Abstract
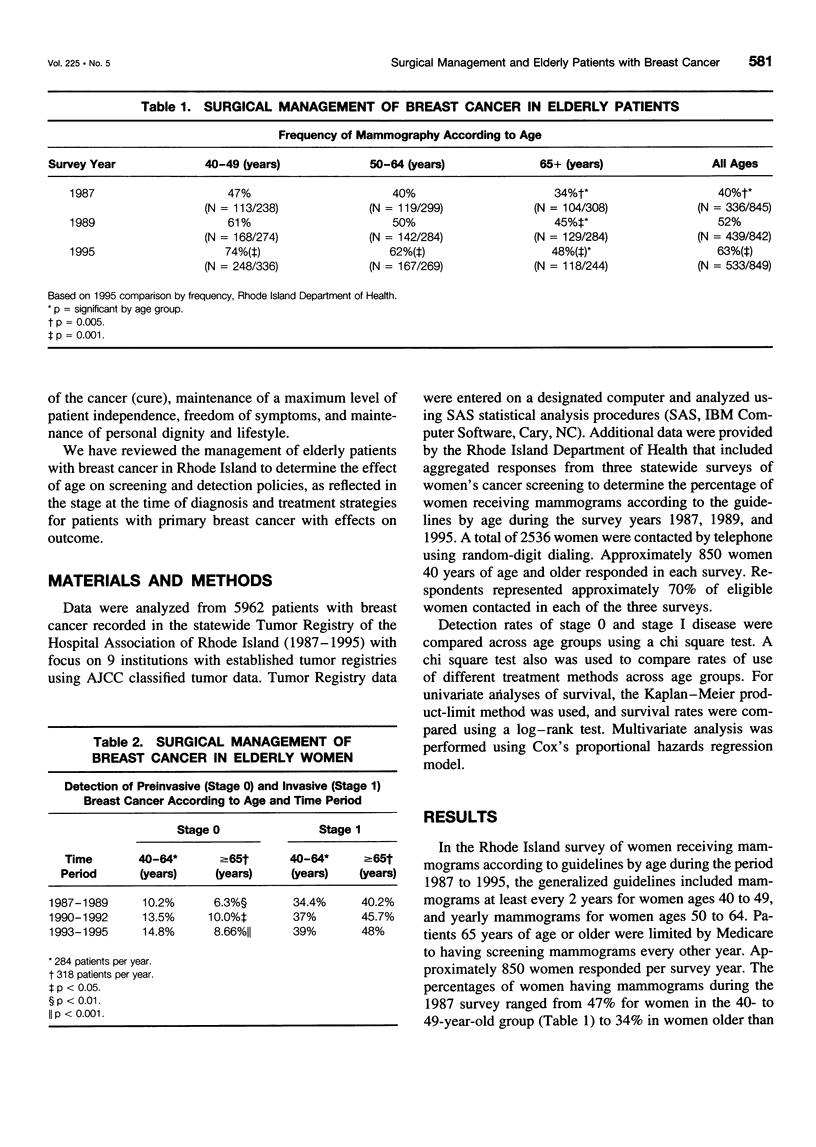
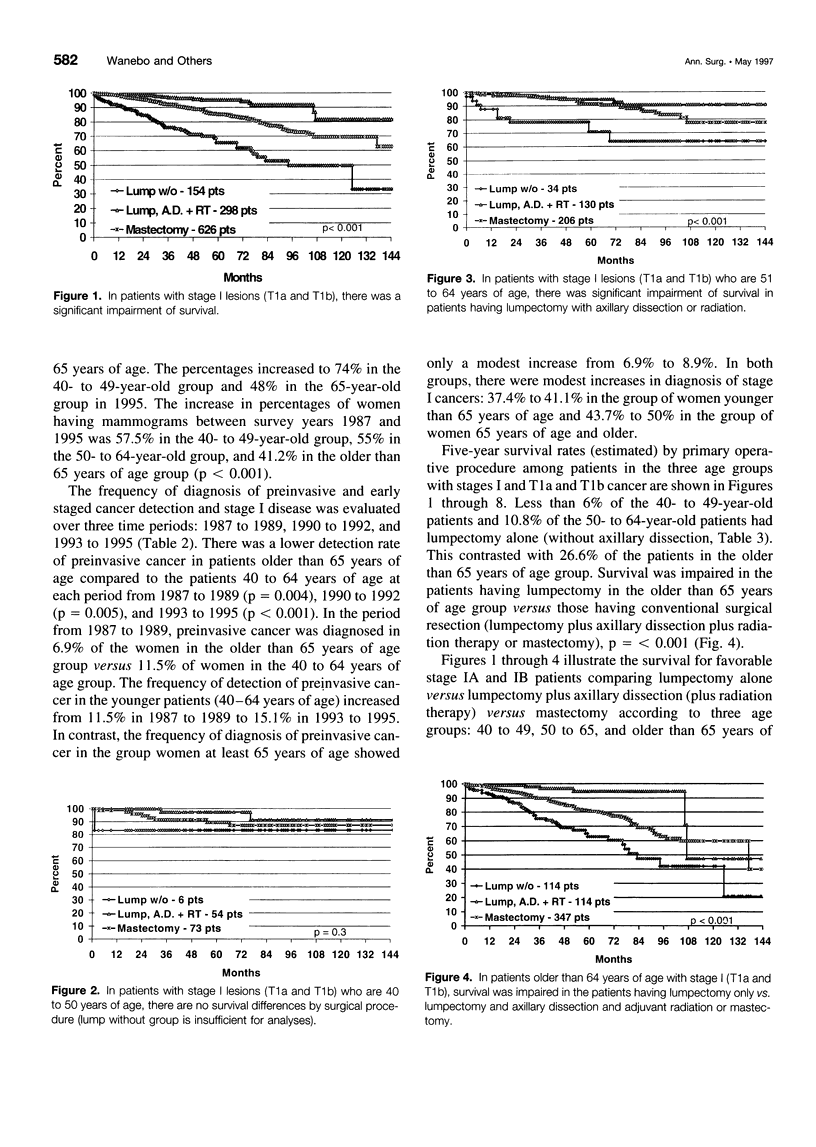
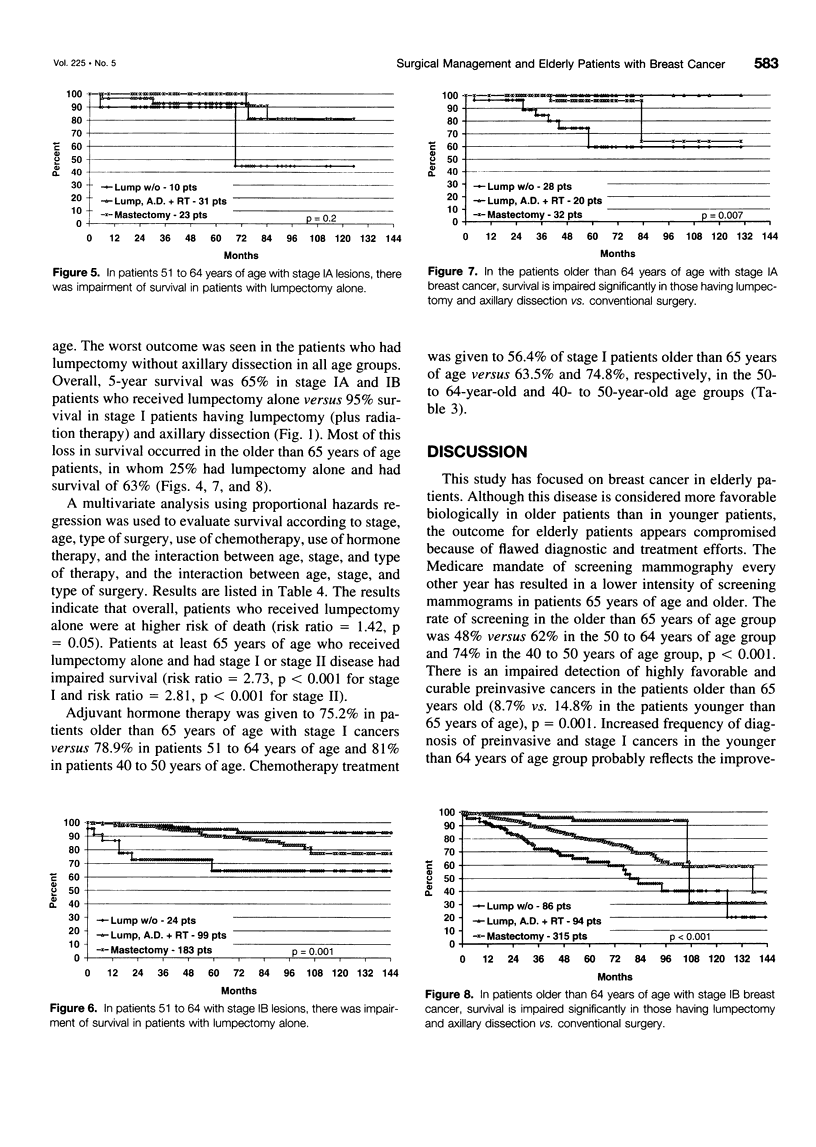
OBJECTIVE: The suggestion that breast cancer management is compromised in elderly patients had prompted our review of the results of policies regarding screening and early detection of breast cancer and the adequacy of primary treatment in older women (> or = 65 years of age) compared to younger women (40 to 64 years of age). SUMMARY BACKGROUND DATA: Although breast cancer in elderly patients is considered biologically less aggressive than similar staged cancer in younger counterparts, outcome still is a matter of stage and adequate treatment of primary cancer. For many reasons, physicians appear reluctant to treat elderly patients according to the same standards used for younger patients. There is even government-mandated alterations in early detection programs. Thus, since 1993, Medicare has mandated screening mammography on a biennial basis for women older than 65 year of age compared to the current accepted standard of yearly mammograms for women older than 50 years of age. Using State Health Department and tumor registry data, the authors reviewed screening practice and management of elderly patients with primary breast cancer to determine the effects of age on screening, detection policies (as reflected in stage at diagnosis), treatment strategies, and outcome. METHODS: Data were analyzed from 5962 patients with breast cancer recorded in the state-wide Tumor Registry of the Hospital Association of Rhoda Island between 1987 and 1995. The focus of the data collection was nine institutions with established tumor registries using AJCC classified tumor data. Additional data were provided by the State Health Department on screening mammography practice in 2536 women during the years 1987, 1989, and 1995. RESULTS: The frequency of mammographic screening for all averaged 40% in 1987, 52% in 1987, and 63% in 1995. In the 65-year-old and older patients, the frequency of screening was 34% in 1987, 45% in 1989, and 48% in 1995, whereas in the 40- to 49-year-old age group, the frequency of mammography was 47% in 1987, 61% in 1989, and 74% in 1995 (p < 0.001). There was a lower detection rate of preinvasive cancer in the 65-year-old and older patients, 8.8% versus 13.7% in patients within the 40- to 64-year-old age group (p < 0.001). There was a higher percentage of treatment by limited surgery among elderly patients with highly curable Stage IA and IB cancer with 26.6% having lumpectomy alone versus 9.4% in the younger patients. Five-year survival in that group was significantly worse (63%) than in patients treated by mastectomy (80%) or lumpectomy with axillary dissection and radiation (95%, < 0.001). A similar effect was seen in patients with Stage II cancer. CONCLUSIONS: Breast cancer management appears compromised in elderly patients (older than 65 years of age). Frequency of mammography screening is significantly less in elderly women older than 65 years of age. Early detection of preinvasive (curative cancers) is significantly less than in younger patients. The recent requirement by Medicare of mammography every other year may further reduce the opportunity to detect potentially curable cancers. Approximately 20% of patients had inferior treatment of favorable stage early primary cancer with worsened survival. Detection and treatment strategy changes are needed to remedy these deficiencies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August D. A., Rea T., Sondak V. K. Age-related differences in breast cancer treatment. Ann Surg Oncol. 1994 Jan;1(1):45–52. doi: 10.1007/BF02303540. [DOI] [PubMed] [Google Scholar]
- Begg C. B., Zelen M., Carbone P. P., McFadden E. T., Brodovsky H., Engstrom P., Hatfield A., Ingle J., Schwartz B., Stolbach L. Cooperative groups and community hospitals. Measurement of impact in the community hospitals. Cancer. 1983 Nov 1;52(9):1760–1767. doi: 10.1002/1097-0142(19831101)52:9<1760::aid-cncr2820520934>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bradbeer J. W., Kyngdon J. Primary treatment of breast cancer in elderly women with Tamoxifen. Clin Oncol. 1983 Mar;9(1):31–34. [PubMed] [Google Scholar]
- Chung M., Chang H. R., Bland K. I., Wanebo H. J. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996 Jan 1;77(1):97–103. doi: 10.1002/(SICI)1097-0142(19960101)77:1<97::AID-CNCR16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Clark G. M. The biology of breast cancer in older women. J Gerontol. 1992 Nov;47(Spec No):19–23. [PubMed] [Google Scholar]
- Dünser M., Häussler B., Fuchs H., Margreiter R. Tumorectomy plus tamoxifen for the treatment of breast cancer in the elderly. Eur J Surg Oncol. 1993 Dec;19(6):529–531. [PubMed] [Google Scholar]
- Fisher B., Bauer M., Margolese R., Poisson R., Pilch Y., Redmond C., Fisher E., Wolmark N., Deutsch M., Montague E. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985 Mar 14;312(11):665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Fisher E. R., Bauer M., Wolmark N., Wickerham D. L., Deutsch M., Montague E., Margolese R., Foster R. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985 Mar 14;312(11):674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- Fleming I. D., Fleming M. D. Breast cancer in elderly women. Cancer. 1994 Oct 1;74(7 Suppl):2160–2164. doi: 10.1002/1097-0142(19941001)74:7+<2160::aid-cncr2820741726>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Haffty B. G., McKhann C., Beinfield M., Fischer D., Fischer J. J. Breast conservation therapy without axillary dissection. A rational treatment strategy in selected patients. Arch Surg. 1993 Dec;128(12):1315–1319. doi: 10.1001/archsurg.1993.01420240023002. [DOI] [PubMed] [Google Scholar]
- Helleberg A., Lundgren B., Norin T., Sander S. Treatment of early localized breast cancer in elderly patients by Tamoxifen. Br J Radiol. 1982 Jul;55(655):511–515. doi: 10.1259/0007-1285-55-655-511. [DOI] [PubMed] [Google Scholar]
- Herbsman H., Feldman J., Seldera J., Gardner B., Alfonso A. E. Survival following breast cancer surgery in the elderly. Cancer. 1981 May 15;47(10):2358–2363. doi: 10.1002/1097-0142(19810515)47:10<2358::aid-cncr2820471006>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Holmes F. F. Clinical evidence for a change in tumor aggressiveness with age. Semin Oncol. 1989 Feb;16(1):34–40. [PubMed] [Google Scholar]
- Horton J. A., Romans M. C., Cruess D. F. Mammography attitudes and usage study, 1992. Womens Health Issues. 1992 Winter;2(4):180–188. doi: 10.1016/s1049-3867(05)80169-0. [DOI] [PubMed] [Google Scholar]
- Hunt K. E., Fry D. E., Bland K. I. Breast carcinoma in the elderly patient: an assessment of operative risk, morbidity and mortality. Am J Surg. 1980 Sep;140(3):339–342. doi: 10.1016/0002-9610(80)90163-4. [DOI] [PubMed] [Google Scholar]
- Kesseler H. J., Seton J. Z. The treatment of operable breast cancer in the elderly female. Am J Surg. 1978 May;135(5):664–666. doi: 10.1016/0002-9610(78)90131-9. [DOI] [PubMed] [Google Scholar]
- Kopans D. B., Moore R. H., McCarthy K. A., Hall D. A., Hulka C. A., Whitman G. J., Slanetz P. J., Halpern E. F. Positive predictive value of breast biopsy performed as a result of mammography: there is no abrupt change at age 50 years. Radiology. 1996 Aug;200(2):357–360. doi: 10.1148/radiology.200.2.8685325. [DOI] [PubMed] [Google Scholar]
- Muss H. B. The role of chemotherapy and adjuvant therapy in the management of breast cancer in older women. Cancer. 1994 Oct 1;74(7 Suppl):2165–2171. doi: 10.1002/1097-0142(19941001)74:7+<2165::aid-cncr2820741727>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tabar L., Duffy S. W., Burhenne L. W. New Swedish breast cancer detection results for women aged 40-49. Cancer. 1993 Aug 15;72(4 Suppl):1437–1448. doi: 10.1002/1097-0142(19930815)72:4+<1437::aid-cncr2820721405>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tabar L., Fagerberg G., Duffy S. W., Day N. E. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J Epidemiol Community Health. 1989 Jun;43(2):107–114. doi: 10.1136/jech.43.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vana J., Bedwani R., Mettlin C., Murphy G. P. Trends in diagnosis and management of breast cancer in the U.S.: from the surveys of the American College of Surgeons. Cancer. 1981 Aug 15;48(4):1043–1052. doi: 10.1002/1097-0142(19810815)48:4<1043::aid-cncr2820480432>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- White R. E., Vezeridis M. P., Konstadoulakis M., Cole B. F., Wanebo H. J., Bland K. I. Therapeutic options and results for the management of minimally invasive carcinoma of the breast: influence of axillary dissection for treatment of T1a and T1b lesions. J Am Coll Surg. 1996 Dec;183(6):575–582. [PubMed] [Google Scholar]
- Yancik R., Ries L. G., Yates J. W. Breast cancer in aging women. A population-based study of contrasts in stage, surgery, and survival. Cancer. 1989 Mar 1;63(5):976–981. doi: 10.1002/1097-0142(19890301)63:5<976::aid-cncr2820630532>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


