Abstract
Osteoarthritis (OA) is a common chronic degenerative joint disease. Recent studies have emphasized the crucial role of macrophages, particularly tissue-resident macrophages (Tissue-Resident Macrophages, TRMs), in the pathogenesis and progression of OA. Under physiological conditions, TRMs maintain joint homeostasis, but under various stimuli, they can polarize into pro-inflammatory M1 or anti-inflammatory M2 phenotypes. An imbalance in macrophage polarization, favoring the M1 phenotype, leads to sustained inflammation, cartilage degradation, and osteophyte formation, further exacerbating OA symptoms and structural damage. This article reviews the current understanding of macrophage polarization in OA, with a particular emphasis on the mechanisms by which TRMs influence the joint microenvironment. It explores the therapeutic potential of drug molecular platforms aimed at regulating macrophage polarization, shifting the balance from pro-inflammatory M1 to anti-inflammatory M2. The discussion includes various pharmacological agents such as corticosteroids, hyaluronic acid derivatives, monoclonal antibodies, and bioactive molecules like Squid Type II Collagen (SCII) in modulating macrophage function and slowing OA progression. Additionally, the article examines advancements in gene therapy methods targeting macrophages, utilizing nanotechnology-based delivery systems to enhance the specificity and efficiency of macrophage phenotype regulation. Targeting TRMs through sophisticated drug molecular platforms presents a promising strategy for developing novel diagnostic and therapeutic interventions for osteoarthritis.
Keywords: Osteoarthritis, Macrophage polarization, Tissue-resident macrophages, Drug molecular platforms, Inflammation, Therapeutic strategies
Introduction
Osteoarthritis (OA) is a prevalent chronic degenerative joint disease characterized primarily by damage to hyaline cartilage, synovial inflammation, and abnormal subchondral bone metabolism [1–3]. The persistent joint pain and stiffness caused by OA can severely reduce patients’ daily activity levels and quality of life, impose a heavy economic burden on society, and pose significant challenges to public health [4–6]. Epidemiological reports indicate that by 2017, there were approximately 303 million hip and knee OA patients worldwide, with around 61.2 million OA patients in China alone. Due to population aging and the exacerbation of global obesity issues, the global prevalence of OA is increasing annually [7–9].
OA is a chronic disease with complex etiologies, and its specific pathogenesis remains not fully understood. It can affect the entire joint function through multiple causal pathways, characterized by pathological changes in all joint tissues, including cartilage, subchondral bone, joint capsule, and synovium [10]. Early OA pathogenesis is often attributed to mechanical wear of these tissues, leading to the release of matrix metalloproteinases (MMPs) and inflammatory mediators, which cause subchondral bone matrix remodeling and secondary inflammatory changes [11]. However, recent clinical evidence suggests that, in addition to mechanical load, sustained low-level inflammation (especially synovitis) exacerbates the radiological and pain progression of OA. OA is now reclassified as a low-grade inflammatory joint disease involving the innate immune system [12, 13]. Macrophages, as the main effectors maintaining joint homeostasis and inflammation, are the most abundant immune cells in the synovium and the primary leukocyte population in the synovial fluid, playing a crucial role in the symptoms and structural progression of OA [12, 14].
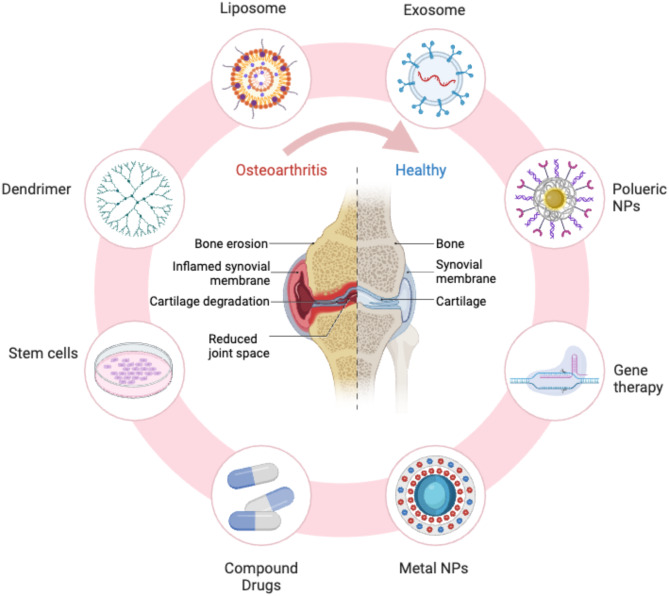
Recent studies have highlighted the significant role of tissue-resident macrophages (TRMs) in maintaining joint homeostasis and contributing to OA pathogenesis. TRMs reside permanently within the synovial tissue, independent of circulating monocytes, and exhibit distinct phenotypic and functional characteristics compared to infiltrating macrophages. These cells originate from embryonic progenitors and self-renew within the synovial environment, providing a stable population that responds rapidly to local changes. TRMs are involved in regulating local immune responses, tissue repair, and remodeling [15]. In the context of OA, TRMs can modulate the inflammatory environment by producing anti-inflammatory cytokines and growth factors that promote tissue repair and maintain joint integrity. However, under chronic inflammatory conditions, TRMs may adopt a pro-inflammatory phenotype, contributing to the persistence of inflammation and joint degradation. Understanding the interactions between TRMs and other synovial cells is essential for elucidating the mechanisms underlying OA progression and for developing targeted therapies that modulate TRM function to restore joint health (Fig. 1).
Fig. 1.
Overview of various therapeutic approaches for osteoarthritis treatment. The diagram highlights different strategies, including liposome, exosome, polyuric nanoparticles (NPs), and metal NPs-based treatments, as well as stem cell therapy, gene therapy, compound drugs, and dendrimer-based interventions
Macrophage polarization and its role in OA
Origin and activation of macrophages
Under normal physiological conditions, macrophages reside in the joint synovium, maintaining a relatively stable population through local proliferation of the monocyte pool. These monocytes embed within the synovial tissue, performing functions related to homeostasis maintenance [6, 16]. During the development of OA, resident synovial macrophages detect the presence of exogenous or endogenous pro-inflammatory stimuli, lose their stable state, and become activated in various ways. One activation pathway involves acute trauma or chronic joint wear that generates damage-associated molecular patterns (DAMPs) such as cartilage fragments, aggrecan (ACAN), and fibronectin [17]. Xie et al. found that these molecules bind to pattern recognition receptors (PRRs) on macrophages, initiating downstream intracellular effects such as the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, thereby secreting pro-inflammatory cytokines and chemokines [18]. Another major activation pathway is through the inflammasome pathway. Schroder and Chang demonstrated that during OA, damaged chondrocytes produce reactive oxygen species (ROS), which mediate the assembly of the NLR family pyrin domain containing protein 3 (NLRP3) and Caspase-1 into the NLRP3 inflammasome within macrophages, inducing the maturation and secretion of pro-inflammatory cytokines (e.g., IL-1β, IL-1α, IL-18), thus generating an inflammatory cascade [19–21].
Polarization and imbalance of macrophages during OA
Once activated, synovial macrophages exert various effects within the joint microenvironment either directly or indirectly (i.e., by stimulating other cells). Macrophages can be classified into classically activated (M1) and alternatively activated (M2) types based on their activation states and functions. M1 macrophages, induced by interferon-γ (IFN-γ), lipopolysaccharide (LPS), or tumor necrosis factor-α (TNF-α), clear pathogens by producing reactive oxygen species, nitric oxide, and releasing lysosomal enzymes. They also secrete various chemokines and pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1, IL-6, IL-12, and cyclooxygenase-2 (COX-2), which participate in inflammatory responses, clear some cell debris, but also damage chondrocytes and tissue repair [22–24]. M2 macrophages, also known as wound-healing macrophages, are activated by IL-4 and IL-13, express pro-chondrogenic genes such as transforming growth factor-β (TGF-β) and insulin-like growth factor (IGF), thereby promoting the synthesis of type II collagen (COL2) and glycosaminoglycans (GAGs), and exhibit anti-inflammatory and cartilage repair functions [22, 25]. Macrophages play a crucial role in the inflammatory response: M1 macrophages phagocytose pathogens, while M2 macrophages regulate the inflammatory microenvironment by secreting anti-inflammatory factors like IL-10, favoring cartilage tissue regeneration and repair. The M1/M2 ratio of macrophages within the joint cavity maintains a dynamic balance, rapidly converting in response to different stimuli. Timely shifts in macrophage polarization states are essential for the resolution of inflammation [18].
Macrophages can amplify inflammatory responses and disrupt the polarization balance by coordinating with other synovial cells [13]. Culemann et al. summarized that macrophages secrete related signaling molecules, such as pro-inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-12, and IL-23) and chemokines (CC-chemokine ligand 2 and CXC-chemokine ligand 8), leading to the continuous recruitment of monocytes and other leukocytes [16]. Zhang et al., by collecting synovial tissues from OA patients, found that newly recruited monocytes differentiated into M1 macrophages through the aforementioned mechanisms. The persistent accumulation of these cells in the synovium ultimately leads to synovial thickening and the occurrence of synovitis [26]. Therefore, during the progression of OA, activated macrophages continuously accumulate and become polarized out of balance within the joint cavity. Liu et al. collected synovial fluid from 80 knee OA patients and 80 healthy controls, and flow cytometry analysis indicated that the M1/M2 macrophage ratio in the synovial fluid positively correlated with the Kellgren-Lawrence grade (severity of OA on imaging) in OA patients [27]. Macrophages play a dual role in the course of osteoarthritis. Inflammation can clear some cell debris and mediate the joint microenvironment towards a normal state, but due to the imbalance in the M1/M2 macrophage ratio, the synthesis of extracellular matrix (ECM) components like ACAN by M2 macrophages is limited, while the continuous release of pro-inflammatory factors by M1 macrophages causes further damage to the cartilage matrix, gradually developing into chronic low-level joint inflammation [28]. Further exploration of the imbalance in macrophage M1/M2 polarization and dynamic polarization mechanisms will aid in preventing OA onset and progression.
TRMs contribute significantly to the polarization imbalance observed in OA. Under homeostatic conditions, TRMs predominantly exhibit an M2-like phenotype, promoting tissue repair and anti-inflammatory responses. However, in the OA environment, persistent exposure to DAMPs and pro-inflammatory cytokines can shift TRMs towards an M1-like phenotype. This phenotypic switch exacerbates the inflammatory milieu, further recruiting and activating additional macrophages and other immune cells, thereby sustaining the inflammatory cascade. Additionally, TRMs interact with synovial fibroblasts and chondrocytes, influencing their behavior and contributing to synovial hyperplasia and cartilage degradation. Understanding the specific factors that drive TRMs towards pro-inflammatory states in OA is crucial for developing strategies to restore their homeostatic functions and rebalance macrophage polarization.
Macrophages promote cartilage degradation and inhibit cartilage formation
Chondrocytes are responsible for the synthesis and degradation balance of the cartilage matrix to ensure the correct distribution of biomechanical loads, thereby reducing joint wear [12]. In OA, M1 macrophages in the joint synovium secrete inflammatory cytokines, MMPs, and reactive oxygen species, which not only induce chondrocyte senescence and apoptosis but also reduce the synthesis of key ECM components such as ACAN, GAG, and COL223. Additionally, pro-inflammatory cytokines promote the synthesis and release of various proteolytic enzymes, including MMPs and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), which degrade joint cartilage. Beyond driving inflammatory responses in OA, Bondeson et al. found that IL-1β and TNF-α also stimulate osteoblasts to secrete MMPs and pro-inflammatory cytokines detrimental to bone and adjacent cartilage [29]. Moreover, macrophages can directly inhibit the differentiation of chondrocytes by downregulating the expression of COL2 and GAG-related genes in mesenchymal stem cells [30]. These effects are primarily exploited by M1-like pro-inflammatory macrophages, as confirmed by selectively removing synovial macrophages [29, 31]. Therefore, synovial macrophages are key inductors of excessive MMPs and inflammatory cytokine expression, ultimately leading to cartilage degradation.
TRMs significantly influence cartilage dynamics in OA. By maintaining an M2-like phenotype under normal conditions, TRMs support cartilage health through the secretion of anti-inflammatory cytokines and growth factors that promote ECM synthesis and chondrocyte survival. However, in the OA environment, the shift of TRMs towards an M1-like phenotype leads to increased secretion of pro-inflammatory cytokines and MMPs, which directly degrade cartilage ECM and inhibit the anabolic processes of chondrocytes. Furthermore, TRMs interact with other synovial cells, such as fibroblasts, enhancing their pro-inflammatory and catabolic activities. Targeting TRMs to maintain or restore their M2-like phenotype could mitigate cartilage degradation and support the reparative processes essential for joint health.
Macrophages promote osteophyte formation
Macrophage polarization is also associated with osteophyte formation. Activated macrophages mediate bone formation under mechanical load by regulating growth factor signaling pathways such as TGF-β [32]. In two mouse OA models induced by collagenase, Van and Blom et al. found that selectively removing synovial macrophages significantly reduced the formation of joint osteophytes and synovial fibrosis [33, 34]. The reduction in bone proliferation due to macrophage depletion was associated with decreased production of TGF-β, bone morphogenetic protein (BMP)-2, and BMP-4 in the synovial lining. This effect could be reversed by intra-articular injection of TGF-β, indicating that macrophages are critical mediators of TGF-β effects [34]. Zhang et al. discovered that R-spondin-2 secreted by M1 macrophages in the synovial tissue of OA patients induced hypertrophy and apoptosis of chondrocytes, promoting cartilage degeneration and osteophyte formation, thereby exacerbating OA structural progression [26]. Aprepati et al., through imaging analysis of OA patients, found that synovitis was significantly associated with osteophyte formation in the anterior and medial tibial regions [35]. These data suggest that research aimed at altering macrophage function in OA can improve osteophyte and synovitis formation, thereby alleviating OA progression. These studies are of significant importance for drug discovery and the identification of new therapeutic targets for OA.
Peripheral sensitization mechanisms mediated by macrophages in OA pain
Synovial macrophages, as the main effectors maintaining joint homeostasis and inflammation, act as sentinel cells sensing joint damage. Studies on macrophage-neuronal receptor interactions have shown that when joint damage occurs, pro-inflammatory mediators released by macrophages can directly activate pain receptors, leading to peripheral sensitization of the joint [36]. M1 macrophages primarily induce the upregulation of voltage-gated channel proteins NaV1.3, NaV1.8, and CaV3.2 by secreting TNF-α, triggering ectopic impulses in dorsal root ganglion (DRG) neurons and mediating pain hypersensitivity [37, 38]. Segond et al. demonstrated that using etanercept or infliximab to neutralize TNF-α expression reduced macrophage infiltration in DRGs and was accompanied by decreased mechanical pain in OA joints [39]. Macrophage-secreted chemokines induce peripheral sensitization by activating the transient receptor potential vanilloid 1 (TRPV1) and recruiting macrophages from actual injury or inflammation sites to the DRG, triggering prolonged neuroinflammation, which is a key factor in OA joint pain [40, 41]. Additionally, TNF-α and IL-1β can regulate the expression of nerve growth factor (NGF) in synovial macrophages. NGF is a primary growth factor causing peripheral pain sensitization, enabling nociceptors to synthesize neuropeptides and sensitize sensory nerve endings, resulting in OA pain [42]. Therefore, the mutual interference between synovial macrophages and dominant primary nociceptors is considered one of the peripheral sensitization mechanisms causing pain in the peripheral nervous system. The presence and activation state of synovial macrophages are of significant clinical importance in the intervention and treatment of OA-related pain.
Applications and limitations of Pharmacological regulation of macrophage polarization in OA treatment
Compound drugs
Corticosteroids, potent anti-inflammatory hormone drugs, exhibit cell type-specific activities, affecting dendritic cells, macrophages, chondrocytes, and other joint tissue cells. Synovial macrophages may be one of the targets of corticosteroids in OA [43]. Utomo et al. established an in vitro acute synovitis model induced by IFN-γ and TNF-α to simulate OA synovium. Gene expression analysis showed that dexamethasone improved synovial inflammation by inhibiting M1 macrophage polarization (downregulating pro-inflammatory factors like IFN-γ and TNF-α) and promoting M2 macrophage polarization (upregulating anti-inflammatory factors like IL-4 and IL-10) compared to the control group, although this finding was derived from an in vitro setup only [44]. Bryan et al. used intra-articular injections of dexamethasone in a surgically induced post-traumatic OA rabbit model, which protected cartilage from degeneration and improved synovial inflammation within the joint [45, 46]. However, the exact mechanism—specifically whether these protective effects were driven by M2 macrophage repolarization—was not directly demonstrated in vivo. Therefore, additional in vivo studies are warranted to clarify whether dexamethasone indeed drives macrophages toward the M2 phenotype in OA. Kinsenoside (KD), another corticosteroid immunosuppressant, was studied by Zhou et al. in a mouse OA model induced by anterior cruciate ligament transection. The results showed that KD dose-dependently upregulated M2 macrophage-related genes arginase-1 (Arg-1), IL-10, and CD206, improved proteoglycan loss, and increased joint cartilage thickness [47]. However, due to various reported side effects of corticosteroid treatments, such as extreme immunosuppression, electrolyte imbalances, abnormal blood lipid metabolism, adrenal cortex dysfunction, mental disorders, osteoporosis, and femoral head necrosis, their use in OA is typically limited to short-term intra-articular injections [48, 49].
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan widely distributed throughout the body, available in various forms. The traditional therapeutic mechanism of HA is to provide a viscoelastic fluid that reduces intra-articular friction and stress, thereby minimizing chondrocyte damage. Recent studies have found that different molecular weights of HA also affect macrophage phenotype changes. Specifically, low molecular weight HA fragments appear to bind Toll-like receptors (TLR-2 and TLR-4), activating NF-κB signaling and upregulating pro-inflammatory genes such as inducible nitric oxide synthase, TNF-α, and IL-12β. By contrast, high molecular weight HA (HMW-HA) tends to engage CD44, a receptor capable of suppressing inflammatory cascades and facilitating the clearance of apoptotic cells, ultimately promoting an M2 (alternatively activated) phenotype. In addition, the HA receptor RHAMM (receptor for hyaluronan-mediated motility) may also play a role in cytoskeletal rearrangements that foster M2 polarization [50, 51]. Shu et al. established a mouse medial meniscus destabilization OA model and used an HMW-HA derivative (Hymovis®) with extended joint residence time for intra-articular injection, resulting in a 25% increase in the proportion of M2 macrophages in the synovium [52]. Jin et al. conducted a prospective single-arm study involving 16 patients, who received intra-articular injections of HMW-HA. Follow-up to the fifth week involved collecting synovial fluid for cytokine detection and flow cytometry analysis. The results showed that the levels of IL-6 and IL-8 cytokines in the synovial fluid rapidly decreased by over 50% post-injection, while the proportion of M2 macrophages continued to increase by 40% [53]. These findings underscore how the molecular weight of HA can selectively influence receptor-binding pathways and thereby shift macrophage polarization states in vivo. However, higher levels of evidence are needed to translate these findings into clinical practice, including more detailed mechanistic studies that validate how HMW-HA engages CD44 (and potentially other receptors) to promote M2 repolarization in human patients with OA.
Bioactive molecules
Monoclonal antibody therapies targeting macrophage polarization have begun to be applied in OA treatment, although the extent to which they induce M2 macrophage polarization remains under investigation. Wang et al. found that subcutaneous injection of adalimumab (an antibody that interferes with macrophage TNF-α pro-inflammatory signaling) effectively improved knee joint pain and function scores in patients with moderate to severe knee OA, but the specific mechanism involving M2 polarization has not been definitively established [54]. Additionally, fesinumab, by inhibiting NGF production by macrophages to induce anti-inflammatory effects, improved walking pain and joint function in knee/hip OA patients within eight weeks through weekly subcutaneous injections [55]. However, clinical trials targeting NGF monoclonal antibodies reported severe adverse events due to osteonecrosis, leading the U.S. Food and Drug Administration to suspend related monoclonal antibody clinical trials [56]. Although molecular engineering has enabled the fine-tuning of monoclonal antibody functions to enhance therapeutic effects and minimize immunogenicity and side effects, monoclonal antibodies targeting TNF-α have also been reported to be associated with other severe infections and malignancies [57]. To clarify whether these effects are directly attributable to M2 repolarization, additional preclinical and clinical studies are crucial for confirming and improving the safety of monoclonal antibody therapies in OA treatment.
Squid type II collagen (SCII) is a classic collagen component that plays an important role in the development and maturation of joint chondrocytes [58]. Studies have found that SCII or SCII-derived bioactive materials can induce macrophage polarization towards the M2 phenotype, thereby inhibiting pathological apoptosis and hypertrophy of chondrocytes, promoting cartilage repair, and alleviating OA progression. Dai et al. established a rat OA model through anterior cruciate ligament transection combined with partial medial meniscectomy. The results showed that SCII significantly reduced MMP-13 immunostaining in cartilage regions, increased proteoglycan content, and improved the structural integrity of cartilage tissue in OA rats [59]. In vitro experiments demonstrated that SCII enhanced the phosphorylation of signal transducer and activator of transcription 6 (STAT6) while inducing the expression of TGF-β and IGF, promoting the transition of macrophages from the M0 to the M2 phenotype, thus exerting immunomodulatory activity. SCII also inhibited pathological apoptosis and hypertrophy of chondrocytes, ultimately promoting cartilage repair [60]. Currently, SCII remains in the preclinical research stage but may represent a novel bioactive material for cartilage repair.
Transient receptor potential vanilloid 1 (TRPV1), a non-specific cation channel, regulates pain perception and immune cell activity, mediating CD4⁺ T cell activation and M2 macrophage polarization [61]. Lv et al. established a rat OA model through medial meniscectomy and intra-articular injection of a TRPV1 agonist. They found that the agonist effectively improved synovial inflammation scores, reduced the content of M1 macrophages, thereby decreasing cartilage destruction and osteophyte formation [62]. In vitro studies indicated that TRPV1 inhibits M1 macrophage polarization and reduces M1 macrophage migration [62]. A Phase IIb clinical trial investigated the effects of a TRPV1 agonist (CNTX-4975) administered via intra-articular injection for treating OA-related knee pain. The results showed that CNTX-4975 significantly improved patients’ knee joint pain and stiffness [63]. Future intervention strategies targeting TRPV1 may become viable treatment methods for OA.
Stem cells
Mesenchymal stem cells (MSCs) are multipotent stem cells found in various tissues, including bone marrow, skeletal muscle, periosteum, and spinal bone. MSCs can regulate various innate immune cells by secreting growth factors, chemokines, cytokines, or other substances, improving the joint cavity microenvironment, promoting inflammation suppression, and repairing damaged cartilage [64, 65]. Accumulating evidence suggests that MSC-induced macrophage reprogramming toward the M2 phenotype involves multiple signaling pathways—most notably the PI3K/AKT, NF-κB, and JAK/STAT pathways. For instance, MSC-derived exosomes have been shown to differentially modulate AKT isoforms by activating phosphorylation of AKT1 (favoring M2 polarization) while suppressing AKT2 phosphorylation (commonly associated with M1 polarization) [66]. Simultaneously, MSC-secreted factors may inhibit the IκB phosphorylation upstream of NF-κB, thereby reducing the nuclear translocation of NF-κB p65 and dampening the expression of pro-inflammatory cytokines [66–68]. Additionally, MSC-conditioned medium has been observed to activate STAT3 while downregulating NF-κB activity, further promoting an anti-inflammatory, M2-like phenotype in macrophages [68]. Topoluk et al. found that after co-culturing macrophages (M1/M2), OA chondrocytes, and placental MSCs, placental MSCs could reduce the M1/M2 macrophage polarization ratio, increase chondrocyte activity, and enhance GAG production [69]. Woo et al. established a mouse OA model with medial meniscus destabilization and injected extracellular vesicles derived from human adipose-derived stem cells into the joint cavity. The results showed that the number of M1 macrophages in the mouse knee synovium decreased by about threefold, IL-1β expression in the synovium and cartilage was inhibited, effectively improving cartilage proteoglycan content and alleviating OA progression. Previous studies have primarily focused on enhancing the chondrogenic differentiation of MSCs, necessitating more research to explore the mechanisms by which stem cell-secreted bioactive factors influence the immune microenvironment within the joint cavity [70].
Prospects and challenges of gene therapy regulating macrophage polarization for OA treatment
Unlike conventional pharmacological methods, gene therapy provides a more ideal treatment approach for OA by achieving long-term expression of nucleic acids, replacing the frequent administration of compounds, bioactive molecules, or stem cell drugs [71]. Gene therapy involves modifying or manipulating gene expression to alter the biological characteristics of living cells to achieve therapeutic effects [72]. Advances in molecular biology methods, synthetic biology, and bioengineering have expanded the repertoire of gene drugs for therapeutic applications, including plasmid DNA (pDNA), clustered regularly interspaced short palindromic repeats (CRISPR), small interfering RNA (siRNA), and microRNA (miRNA) [73].
The uptake of nucleic acid molecules primarily relies on endocytosis mechanisms, necessitating the use of carriers for effective transport to the cell nucleus or cytoplasm. Free nucleic acids face poor macrophage targeting, easy degradation in joint cavity/cell contents, and low bioavailability. Viral vectors, including lentivirus, adenovirus, and adeno-associated virus, can be used for efficient targeted transfection of macrophages. However, their immunogenicity, limited gene loading capacity, and complex preparation processes restrict their clinical application [74]. Nanotechnology-based non-viral gene carriers (nano gene delivery systems) offer more ideal delivery systems with advantages such as low immunogenicity, extended in vivo half-life, simple preparation, and targeted delivery [75].
Research progress in gene therapy regulating macrophage polarization
Multiple transcription pathways are involved in macrophage polarization within the molecular network of activated macrophages. Transcription factors NF-κB, nuclear transcription factor-activated protein 1, CCAAT/Enhancer Binding Protein Alpha (C/EBPa), and IFN-γ participate in TLR-induced macrophage polarization towards the M1 type; whereas STAT6, peroxisome proliferator-activated receptor γ (PPAR-γ), IFN-4, and Kruppel-like factor 4 (KLF4) are involved in macrophage polarization towards the M2 type [76]. With a deeper understanding of the molecular mechanisms controlling macrophage polarization and function, precise regulation of macrophage phenotypes through gene therapy holds promise as a therapeutic approach to improve OA [36].
miRNAs are a class of novel non-coding small RNAs that regulate the expression of various proteins by inhibiting mRNA translation at the post-transcriptional level. As key factors in epigenetic regulation, miRNAs can alter gene expression without changing the DNA sequence encoding proteins, potentially offering a safer method for regulating macrophage gene expression [77]. Among known miRNAs, miRNA-let7c, when overexpressed in M1 macrophages, targets C/EBPδ to reduce M1 phenotype expression while promoting polarization towards the M2 phenotype [78]. Additionally, multiple in vitro studies targeting macrophages have indicated that miR-223, miR-124, and miR-125a-5p are potential therapeutic strategies for repolarizing M1 macrophages to M2 [79–81].
Similarly, siRNA-mediated transient inhibition of pro-inflammatory cytokines has been shown to effectively alleviate the progression of joint inflammation. Silencing related pro-inflammatory pathways with siRNA can reduce the expression of M1 macrophage markers, repolarizing M1 macrophages to M2, thereby helping to alleviate inflammation. Among various pro-inflammatory cytokine-specific siRNAs, TNF-α siRNA-based therapies have been widely used to treat inflammatory diseases. Intracellular injection of TNF-α siRNA via electroporation needles can partially alleviate collagen-induced arthritis (CIA) in mice [82]. Khoury et al. used liposome-encapsulated siRNAs targeting three cytokines—IL-1, IL-6, and IL-18—and effectively improved arthritis inflammation and cartilage destruction in CIA mice [83].
Plasmid DNA (pDNA) expressing anti-inflammatory mediators represents another gene therapy strategy. IL-10 is a cytokine with potent anti-inflammatory activity, but its clinical application is increasingly limited due to severe cytokine-related side effects. pDNA expressing IL-10 has become a preferred therapeutic strategy for upregulating IL-10 cytokines in CIA inflammatory tissues. Fellowes et al. constructed a CIA mouse model and administered intra-peritoneal injections of cationic liposome-encapsulated IL-10 pDNA. The results showed that IL-10 pDNA effectively improved the degree of arthritis in mice [84]. Additionally, Zheng et al. prepared human serum albumin-loaded IL-10 pDNA and dexamethasone phosphate nanoparticles, treating CIA mouse models via intravenous injection. The results demonstrated that this treatment reduced the secretion of pro-inflammatory factors (TNF-α, IL-1β), increased the expression of anti-inflammatory factors (IL-10), promoted the polarization of macrophages from the M1 to the M2 phenotype, and alleviated joint swelling and bone erosion [85].
Biological barriers in gene therapy regulating macrophage polarization for OA treatment
Bioactive therapeutic genes encounter multiple biological barriers when reaching synovial macrophages and exhibiting regulatory effects. Due to the presence of nucleases in body fluids such as synovial fluid, naked therapeutic genes are rapidly degraded within the joint cavity [86]. The limited quantity of therapeutic genes taken up by macrophages is captured in lysosomes, where the acidic environment and abundant enzymes lead to the degradation of therapeutic genes [87]. The active sites of siRNA are located in the cytoplasm, and other therapeutic genes must further transfer from the cytoplasm to the nucleus for gene expression. However, the nuclear membrane and nuclear pore complexes only allow molecules smaller than 40 kDa to pass through, thus limiting the penetration of nucleic acid molecules [88, 89]. Compared to most other primary mammalian cells, therapeutic genes entering the nucleus in macrophages face greater challenges. In other animal cells, the nuclear membrane disassembles during mitosis, allowing exogenous therapeutic genes to freely enter and exit the nucleus. However, macrophages are typically non-dividing terminally differentiated cells [90]. Additionally, macrophages possess pattern recognition receptors that can detect therapeutic genes as potential foreign and dangerous viral invaders, triggering inflammatory signaling cascades that lead to nucleic acid denaturation or macrophage apoptosis [91]. Therefore, designing nano gene delivery systems to ensure effective nucleic acid payloads and circumvent macrophage phagocytosis and digestion effects is essential [92].
Advances in nano gene delivery systems transfecting macrophages
Liposomes, as nano gene delivery systems, offer advantages such as simple preparation, biodegradability, biocompatibility, and large gene-loading capacity. Cationic liposomes can neutralize the negative charges on therapeutic genes like DNA, compressing and encapsulating them to reduce electrostatic repulsion between therapeutic genes and cell membranes, facilitating transmembrane entry of therapeutic genes. However, attempts to transfect primary macrophages or bone marrow cell lines using lipid-based reagents in vitro typically result in low transfection efficiency (< 5%), with transgene expression lasting no more than 24 h. Further improvements to liposomes are necessary to enhance their transfection capabilities in macrophages [93].
Metal nanoparticles, with their excellent stability and biocompatibility, hold great potential in gene delivery. Representative metal nanoparticles include gold nanoparticles and magnetic iron oxide ions. Gold nanoparticles possess surface inertness but can be covalently functionalized through various techniques, allowing for the binding of specific groups like amino groups and interaction with therapeutic genes for gene delivery. Lee et al. used oligodeoxynucleotides (glutamate) end-labeled Cas9 proteins to facilitate their loading onto arginine-functionalized gold nanoparticles containing CRISPR-Cas9 pDNA. This carrier complex was intravenously injected into mice, resulting in gene editing efficiencies of 8% and 4% in liver and spleen macrophages, respectively [94]. This formulation lays the foundation for developing macrophage-mediated immunotherapies.
To overcome the low transfection efficiency of macrophages, researchers have begun utilizing various receptors on macrophages (mannose, scavenger, and cluster of differentiation proteins) to target specific endocytic pathways within macrophages, thereby increasing the gene transfection efficiency of existing gene carriers. Jain et al. evaluated the gene transfection efficiency of mannose-modified alginate nanoparticles delivering IL-10 pDNA to macrophages. The results showed that approximately 9% of macrophages were transfected 24 h after nanoparticle transfection [95]. Zhang et al. used polyglutamic acid as a linker to engineer mannose segments onto the surface of their gene delivery system, promoting interaction with macrophage mannose receptors. This method provided a higher transfection efficiency in primary macrophages (approximately 30%) [96]. Nano gene delivery systems targeting specific endocytic pathways in macrophages hold potential for successfully editing macrophage repolarization.
Summary and outlook
Synovial macrophages, as immune cells, play a crucial role in the progression of OA symptoms and structural changes through various mechanisms. DAMPs in the synovial fluid activate macrophages, which differentiate into different phenotypes, predominantly M1 macrophages that produce pro-inflammatory mediators and various MMPs. These factors further affect chondrocytes and the cartilage matrix, promoting the release of cartilage fragments and fibronectin into the synovial fluid, continuously inducing macrophage differentiation into the M1 phenotype. This ongoing interaction between the synovium and cartilage forms a vicious cycle that drives OA progression. Additionally, tissue-resident macrophages (TRMs) are integral to maintaining joint homeostasis but can become pro-inflammatory under chronic OA conditions, further exacerbating inflammation and joint degradation.
Due to the formation of a chronic low-level inflammatory state in OA, which may be related to an imbalance in the M1/M2 macrophage polarization ratio, promoting macrophage differentiation towards the M2 phenotype or inducing the conversion of M1 macrophages to M2 macrophages could be effective therapeutic strategies for alleviating OA structural and symptom progression. Corticosteroids, HMW-HA, monoclonal antibodies, SCII, and stem cells are potential therapeutic agents targeting this intervention mechanism to alleviate OA. However, specific therapeutic effects and biological safety require further research and validation. Recent studies suggest that gene therapy interventions targeting macrophage repolarization, particularly focusing on tissue-resident macrophages, may offer a more ideal OA treatment method. Despite the multiple biological barriers to safely and efficiently delivering therapeutic genes to macrophages, significant progress has been made in nano gene delivery systems’ ability to target specific endocytic pathways in macrophages. With advancements and applications in nano gene delivery systems, gene therapy research and application in OA macrophages, especially TRMs, will reach new heights.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFB4606700, 2023YFE0126900, 2023YFB3813000, 2022YFC2409705), the National Natural Science Foundation of China (82272561 and 82402868), Sichuan Provincial Natural Science Foundation Project (2025ZNSFSC1788), and Sichuan University West China Hospital Dedicated Postdoctoral Research Fund (2024HXBH150).
Author contributions
Limin Wu: Conceptualized and designed the manuscript framework, conducted an extensive literature review, and drafted the manuscript. Wu also contributed to the critical analysis and interpretation of the reviewed studies, ensuring the scientific rigor and coherence of the content.Xiaotao Cao: Contributed to the manuscript structure, assisted with data collection, and provided critical revisions to the manuscript for important intellectual content. Cao also supported the analysis of therapeutic strategies discussed in the review and ensured the accuracy of cited references.Bin Shen: Supervised the overall development of the manuscript, provided expert insights into osteoarthritis pathophysiology and macrophage biology, and contributed to the refinement of the manuscript. Shen also reviewed and approved the final version of the manuscript, ensuring accountability for all aspects of the work.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFB4606700, 2023YFE0126900, 2023YFB3813000, 2022YFC2409705), the National Natural Science Foundation of China (82272561 and 82402868 ), Sichuan Provincial Natural Science Foundation Project (2025ZNSFSC1788), and Sichuan University West China Hospital Dedicated Postdoctoral Research Fund (2024HXBH150).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final manuscript for publication.
Competing interests
All authors declare that they have no conflict of interest with other people or organizations that could inappropriately influence this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthr Lancet. 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthr Lancet. 2015;386:376–87. [DOI] [PubMed] [Google Scholar]
- 3.Andia I, Atilano L, Maffulli N. Biological targets of multimolecular therapies in Middle-Age osteoarthritis. Sports Med Arthrosc Rev. 2022;30:141–6. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–41. [DOI] [PubMed] [Google Scholar]
- 5.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15:S230–5. [PubMed] [Google Scholar]
- 6.Yin X, Wang Q, Tang Y, Wang T, Zhang Y, Yu T. Research progress on macrophage polarization during osteoarthritis disease progression: a review. J Orthop Surg Res. 2024;19:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global regional. National incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a lancet commission. Lancet. 2020;396:1711–2. [DOI] [PubMed] [Google Scholar]
- 9.Chisari E, Rehak L, Khan WS, Maffulli N. The role of the immune system in tendon healing: a systematic review. Br Med Bull. 2020;133:49–64. [DOI] [PubMed] [Google Scholar]
- 10.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egloff C, Hügle T, Valderrabano V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly. 2012;142:w13583. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28:555–61. [DOI] [PubMed] [Google Scholar]
- 14.Sarmanova A, Hall M, Moses J, Doherty M, Zhang W. Synovial changes detected by ultrasound in people with knee osteoarthritis - a meta-analysis of observational studies. Osteoarthritis Cartilage. 2016;24:1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Zheng M, Feng Z, Lin Q. CCL4L2 participates in tendinopathy progression by promoting macrophage inflammatory responses: a single-cell analysis. J Orthop Surg Res. 2024;19:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culemann S, Grüneboom A, Nicolás-Ávila J, Weidner D, Lämmle KF, Rothe T, et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie J, Huang Z, Yu X, Zhou L, Pei F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019;46:36–44. [DOI] [PubMed] [Google Scholar]
- 19.Schroder K, Tschopp J. Inflammasomes Cell. 2010;140:821–32. [DOI] [PubMed] [Google Scholar]
- 20.Chang WC, Chu MT, Hsu CY, Wu YJ, Lee JY, Chen TJ, et al. Rhein, an anthraquinone drug, suppresses the NLRP3 inflammasome and macrophage activation in urate Crystal-Induced gouty inflammation. Am J Chin Med. 2019;47:135–51. [DOI] [PubMed] [Google Scholar]
- 21.Yuan N, Zhang W, Yang W, Ji W, Li J. Exosomes derived from M2 macrophages prevent steroid-induced osteonecrosis of the femoral head by modulating inflammation, promoting bone formation and inhibiting bone resorption. J Orthop Surg Res. 2024;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utomo L, Bastiaansen-Jenniskens YM, Verhaar JA, van Osch GJ. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis Cartilage. 2016;24:2162–70. [DOI] [PubMed] [Google Scholar]
- 23.Haltmayer E, Ribitsch I, Gabner S, Rosser J, Gueltekin S, Peham J, et al. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS ONE. 2019;14:e0214709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, Kong F, Zhao G, Jin J, Feng S, Li M. SP1 transcriptionally activates HTR2B to aggravate traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Orthop Surg Res. 2024;19:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Lin C, Zeng C, Wang Z, Wang H, Lu J, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77:1524–34. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Zhang M, Zhao J, Zheng M, Yang H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med. 2018;16:5009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai Y, Fujita M, Kawasaki S, Sanaki T, Yoshioka T, Higashino K, et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain. 2019;160:895–907. [DOI] [PubMed] [Google Scholar]
- 29.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horkay F, Basser PJ, Hecht AM, Geissler E. Structure and properties of cartilage proteoglycans. Macromol Symp. 2017;372:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E, et al. Human Osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22:1167–75. [DOI] [PubMed] [Google Scholar]
- 32.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. [DOI] [PubMed] [Google Scholar]
- 33.van Lent PL, Blom AB, van der Kraan P, Holthuysen AE, Vitters E, van Rooijen N, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50:103–11. [DOI] [PubMed] [Google Scholar]
- 34.Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM, Roth J, van Rooijen N, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:627–35. [DOI] [PubMed] [Google Scholar]
- 35.Arepati A, Ishijima M, Kanako H, Aoki T, Liu L, Negishi Y, et al. Osteophyte formation is associated with synovitis in osteoarthritis-Thebunkyo health study. Osteoarthr Cartil. 2020;28:S112–3. [Google Scholar]
- 36.Wang D, Chai XQ, Hu SS, Pan F. Joint synovial macrophages as a potential target for intra-articular treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2022;30:406–15. [DOI] [PubMed] [Google Scholar]
- 37.He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010;151:266–79. [DOI] [PubMed] [Google Scholar]
- 38.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Segond G, Boettger MK, Fischer N, Gajda M, Bräuer R, Schaible HG. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain. 2009;145:151–9. [DOI] [PubMed] [Google Scholar]
- 40.Jiang BC, Liu T, Gao YJ. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 2020;212:107581. [DOI] [PubMed] [Google Scholar]
- 41.Wu XB, Jing PB, Zhang ZJ, Cao DL, Gao MH, Jiang BC, et al. Chemokine receptor CCR2 contributes to neuropathic pain and the associated depression via increasing NR2B-mediated currents in both D1 and D2 dopamine receptor-containing medium spiny neurons in the nucleus accumbens shell. Neuropsychopharmacology. 2018;43:2320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takano S, Uchida K, Inoue G, Miyagi M, Aikawa J, Iwase D, et al. Nerve growth factor regulation and production by macrophages in Osteoarthritic synovium. Clin Exp Immunol. 2017;190:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baschant U, Lane NE, Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:645–55. [DOI] [PubMed] [Google Scholar]
- 44.Utomo L, van Osch GJ, Bayon Y, Verhaar JA, Bastiaansen-Jenniskens YM. Guiding synovial inflammation by macrophage phenotype modulation: an in vitro study towards a therapy for osteoarthritis. Osteoarthritis Cartilage. 2016;24:1629–38. [DOI] [PubMed] [Google Scholar]
- 45.Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226–42. [DOI] [PubMed] [Google Scholar]
- 46.Heard BJ, Barton KI, Chung M, Achari Y, Shrive NG, Frank CB, et al. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J Orthop Res. 2015;33:1826–34. [DOI] [PubMed] [Google Scholar]
- 47.Zhou F, Mei J, Han X, Li H, Yang S, Wang M, et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9:973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015;1:e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn JK, Dankers W, Morand EF. Could GILZ be the answer to glucocorticoid toxicity in lupus?? Front Immunol. 2019;10:1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Cha J, Jang M, Kim P. Hyaluronic acid-based extracellular matrix triggers spontaneous M2-like Polarity of monocyte/macrophage. Biomater Sci. 2019;7:2264–71. [DOI] [PubMed] [Google Scholar]
- 51.Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1:481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shu CC, Zaki S, Ravi V, Schiavinato A, Smith MM, Little CB. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: differential effect of stem cell and hyaluronan treatment. Arthritis Res Ther. 2020;22:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin L, Xu K, Liang Y, Du P, Wan S, Jiang C. Effect of hyaluronic acid on cytokines and immune cells change in patients of knee osteoarthritis. BMC Musculoskelet Disord. 2022;23:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J. Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: an open-label randomized controlled trial. J Int Med Res. 2018;46:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155:1245–52. [DOI] [PubMed] [Google Scholar]
- 56.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. 2015;23(Suppl 1):S18–21. [DOI] [PubMed] [Google Scholar]
- 57.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38. [DOI] [PubMed] [Google Scholar]
- 58.Fernandes TL, Gomoll AH, Lattermann C, Hernandez AJ, Bueno DF, Amano MT. Macrophage: A potential target on cartilage regeneration. Front Immunol. 2020;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dai M, Liu X, Wang N, Sun J. Squid type II collagen as a novel biomaterial: isolation, characterization, immunogenicity and relieving effect on degenerative osteoarthritis via inhibiting STAT1 signaling in pro-inflammatory macrophages. Mater Sci Eng C Mater Biol Appl. 2018;89:283–94. [DOI] [PubMed] [Google Scholar]
- 60.Dai M, Sui B, Xue Y, Liu X, Sun J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via Immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018;180:91–103. [DOI] [PubMed] [Google Scholar]
- 61.Mickle AD, Shepherd AJ, Mohapatra DP. Sensory TRP channels: the key transducers of nociception and pain. Prog Mol Biol Transl Sci. 2015;131:73–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lv Z, Xu X, Sun Z, Yang YX, Guo H, Li J, et al. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca(2+)/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021;12:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayorga AJ, Flores CM, Trudeau JJ, Moyer JA, Shalayda K, Dale M, et al. A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis. Scand J Pain. 2017;17:134–43. [DOI] [PubMed] [Google Scholar]
- 64.van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term Osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186–96. [DOI] [PubMed] [Google Scholar]
- 65.Scala P, Rehak L, Giudice V, Ciaglia E, Puca AA, Selleri C et al. Stem cell and macrophage roles in skeletal muscle regenerative medicine. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed]
- 66.Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu B, et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 2019;23:7617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [DOI] [PubMed] [Google Scholar]
- 68.Gao S, Mao F, Zhang B, Zhang L, Zhang X, Wang M, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood). 2014;239:366–75. [DOI] [PubMed] [Google Scholar]
- 69.Topoluk N, Steckbeck K, Siatkowski S, Burnikel B, Tokish J, Mercuri J. Amniotic mesenchymal stem cells mitigate osteoarthritis progression in a synovial macrophage-mediated in vitro explant coculture model. J Tissue Eng Regen Med. 2018;12:1097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang S, Chen P, Zhang H, Weng H, Fang Z, Chen C, et al. Comparison of curative effect of human umbilical Cord-Derived mesenchymal stem cells and their small extracellular vesicles in treating osteoarthritis. Int J Nanomed. 2021;16:8185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–14. [DOI] [PubMed] [Google Scholar]
- 72.Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol. 2011;7:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vannucci L, Lai M, Chiuppesi F, Ceccherini-Nelli L, Pistello M. Viral vectors: a look back and ahead on gene transfer technology. New Microbiol. 2013;36:1–22. [PubMed] [Google Scholar]
- 75.Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012;20:1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. [DOI] [PubMed] [Google Scholar]
- 77.Carissimi C, Fulci V, Macino G. MicroRNAs: novel regulators of immunity. Autoimmun Rev. 2009;8:520–4. [DOI] [PubMed] [Google Scholar]
- 78.Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, et al. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, et al. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288:35428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–903. [DOI] [PubMed] [Google Scholar]
- 81.Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS ONE. 2013;8:e81774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schiffelers RM, Xu J, Storm G, Woodle MC, Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis Rheum. 2005;52:1314–8. [DOI] [PubMed] [Google Scholar]
- 83.Khoury M, Escriou V, Courties G, Galy A, Yao R, Largeau C, et al. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum. 2008;58:2356–67. [DOI] [PubMed] [Google Scholar]
- 84.Fellowes R, Etheridge CJ, Coade S, Cooper RG, Stewart L, Miller AD, et al. Amelioration of established collagen induced arthritis by systemic IL-10 gene delivery. Gene Ther. 2000;7:967–77. [DOI] [PubMed] [Google Scholar]
- 85.Zheng X, Yu X, Wang C, Liu Y, Jia M, Lei F, et al. Targeted co-delivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Deliv. 2022;29:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A, Talekar M, Raikar A, Amiji M. Macrophage-targeted delivery systems for nucleic acid therapy of inflammatory diseases. J Control Release. 2014;190:515–30. [DOI] [PubMed] [Google Scholar]
- 87.Needham D, Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990;58:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leonetti JP, Mechti N, Degols G, Gagnor C, Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991;88:2702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bieber T, Meissner W, Kostin S, Niemann A, Elsasser HP. Intracellular route and transcriptional competence of polyethylenimine-DNA complexes. J Control Release. 2002;82:441–54. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X, Edwards JP, Mosser DM. The expression of exogenous genes in macrophages: Obstacles and opportunities. Methods Mol Biol. 2009;531:123–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keller AA, Maeß MB, Schnoor M, Scheiding B, Lorkowski S. Transfecting Macrophages Methods Mol Biol. 2018;1784:187–95. [DOI] [PubMed] [Google Scholar]
- 93.Kusumawati A, Commes T, Liautard JP, Widada JS. Transfection of myelomonocytic cell lines: cellular response to a lipid-based reagent and electroporation. Anal Biochem. 1999;269:219–21. [DOI] [PubMed] [Google Scholar]
- 94.Lee YW, Mout R, Luther DC, Liu Y, Castellanos-García L, Burnside AS et al. In vivo editing of macrophages through systemic delivery of CRISPR-Cas9-Ribonucleoprotein-Nanoparticle nanoassemblies. Adv Ther (Weinh) 2019;2. [DOI] [PMC free article] [PubMed]
- 95.Jain S, Amiji M. Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules. 2012;13:1074–85. [DOI] [PubMed] [Google Scholar]
- 96.Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.