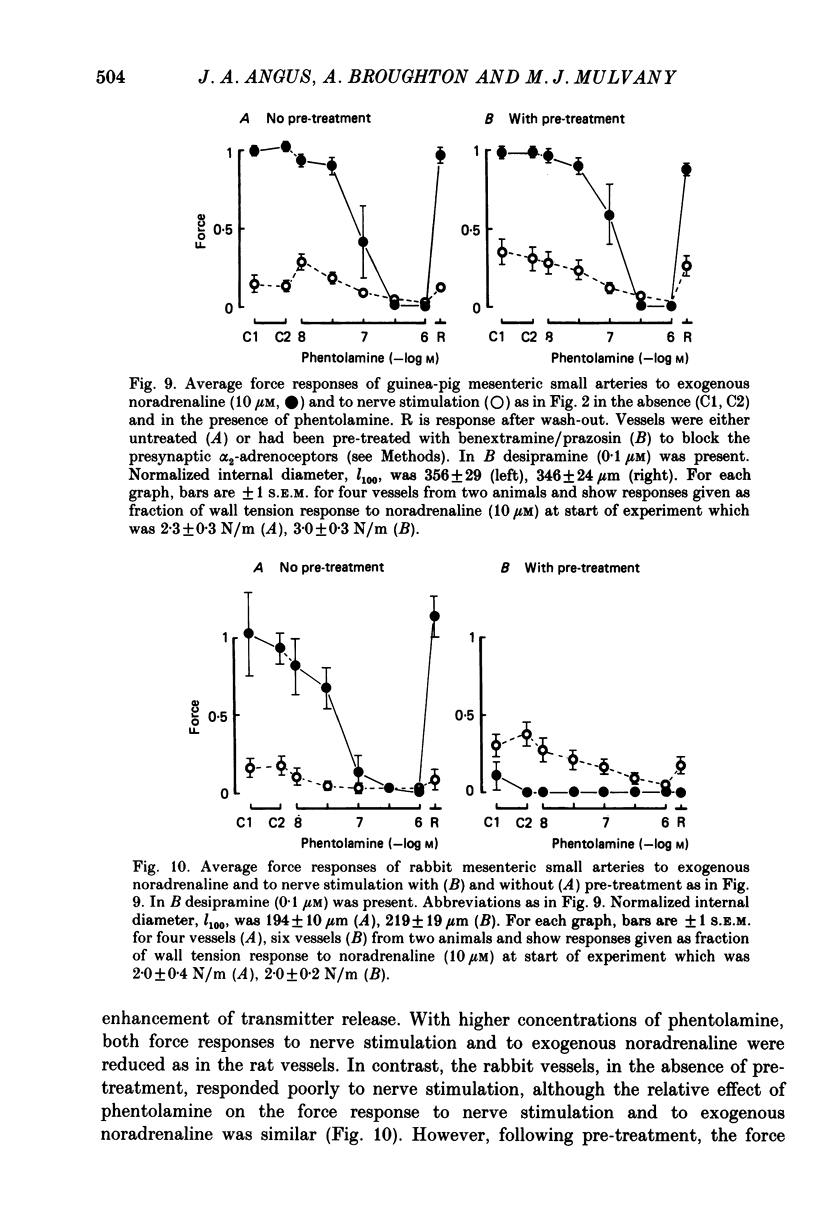
1. We have investigated the adrenoceptors mediating the force and electrical responses of rat mesenteric small arteries (i.d. 100-300 microns). Some mechanical experiments were also performed using guinea-pig and rabbit mesenteric small arteries. 2. Vessels were mounted on an isometric myograph and stimulated either with short (3 s) trains of electric field stimuli (ca. 0.2 ms pulse width) at 25 Hz (nerve stimulation) or with 10 microM-exogenous noradrenaline. 3. Nerve stimulation caused a force response equal to ca. 40% of the response to exogenous noradrenaline and, in the rat vessels, excitatory junction potentials (EJPs), which normally summated to give a depolarization of ca. 10 mV (although action potentials were sometimes seen). 4. Almost complete and reversible inhibition of the force responses of all vessels to both exogenous noradrenaline and to nerve stimulation was obtained using prazosin (0.1 microM) or phentolamine (1 microM). 5. Irreversible blockade of alpha 2-receptors enhanced the force response of all vessels to nerve stimulation by ca. 50%, but did not affect the force response of rat and guinea-pig vessels to exogenous noradrenaline. In the rabbit vessels this force response was abolished by alpha 2-blockade. 6. Following alpha 2-blockade, in the rat vessels the alpha-antagonists prazosin (0.1 microM), phentolamine (0.1 microM), phenoxybenzamine (0.01 microM) and benextramine (10 microM) all totally abolished the force response to exogenous noradrenaline, and inhibited the response to nerve stimulation by at least 80%. Similar effects of phentolamine were seen in the guinea-pig and, for the response to nerve stimulation, in the rabbit vessels. 7. In the rat vessels, alpha-adrenoceptor antagonists did not affect the EJPs, but did inhibit the small depolarization which resulted from several seconds of nerve stimulation. The ATP analogue alpha, beta-methylene-ATP (3 microM) abolished the EJPs, but only slightly reduced the force responses. 8. The results suggest that the force response to nerve stimulation of the rat mesenteric small arteries is mediated primarily through alpha-adrenoceptors, but also to a small degree through non-alpha-adrenoceptors, possibly ATP receptors.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. Comparison of the antagonistic effects of phentolamine on vasoconstrictor responses to exogenous and neurally released noradrenaline in vivo. Br J Pharmacol. 1985 May;85(1):249–253. doi: 10.1111/j.1476-5381.1985.tb08853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Su C. Distribution theory of resistance of neurogenic vasoconstriction to alpha-receptor blockade in the rabbit. Circ Res. 1971 Feb;28(2):179–187. doi: 10.1161/01.res.28.2.179. [DOI] [PubMed] [Google Scholar]
- Bohlen H. G. Intestinal microvascular adaptation during maturation of spontaneously hypertensive rats. Hypertension. 1983 Sep-Oct;5(5):739–745. doi: 10.1161/01.hyp.5.5.739. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976 Aug;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Cheung D. W. Neural regulation of electrical and mechanical activities in the rat tail artery. Pflugers Arch. 1984 Mar;400(3):335–337. doi: 10.1007/BF00581570. [DOI] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. R., Starke K. An examination of the pre- and postsynaptic alpha-adrenoceptors involved in neuroeffector transmission in rabbit aorta and portal vein. Br J Pharmacol. 1982 Jun;76(2):327–335. doi: 10.1111/j.1476-5381.1982.tb09224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F. Dibenamine blockade in strips of rabbit aorta and its use in differentiating receptors. J Pharmacol Exp Ther. 1954 Jul;111(3):265–284. [PubMed] [Google Scholar]
- Flavahan N. A., Grant T. L., Greig J., McGrath J. C. Analysis of the alpha-adrenoceptor-mediated, and other, components in the sympathetic vasopressor responses of the pithed rat. Br J Pharmacol. 1985 Sep;86(1):265–274. doi: 10.1111/j.1476-5381.1985.tb09458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Lew M. J. Lack of involvement of alpha-adrenoceptors in sympathetic neural vasoconstriction in the hindquarters of the rabbit. Br J Pharmacol. 1987 Jan;90(1):51–60. doi: 10.1111/j.1476-5381.1987.tb16824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O., Silverberg G. D. Noradrenaline receptors on the rat basilar artery. J Physiol. 1982 Jul;328:351–360. doi: 10.1113/jphysiol.1982.sp014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan T., Hjemdahl P. Pre- and postjunctional alpha-adrenoceptor-mediated effects of prazosin, methoxamine and 6-fluoronoradrenaline in blood-perfused canine skeletal muscle in situ. Eur J Pharmacol. 1987 Jan 6;133(1):9–20. doi: 10.1016/0014-2999(87)90200-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Saville V. L., Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986 Apr 2;122(3):291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Laher I., Khayal M. A., Bevan J. A. Norepinephrine-sensitive, phenoxybenzamine-resistant receptor sites associated with contraction in rabbit arterial but not venous smooth muscle: possible role in adrenergic neurotransmission. J Pharmacol Exp Ther. 1986 May;237(2):364–368. [PubMed] [Google Scholar]
- Madjar H., Docherty J. R., Starke K. An examination of pre- and postsynaptic alpha-adrenoceptors in the autoperfused rabbit hindlimb. J Cardiovasc Pharmacol. 1980 Sep-Oct;2(5):619–627. doi: 10.1097/00005344-198009000-00011. [DOI] [PubMed] [Google Scholar]
- Medgett I. C., Langer S. Z. Heterogeneity of smooth muscle alpha adrenoceptors in rat tail artery in vitro. J Pharmacol Exp Ther. 1984 Jun;229(3):823–830. [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Nyborg N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol. 1980;71(2):585–596. doi: 10.1111/j.1476-5381.1980.tb10977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Warshaw D. M. The active tension-length curve of vascular smooth muscle related to its cellular components. J Gen Physiol. 1979 Jul;74(1):85–104. doi: 10.1085/jgp.74.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Keef K. Measurements of the membrane potential of arterial smooth muscle in anesthetized animals and its relationship to changes in artery diameter. Microvasc Res. 1985 Jul;30(1):19–28. doi: 10.1016/0026-2862(85)90034-2. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Kotecha N. Relation between membrane potential and contractile force in smooth muscle of the rat tail artery during stimulation by norepinephrine, 5-hydroxytryptamine, and potassium. Circ Res. 1987 May;60(5):791–795. doi: 10.1161/01.res.60.5.791. [DOI] [PubMed] [Google Scholar]
- Neild T., Kotecha N. Two-component responses to sympathetic nerve stimulation in the rat tail artery. Comp Biochem Physiol C. 1985;81(2):311–317. doi: 10.1016/0742-8413(85)90012-x. [DOI] [PubMed] [Google Scholar]
- Nilsson H. Different nerve responses in consecutive sections of the arterial system. Acta Physiol Scand. 1984 Aug;121(4):353–361. doi: 10.1111/j.1748-1716.1984.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Ljung B., Sjöblom N., Wallin B. G. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol Scand. 1985 Mar;123(3):303–309. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Owen M. P., Quinn C., Bevan J. A. Phentolamine-resistant neurogenic constriction occurs in small arteries at higher frequencies. Am J Physiol. 1985 Aug;249(2 Pt 2):H404–H414. doi: 10.1152/ajpheart.1985.249.2.H404. [DOI] [PubMed] [Google Scholar]
- SPEDEN R. N. ELECTRICAL ACTIVITY OF SINGLE SMOOTH MUSCLE CELLS OF THE MESENTERIC ARTERY PRODUCED BY SPLANCHNIC NERVE STIMULATION IN THE GUINEA PIG. Nature. 1964 Apr 11;202:193–194. doi: 10.1038/202193a0. [DOI] [PubMed] [Google Scholar]
- SU C., BEVAN J. A., URSILLO R. C. ELECTRICAL QUIESCENCE OF PULMONARY ARTERY SMOOTH MUSCLE DURING SYMPATHOMIMETIC STIMULATION. Circ Res. 1964 Jul;15:26–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steedman W. M. Micro-electrode studies on mammalian vascular muscle. J Physiol. 1966 Oct;186(2):382–400. doi: 10.1113/jphysiol.1966.sp008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T., Nakajima M., Miyazaki M. Modification by yohimbine and prazosin of the mechanical response of isolated dog mesenteric, renal and coronary arteries to transmural stimulation and norepinephrine. Eur J Pharmacol. 1984 Feb 10;98(1):69–78. doi: 10.1016/0014-2999(84)90110-9. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


