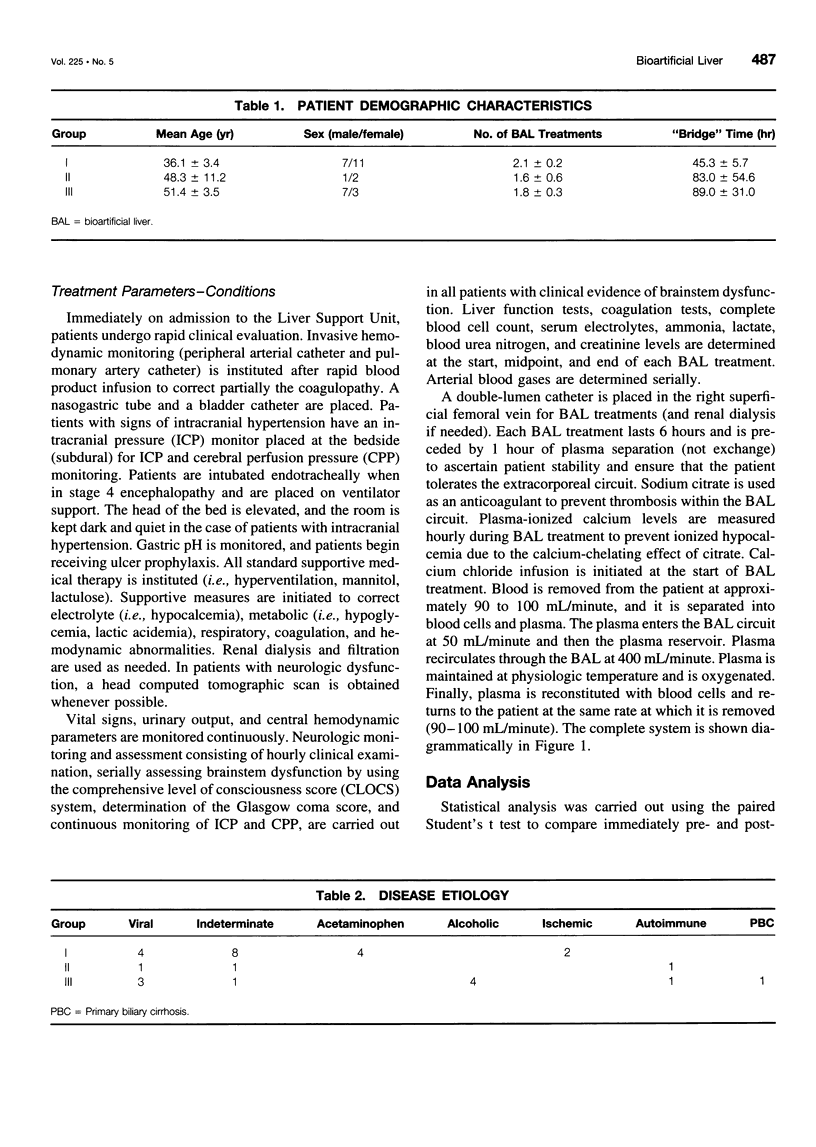
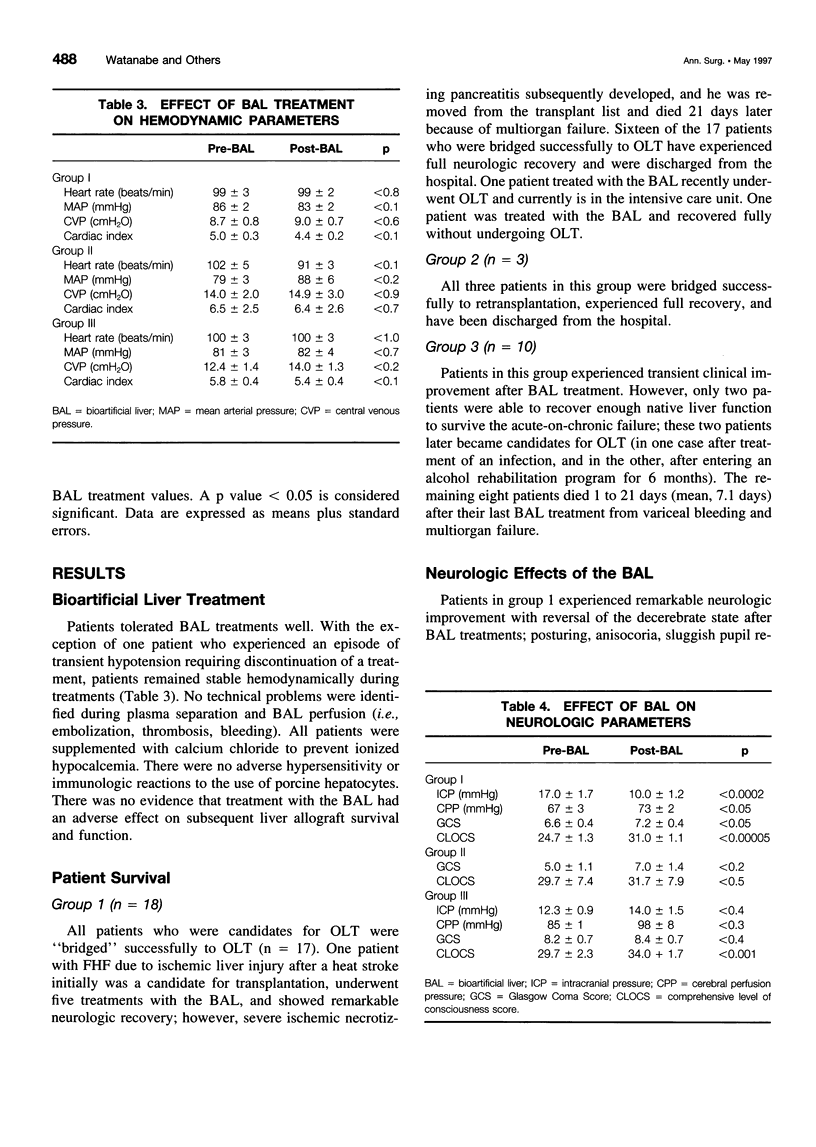
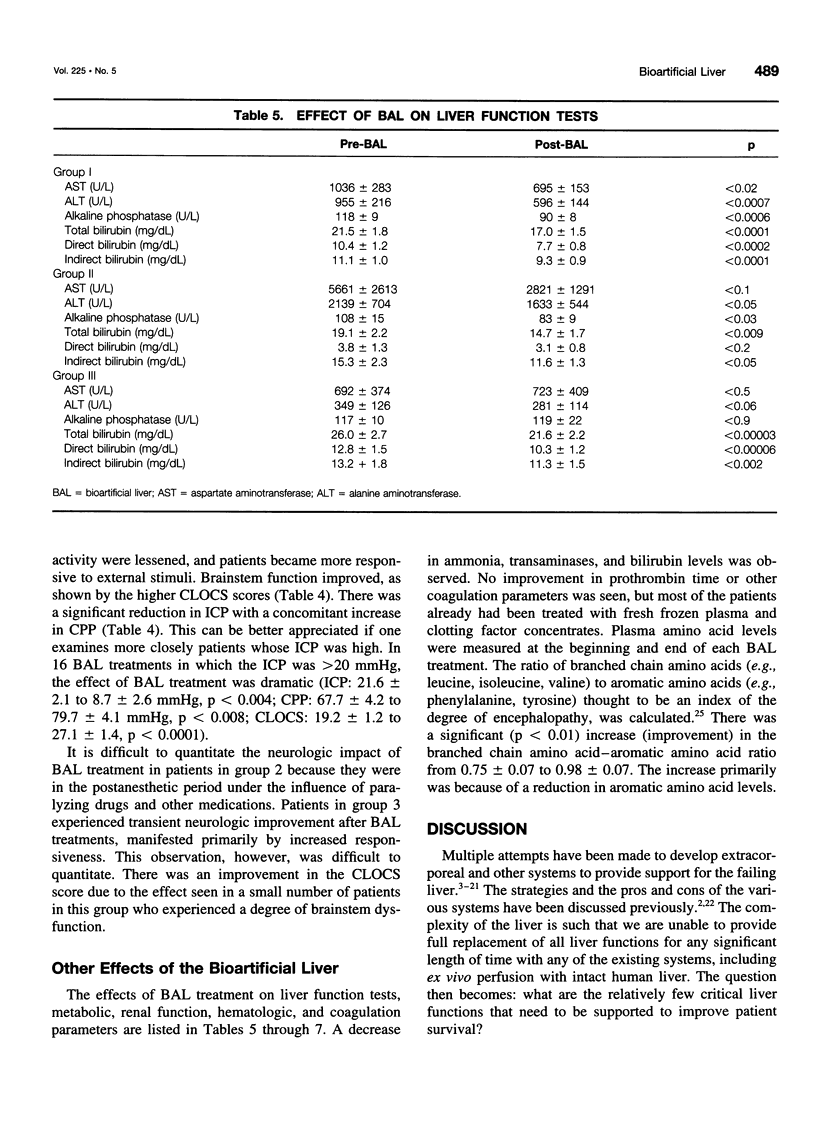
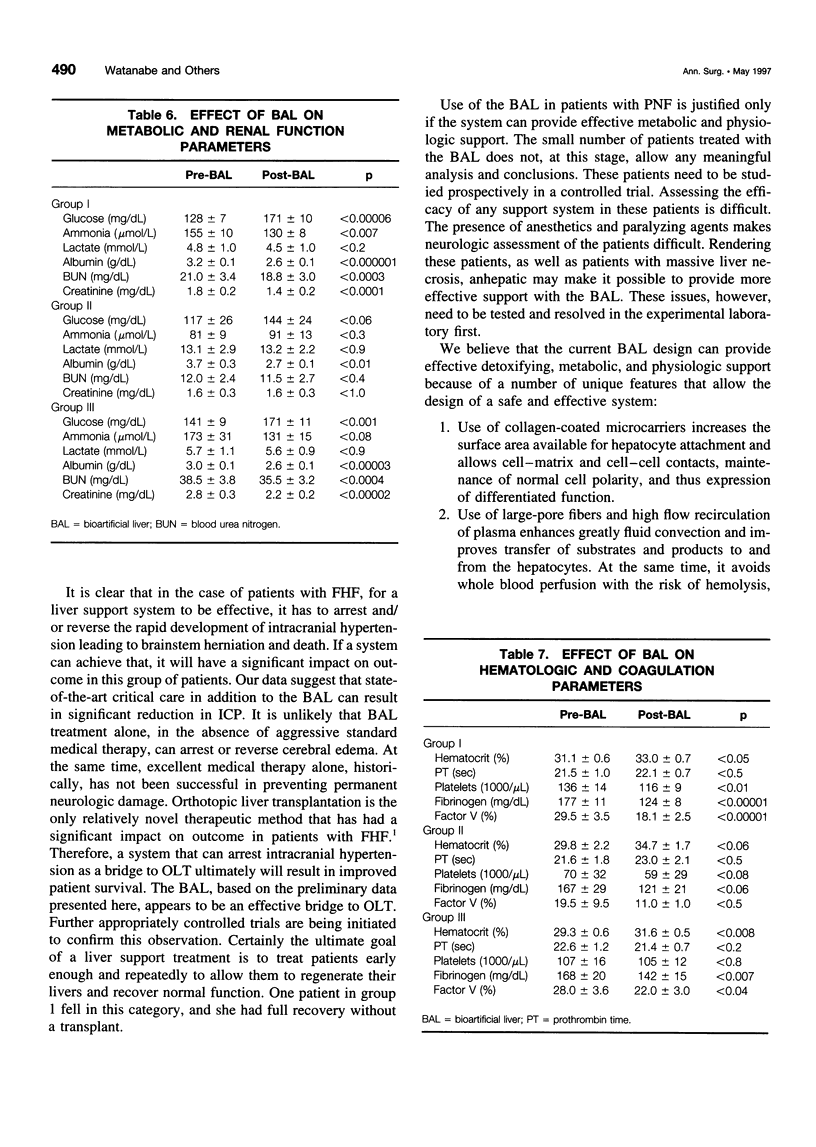
Abstract
OBJECTIVE: The purpose of this study was to develop a bioartificial liver (BAL) to treat patients with severe liver failure until they can be either transplanted or recover spontaneously. SUMMARY BACKGROUND DATA: Severe acute liver failure is associated with high mortality. Liver transplantation has emerged as an effective therapy for patients who did not respond to standard management. However, because of the donor organ shortage and urgent need for transplantation, many patients die before they can be transplanted and others do not survive after transplantation, primarily because of intracranial hypertension. METHODS: Three groups of patients with severe acute liver failure were treated with the BAL. In group 1 (n = 18) were patients with fulminant hepatic failure (FHF), in group 2 (n = 3) were patients with primary nonfunction (PNF) of a transplanted liver, and in group 3 (n = 10) were patients with acute exacerbation of chronic liver disease. Patients in groups 1 and 2 were candidates for transplantation at the time they entered the study, whereas patients in group 3 were not. RESULTS: In group 1, 16 patients were "bridged" successfully to transplantation, 1 patient was bridged to recovery without a transplant, and 1 patient died because of concomitant severe pancreatitis. In group 2, all patients were bridged successfully to retransplantation. In group 3, two patients were supported to recovery and successful transplants at later dates; the other eight patients, although supported temporarily with the BAL, later died because they were not candidates for transplantation. CONCLUSIONS: The authors' clinical experience with the BAL has yielded encouraging results. A randomized, controlled, prospective trial (phase II-III) is being initiated to determine the efficacy of the system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bihari D., Hughes R. D., Gimson A. E., Langley P. G., Ede R. J., Eder G., Williams R. Effects of serial resin hemoperfusion in fulminant hepatic failure. Int J Artif Organs. 1983 Nov;6(6):299–302. [PubMed] [Google Scholar]
- Chari R. S., Collins B. H., Magee J. C., DiMaio J. M., Kirk A. D., Harland R. C., McCann R. L., Platt J. L., Meyers W. C. Brief report: treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N Engl J Med. 1994 Jul 28;331(4):234–237. doi: 10.1056/NEJM199407283310404. [DOI] [PubMed] [Google Scholar]
- Cooper G. N., Jr, Karlson K. E., Clowes G. H., Martin H., Randall H. T. Total blood washout and exchange. A valuable tool in acute hepatic coma and Reye's syndrome. Am J Surg. 1977 Apr;133(4):522–530. doi: 10.1016/0002-9610(77)90142-8. [DOI] [PubMed] [Google Scholar]
- Demetriou A. A., Rozga J., Podesta L., Lepage E., Morsiani E., Moscioni A. D., Hoffman A., McGrath M., Kong L., Rosen H. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995;208:111–117. doi: 10.3109/00365529509107771. [DOI] [PubMed] [Google Scholar]
- Fox I. J., Langnas A. N., Fristoe L. W., Shaefer M. S., Vogel J. E., Antonson D. L., Donovan J. P., Heffron T. G., Markin R. S., Sorrell M. F. Successful application of extracorporeal liver perfusion: a technology whose time has come. Am J Gastroenterol. 1993 Nov;88(11):1876–1881. [PubMed] [Google Scholar]
- Lee W. M. Acute liver failure. N Engl J Med. 1993 Dec 16;329(25):1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- Matsumura K. N., Guevara G. R., Huston H., Hamilton W. L., Rikimaru M., Yamasaki G., Matsumura M. S. Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery. 1987 Jan;101(1):99–103. [PubMed] [Google Scholar]
- Morsiani E., Rozga J., Scott H. C., Lebow L. T., Moscioni A. D., Kong L. B., McGrath M. F., Demetriou A. A. Automated liver cell processing facilitates large scale isolation and purification of porcine hepatocytes. ASAIO J. 1995 Apr-Jun;41(2):155–161. [PubMed] [Google Scholar]
- Neuzil D. F., Rozga J., Moscioni A. D., Ro M. S., Hakim R., Arnaout W. S., Demetriou A. A. Use of a novel bioartificial liver in a patient with acute liver insufficiency. Surgery. 1993 Mar;113(3):340–343. [PubMed] [Google Scholar]
- Nyberg S. L., Remmel R. P., Mann H. J., Peshwa M. V., Hu W. S., Cerra F. B. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994 Jul;220(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- Olumide F., Eliashiv A., Kralios N., Norton L., Eiseman B. Hepatic support with hepatocyte suspensions in a permeable membrane dialyzer. Surgery. 1977 Nov;82(5):599–606. [PubMed] [Google Scholar]
- Record C. O., Buxton B., Chase R. A., Curzon G., Murray-Lyon I. M., Williams R. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976 Sep 10;6(5):387–394. doi: 10.1111/j.1365-2362.1976.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Redeker A. G., Yamahiro H. S. Controlled trial of exchange-transfusion therapy in fulminant hepatitis. Lancet. 1973 Jan 6;1(7793):3–6. doi: 10.1016/s0140-6736(73)91220-8. [DOI] [PubMed] [Google Scholar]
- Rozga J., Holzman M. D., Ro M. S., Griffin D. W., Neuzil D. F., Giorgio T., Moscioni A. D., Demetriou A. A. Development of a hybrid bioartificial liver. Ann Surg. 1993 May;217(5):502–511. doi: 10.1097/00000658-199305010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga J., Podesta L., LePage E., Morsiani E., Moscioni A. D., Hoffman A., Sher L., Villamil F., Woolf G., McGrath M. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994 May;219(5):538–546. doi: 10.1097/00000658-199405000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga J., Williams F., Ro M. S., Neuzil D. F., Giorgio T. D., Backfisch G., Moscioni A. D., Hakim R., Demetriou A. A. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993 Feb;17(2):258–265. [PubMed] [Google Scholar]
- Saito S., Sakagami K., Orita K. A new hybrid artificial liver using a combination of hepatocytes and biomatrix. ASAIO Trans. 1987 Jul-Sep;33(3):459–462. [PubMed] [Google Scholar]
- Silk D. B., Trewby P. N., Chase R. A., Mellon P. J., Hanid M. A., Davies M., Langley P. G., Wheeler P. G., Williams R. Treatment of fulminant hepatic failure by polyacrylonitrile-membrane haemodialysis. Lancet. 1977 Jul 2;2(8027):1–3. doi: 10.1016/s0140-6736(77)90001-0. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Webster D. R., Wilson J., Lingham S. An X-linked syndrome characterised by hyperuricaemia, deafness, and neurodevelopmental abnormalities. Lancet. 1982 Jul 10;2(8289):68–70. doi: 10.1016/s0140-6736(82)91690-7. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Chong M. G., Koussayer T., He D. E., Shang T. A., Whisennand H. H., Kelly J. H. Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology. 1992 Jul;16(1):60–65. doi: 10.1002/hep.1840160112. [DOI] [PubMed] [Google Scholar]
- Watanabe F. D., Demetriou A. A. Support of acute liver failure patients with a bioartificial liver. J Clin Apher. 1996;11(3):138–142. doi: 10.1002/(SICI)1098-1101(1996)11:3<138::AID-JCA4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]



