Abstract
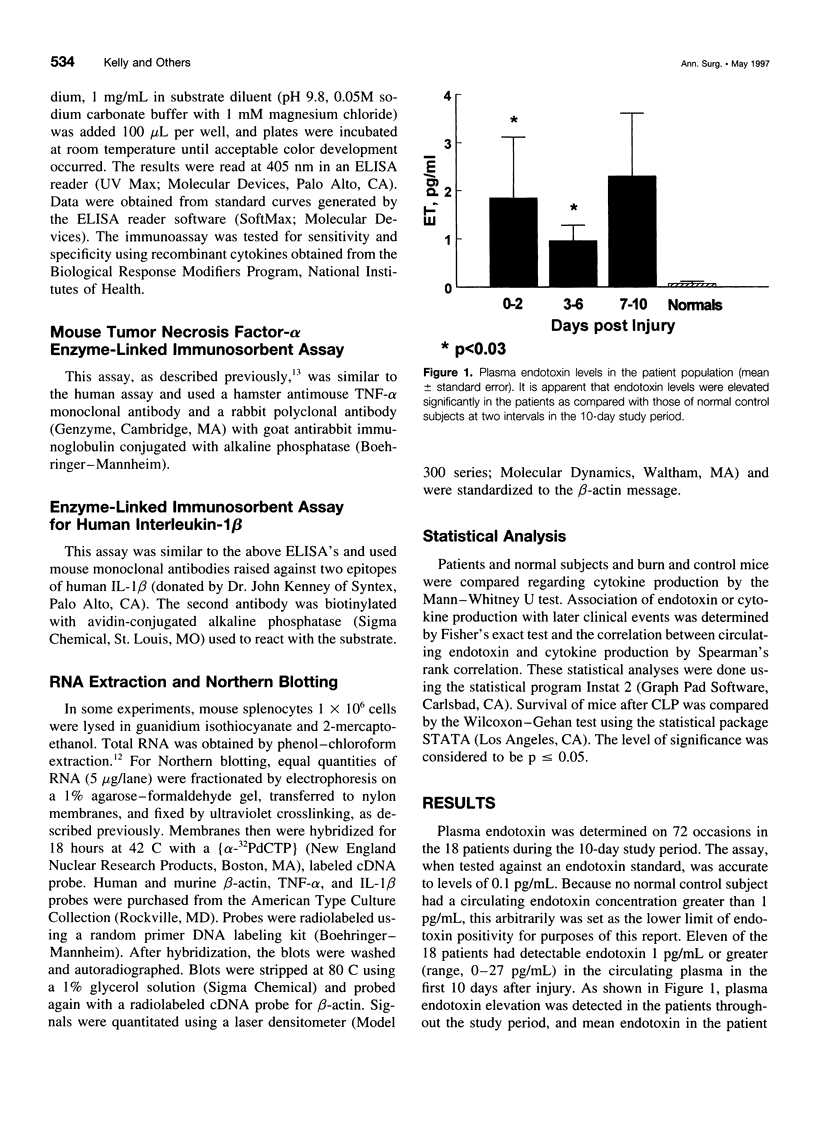
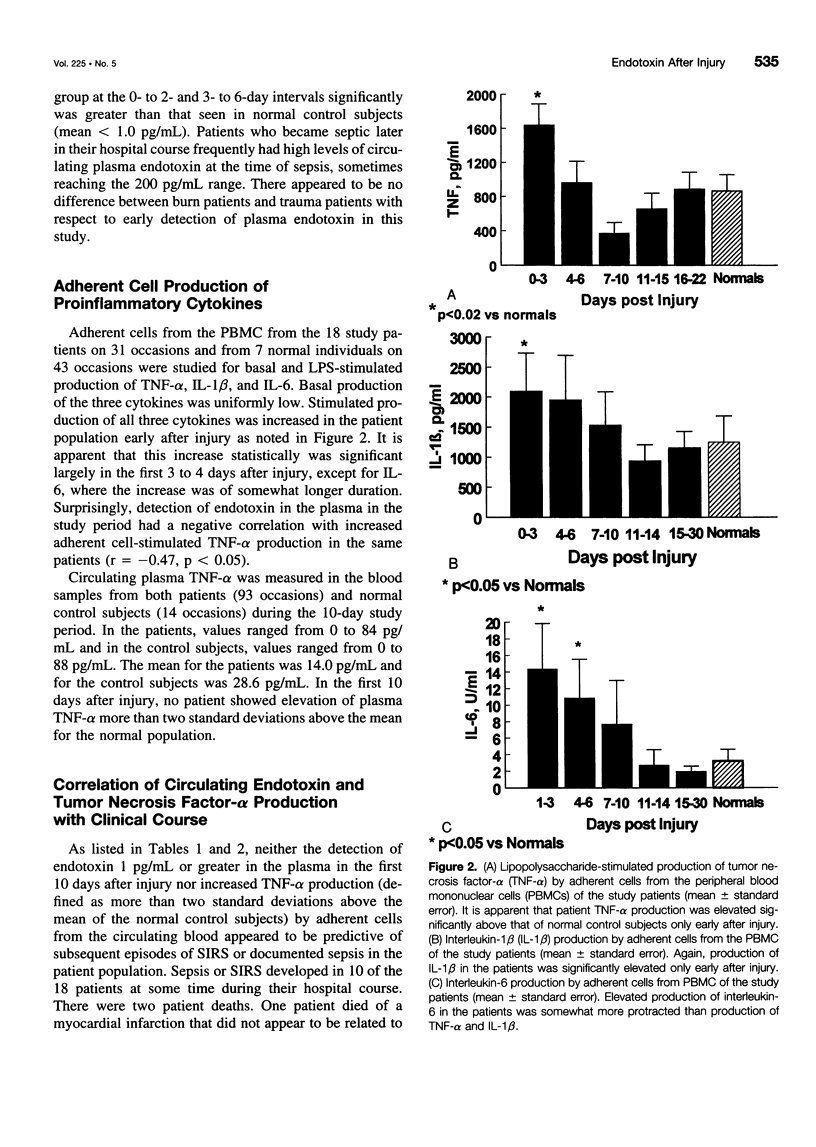
OBJECTIVE: Patients with severe traumatic or burn injury and a mouse model of burn injury were studied early after injury to determine the relation of plasma endotoxin (lipopolysaccharide [LPS]) to the production of proinflammatory cytokines and subsequent resistance to infection. SUMMARY BACKGROUND DATA: Elevated levels of plasma LPS have been reported in patients after serious injury. It has been suggested that circulating LPS may be a trigger for increased proinflammatory cytokine production and may play a role in the septic syndromes seen in a substantial portion of such patients. Yet, despite multiple reports of leakage of LPS from the gut and bacterial translocation after injury in animal models, there is little direct evidence linking circulating LPS with production of inflammatory mediators. METHODS: The authors studied serial samples of peripheral blood from 10 patients with 25% to 50% surface area burns and 8 trauma patients (injury Severity Score, 25-57). Patients were compared with 18 healthy volunteers. The study was focused on the first 10 days after injury before the onset of sepsis or the systemic inflammatory response syndrome. Plasma samples were assayed for LPS, and adherent cells from the blood were studied for basal and LPS-stimulated production of tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1 beta), and interleukin-6 (IL-6). The correlation of increased plasma LPS with TNF-alpha production was studied as was the association of increased plasma LPS and increased TNF-alpha production with subsequent septic complications. We also studied a mouse model of 25% burn injury. Burn mice were compared with sham burn control subjects. Plasma samples were assayed at serial intervals for LPS, and adherent cells from the spleens were studied for basal- and LPS-stimulated production of TNF-alpha, IL-1 beta, and IL-6. Expression of the messenger RNAs for IL-1 beta and TNF-alpha also was measured. The relation of increased TNF-alpha production with mortality from a septic challenge, cecal ligation and puncture (CLP), was determined. Finally, the effect of administration of LPS to normal mice on subsequent mortality after CLP and on TNF-alpha production was studied. RESULTS: Elevated plasma LPS (> 1 pg/mL) was seen in 11 of the 18 patients within 10 days of injury and in no normal control subjects. In this period, patients as compared with control subjects showed increased stimulated production of TNF-alpha, IL-1 beta, and IL-6. Increased TNF-alpha production was not correlated with elevated plasma LPS in the same patients. Neither increased plasma LPS nor increased TNF-alpha production early after injury was correlated with subsequent development of systemic inflammatory response syndrome or sepsis in the patients. Burn mice, as compared with sham burn control subjects, showed elevated plasma LPS levels chiefly in the first 3 days after injury. Increased stimulated production of proinflammatory cytokines by adherent splenocytes from the burn mice also was seen at multiple intervals after injury and did not correlate with mortality from CLP. Increased production of TNF-alpha and IL-1 beta was associated with increased expression of messenger RNAs for these cytokines. Finally, two doses of 1 ng LPS administered 24 hours apart to normal mice had no effect on mortality from CLP performed 7 days later nor on the production of TNF-alpha at the time of CLP. CONCLUSIONS: These findings call into question the idea that circulating LPS is the trigger for increased proinflammatory cytokine production, systemic inflammatory response syndrome, and septic complications in injured patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone R. C. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992 Dec 23;268(24):3452–3455. [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992 Aug;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditter B., Becker K. P., Urbaschek R., Urbaschek B. Quantitativer Endotoxin-Nachweis. Automatisierter, kinetischer Limulus-Amöbozyten-Lysat-Mikrotiter-Test mit Messung probenabhängiger Interferenzen. Arzneimittelforschung. 1983;33(5):681–687. [PubMed] [Google Scholar]
- Gianotti L., Alexander J. W., Pyles T., Fukushima R. Arginine-supplemented diets improve survival in gut-derived sepsis and peritonitis by modulating bacterial clearance. The role of nitric oxide. Ann Surg. 1993 Jun;217(6):644–654. doi: 10.1097/00000658-199306000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch R. C., Rodriguez R., Manning T., Bishop M., Mead P., Shoemaker W. C., Abraham E. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993 Jun;21(6):839–845. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- Horgan A. F., Mendez M. V., O'Riordain D. S., Holzheimer R. G., Mannick J. A., Rodrick M. L. Altered gene transcription after burn injury results in depressed T-lymphocyte activation. Ann Surg. 1994 Sep;220(3):342–352. doi: 10.1097/00000658-199409000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. G., 2nd, Minei J. P., Barber A. E., Rayburn J. L., Fahey T. J., 3rd, Shires G. T., 3rd, Shires G. T. Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann Surg. 1990 Apr;211(4):399–405. doi: 10.1097/00000658-199004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk K. A., Croce M. A., Fabian T. C., Minard G., Tolley E. A., Poret H. A., Kuhl M. R., Brown R. O. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992 May;215(5):503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. H., Seatter S. C., Manthei R., Bubrick M., West M. A. Macrophage endotoxin tolerance: effect of TNF or endotoxin pretreatment. J Surg Res. 1994 Jul;57(1):85–92. doi: 10.1006/jsre.1994.1115. [DOI] [PubMed] [Google Scholar]
- Maejima K., Deitch E., Berg R. Promotion by burn stress of the translocation of bacteria from the gastrointestinal tracts of mice. Arch Surg. 1984 Feb;119(2):166–172. doi: 10.1001/archsurg.1984.01390140032006. [DOI] [PubMed] [Google Scholar]
- Moore F. A., Moore E. E., Jones T. N., McCroskey B. L., Peterson V. M. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989 Jul;29(7):916–923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- Munster A. M., Smith-Meek M., Dickerson C., Winchurch R. A. Translocation. Incidental phenomenon or true pathology? Ann Surg. 1993 Sep;218(3):321–327. doi: 10.1097/00000658-199309000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Riordain M. G., Collins K. H., Pilz M., Saporoschetz I. B., Mannick J. A., Rodrick M. L. Modulation of macrophage hyperactivity improves survival in a burn-sepsis model. Arch Surg. 1992 Feb;127(2):152–158. doi: 10.1001/archsurg.1992.01420020034005. [DOI] [PubMed] [Google Scholar]
- Pfeiffer L., Ehrhardt N., Kretzschmar M., Urbaschek R., Schubert K., Schirrmeister W. Endotoxinämie und Multiorganversagen nach Polytrauma. Anaesthesiol Reanim. 1996;21(4):91–96. [PubMed] [Google Scholar]
- Rodrick M. L., Moss N. M., Grbic J. T., Revhaug A., O'Dwyer S. T., Michie H. R., Gough D. B., Dubravec D., Manson J. M., Saporoschetz I. B. Effects of in vivo endotoxin infusions on in vitro cellular immune responses in humans. J Clin Immunol. 1992 Nov;12(6):440–450. doi: 10.1007/BF00918856. [DOI] [PubMed] [Google Scholar]
- Roumen R. M., Hendriks T., Wevers R. A., Goris J. A. Intestinal permeability after severe trauma and hemorrhagic shock is increased without relation to septic complications. Arch Surg. 1993 Apr;128(4):453–457. doi: 10.1001/archsurg.1993.01420160095016. [DOI] [PubMed] [Google Scholar]
- Winchurch R. A., Thupari J. N., Munster A. M. Endotoxemia in burn patients: levels of circulating endotoxins are related to burn size. Surgery. 1987 Nov;102(5):808–812. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Wedel A., Schraut W., Ströbel M., Wendelgass P., Sternsdorf T., Bäuerle P. A., Haas J. G., Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994 Jun 24;269(25):17001–17004. [PubMed] [Google Scholar]