Abstract
Objective
Brucellosis-induced aortic aneurysm (BIA), as a rare yet highly life-threatening type of infectious aortic aneurysm, currently lacks standardized treatment protocols. The primary objective of this study is to thoroughly evaluate the safety and efficacy of endovascular therapy using a combination of covered stents and drug irrigation techniques for the treatment of BIA. This endeavor aims to provide a scientific basis for the clinical management of this disease.
Methods
This study employed a retrospective analysis approach to systematically collect comprehensive clinical data from patients with infectious aortic aneurysms admitted to three independent campuses of the same hospital from January 2016 to January 2024. The analysis encompassed a wide range of aspects, including patients' epidemiological characteristics, diverse clinical manifestations, detailed laboratory test reports, computed tomography angiography imaging data of the thoracoabdominal aorta(CTATA), specific treatment strategies, and prognosis during follow-up. The aim of this study is to provide robust support for optimizing the treatment strategies of Brucella-induced aortic aneurysms through comprehensive and in-depth data mining.
Results
Among the 27 confirmed cases of infectious aortic aneurysm, a striking 51.7% (n = 15) were unequivocally diagnosed as a consequence of brucellosis-induced aneurysmal formation. Direct confirmation of brucellosis via blood culture succeeded in merely 6 cases (40%), with the remainder being verified through the combination of the Rose Bengal plate agglutination and tube agglutination tests specific to Brucella. Gender distribution among these 15 patients was heavily skewed, with a significant majority being male (n = 13) contrasting with just 2 females. Their ages spanned a range from 47 to 75 years. Delving deeper, 11 patients had a documented history of contact with cattle, sheep, pigs, or their derivatives, whereas 4 did not present with such definitive exposure. The study exhibited diversity in aneurysm locations, including 6 cases of simple abdominal aortic aneurysms (AAAs), 2 in the iliac arteries, 4 involving both abdominal and iliac arteries, a unique abdominal aortic segment harboring two distinct pseudoaneurysms, a complex case where pseudoaneurysms impacted the thoracic, abdominal, and iliac arteries concurrently, and a solitary thoracic aortic aneurysm. All patients underwent successful endovascular aneurysm repair (EVAR) utilizing a stent-graft in conjunction with drug flushing techniques. Notably, 4 patients necessitated emergency surgical intervention due to impending rupture or aneurysmal rupture. No perioperative deaths were recorded. Postoperatively, all patients received comprehensive, long-term antibiotic therapy. One patient, three days following EVAR, underwent percutaneous endoscopic lumbar discectomy and drainage for brucellar spondylitis, subsequently being transferred to the orthopedics department for further care. Another case required inferior vena cava filter implantation due to pulmonary embolism and deep venous thrombosis of the lower extremities at admission, with the filter successfully removed three months later. Furthermore, one patient was readmitted six months post-discharge for acute myocardial infarction and underwent a successful percutaneous coronary intervention (PCI).During a two-year follow-up, a patient presented with enlargement of the original pseudoaneurysm in both abdominal and iliac arteries, which was effectively addressed through repeat EVAR with a stent-graft.
Conclusion
The diagnosis of Brucella-induced aortic aneurysm is prone to being overlooked, and some patients present with severe conditions at their initial diagnosis. Therefore, emphasizing early diagnosis and timely antibacterial treatment is crucial for containing disease progression. The application of endovascular repair with drug flushing covered stent grafts combined with long-term, regular antibiotic therapy has proven to be a safe, effective, and feasible treatment option for Brucella-induced aortic aneurysm, worthy of widespread promotion and application in clinical practice.
Keywords: Brucellosis, Aortic aneurysm, Drug flushing technique of covered stent, Endovascular repair
Background
Brucellosis stems from the bacterium Brucella and constitutes a zoonotic infection manifesting as both acute and chronic forms, affecting humans and animals globally. Annually, approximately half a million individuals worldwide contract the disease, posing a potential health hazard to a staggering 2.4 billion peoples [1, 2]. Its historical prevalence was particularly notable along the Mediterranean rim and in Eastern Europe; however, the past decade has witnessed significant geographical shifts in its occurrence. Table 1 offers a comprehensive glimpse into the epidemic landscape across the top ten brucellosis-endemic nations from 2014 to 2017 [3]. while Table 2 zeros in on the situation within China's ten most severely affected provinces spanning 2004 to 2018 [4]. Literature indicates that cardiovascular sequelae of Brucella infection occur in roughly 1–2% of cases, with infectious aortic aneurysms comprising a mere 0.06% of these instances [5, 6]. Nevertheless, this subtype carries a grim prognosis, characterized by a high mortality rate hovering between 21 and 22% [7–9]. Notably, over the last seven decades, merely 71 reports detailing diverse pathological types of brucellosis-linked thoracic and abdominal aortic aneurysms have surfaced [10], likely due to resource constraints, lack of financial backing, especially in developing nations where research publication opportunities are scarce, contributing to a global dearth of awareness, underreporting, and thus, an underestimation of the true burden of Brucella-induced aortic aneurysms.
Table 1 .
Overview of the Top Ten Countries Globally Experiencing Human Brucellosis Epidemics from 2014–2017
| Country | Years | Number of brucellosis cases | Incidence rate per (100,000) |
|---|---|---|---|
| Kenya | 2017 | 101985 | 203.07 |
| Yemen | 2016 | 25041 | 89.96 |
| Syria | 2017 | 8067 | 47.26 |
| Greece | 2015 | 4620 | 42.96 |
| Eritrea | 2017 | 1132 | 21.82 |
| Palestine | 2017 | 894 | 20.07 |
| Iran | 2017 | 15410 | 19.1 |
| Saudi Arabia | 2017 | 4692 | 14.18 |
| Kyrgyzstan | 2018 | 787 | 12.83 |
| Armenia | 2017 | 362 | 12.29 |
Table 2 .
The situation of the ten provinces in China that were most severely affected by brucellosis from 2004 to 2018
| Region | Number of brucellosis cases | Incidence rate (per 100,000) |
|---|---|---|
| Inner Mongolia Autonomous Region | 151613 | 41.21 |
| Xinjiang Uyghur Autonomous Region | 45164 | 13.82 |
| Ningxia Hui Autonomous Region | 12711 | 13.42 |
| Shanxi Province | 69056 | 13.1 |
| Heilongjiang Province | 69875 | 12.21 |
| Jilin province | 24259 | 5.92 |
| Hebei Province | 49318 | 4.6 |
| Liaoning Province | 20445 | 3.15 |
| Gansu Province | 9409 | 2.41 |
| Shaanxi Province | 11581 | 2.06 |
| China | 524980 | 2.61 |
Presently, literature predominantly features isolated case studies of Brucella-induced aortic aneurysms, with treatment strategies primarily centered on high-risk open surgical interventions, including aneurysmectomy, local debridement coupled with extra-anatomic bypass grafting, or in situ graft reconstruction. While these treatments demonstrate notable success, they are fraught with significant surgical stress, high complication rates, and prolonged postoperative recuperation, significantly compromising patients' work capacity. Alternatively, sole reliance on EVAR poses the threat of post-operative stent graft infection, and patients residing in endemic regions face an elevated risk of brucellosis recurrence and reinfection. Acknowledging the unique characteristics of our regional patient population (elaborated in Table 3), we embarked on an innovative endeavor, devising a novel endovascular repair protocol that integrates systemic and localized antimicrobial therapy. The present study endeavors to appraise the outcomes of endovascular stent repair administered to 15 patients with Brucella-induced aortic aneurysms, treated using this pioneering approach at our hospital from January 2016 to January 2024.
Table 3 .
Preoperative clinical data of 15 patients
| Case | Sex/Age | Job | Past history | Contact hidtory | Symptoms | Symptom-to-diagnosis interval (day) | SAT | RBPT | Blood culture | WBC | NEUT | ALT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/70 | Farmer | Hypertension VTE | Cattle Sheep | Abdominal and lower extremity pain and fever | 60 | 1:400(+++) | + | + | 2.76*109/L | 49.10% | 19U/L |
| 2 | F/61 | Farmer | _ | Cattle Sheep | Aneurysm rupture and abdominal pain | 41 | 1:100(++) | + | + | 5.38*109/L | 59.40% | 29.1U/L |
| 3 | M/55 | Civil servant | Hypertension Atherosclerosis | _ | Aneurysm rupture and shock and fever | 9 | 1:400(+++) | - | + | 5.24*109/L | 72.30% | 53U/L |
| 4 | M/47 | Transport driver | _ | Cow milk | Abdominal and low back pain and fever | 27 | 1:400(+++) | + | - | 8.21*109/L | 79% | 32.2U/L |
| 5 | M/54 | Farmer | _ | Cattle Sheep | Abdominal distention and pain and fever | 10 | 1:200(+++) | + | - | 14.27*109/L | 53.60% | 27U/L |
| 6 | M/67 | Farmer | Hypertension CAD Type 2 diabetes | Pigs Sheep | Aneurysm rupture and vomit blood and fever | 18 | 1:400(++) | + | + | 6.74*109/L | 74.80% | 16.2U/L |
| 7 | M/67 | junkman | COPD BPH | _ | Abdominal pain and fever | 30 | 1:400(++) | + | - | 4.74*109/L | 51.50% | 34.1U/L |
| 8 | M/54 | Butcher | CAD | Raw beef and mutton | Abdominal and lift limbs pain and fever | 10 | 1:400(++++) | - | - | 8.77*109/L | 75.20% | 11.1U/L |
| 9 | M/70 | Farmer | CRF | _ | Abdominal pain and pulsation mass | 14 | 1:200(++) | + | - | 6.04*109/L | 56.70% | 29U/L |
| 10 | M/64 | Butcher | _ | Raw beef and mutton | Abdominal pain | 24 | 1:400(++) | + | - | 3.28*109/L | 69.30% | 7.8U/L |
| 11 | F/73 | Farmer | Type 2 diabetes | Cattle Sheep | Chest pain and tightness | 30 | 1:400(++) | + | + | 3.1*109/L | 91% | 30.8U/L |
| 12 | M/59 | Herdsman | _ | Cattle Sheep Horses | Lower back pain and weakness | 20 | 1:400(++) | + | - | 9.35*109/L | 76.90% | 43U/L |
| 13 | M/66 | Farmer | Hypertension Rheumatoid arthritis | Cattle Sheep | Muscle pain and fever | 20 | 1:200(++) | + | + | 3.39*109/L | 59.30% | 106U/L |
| 14 | M/75 | Retirement | Hypertension | _ | Abdominal pain and pulsation mass | 10 | 1:200(+++) | + | - | 9.8*109/L | 87.30% | 6.6U/L |
| 15 | M/58 | Herdsman | Type 2 diabetes | Cattle Sheep Horses | Aneurysm rupture and shock | 7 | 1:200(+++) | + | - | 11.25*109/L | 89.10% | 9U/L |
| Case | AST | Albumin | SCR | BUN | ESR | CRP | PCT | Lesion site | Maximum aneurysm diameter (mm) | Involved site | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40U/L | 30.2U/L | 95umol/L | 9.89mmol/L | 38mm/H | 15.1mg/L | 0.5 | AA+ right IA | 48.6 44.6 | L5 vertebral body destruction | 40 |
| 2 | 28.7U/L | 46.6U/L | 62umol/L | 5.34mmol/L | 27mm/H | 32mg/L | <0.05 | AA | 87 | _ | 19 |
| 3 | 45U/L | 37.7U/L | 75umol/L | 5.39mmol/L | 46mm/H | 88.5mg/L | <0.05 | AA+ lift IA | 61.7 43 | _ | 28 |
| 4 | 25U/L | 46.3U/L | 66umol/L | 6.8mmol/L | 29mm/H | 41.1mg/L | 1 | AA+ right IA | 57.2 32.5 | _ | 23 |
| 5 | 16U/L | 36.2U/L | 59umol/L | 5.89mmol/L | 22mm/H | 25.3mg/L | 0.5 | AA | 48.3 | _ | 20 |
| 6 | 14.7U/L | 28.8U/L | 73.6umol/L | 5.09mmol/L | 58mm/H | 83.26mg/L | ≤0.05 | TA+AA+lift IA | 96.8 42 13.9 | _ | 33 |
| 7 | 46.6U/L | 38.8U/L | 58umol/L | 3.67mmol/L | 71mm/H | 148.26mg/L | 0.04 | AA | 44.5 | _ | 20 |
| 8 | 12.6U/L | 41.3U/L | 65.1umol/L | 5.95mmol/L | 64mm/H | 138.8mg/L | 0.23 | lift IA | 38.5 | _ | 21 |
| 9 | 22U/L | 39.4U/L | 177umol/L | 10.08mmol/L | 27mm/H | 6.54mg/L | 0.05 | AA | 49.3 | _ | 19 |
| 10 | 16.5U/L | 34.9U/L | 53.4umol/L | 3.99mmol/L | 27mm/H | 12.73mg/L | ≤0.05 | AA+AA | 49.6 52.9 | _ | 30 |
| 11 | 25U/L | 29.9U/L | 73umol/L | 4.46mmol/L | 59mm/H | 210mg/L | 0.2 | TA | 62 | _ | 14 |
| 12 | 21U/L | 35.5U/L | 70umol/L | 5.46mmol/L | 70mm/H | 57.1mg/L | <0.05 | AA | 53.4 | L3,L4 vertebral body destruction | 28 |
| 13 | 127U/L | 30.4U/L | 80umol/L | 5.18mmol/L | 37mm/H | 31.7mg/L | 0.117 | AA+ right IA | 47.1 36.1 | _ | 8 |
| 14 | 12.8U/L | 29.3U/L | 204umol/L | 16.4mmol/L | 43mm/H | 69.9mg/L | 0.61 | AA | 46.6 | _ | 35 |
| 15 | 21U/L | 32.7U/L | 42umol/L | 7.78mmol/L | 41mm/H | 47.5mg/L | 0.13 | lift IA | 101 | _ | 16 |
Abbreviations: TA thoracic aorta, AA abdominal artery, IA iliac artery, VTE venous thrombus embolism, COPD chronic obstructive pulmonary disease, BPH benign prostate hyperplasia, CAD coronary artery disease, CRF chronic renal failure, SAT serum agglutination test, RBPT rose bengal plate agglutination test
Materials and methods
This study rigorously conducted a comprehensive retrospective evaluation of 27 confirmed cases of infectious aortic aneurysms admitted to the JinFeng, XiXia, and NingNan campuses of people's hospital of ningxia hui autonomous region between January 2016 and January 2024. With meticulous precision, the investigation delved into the specifics of 15 cases definitively diagnosed with Brucella-induced aortic aneurysms. Between June to July 2024, follow-up assessments were methodically executed via telephonic and face-to-face interviews. The study adhered to stringent ethical guidelines, gaining prior approval from the hospital's ethics review committee and adhering to the Helsinki Declaration, thereby ensuring informed consent from each surviving participant. For inclusion in this study, stringent criteria were employed: patients presenting with brucella-associated aortic aneurysms who underwent endovascular stent-graft placement in conjunction with drug infusion therapy. Diagnosis was established on the basis of: (1) a constellation of infectious symptoms, including fever, flank or abdominal pain, and pain in the lower extremities; (2) laboratory markers indicative of infection, such as elevated white blood cell (WBC) count, hypersensitive C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT) levels; (3) confirmatory evidence of brucella infection through positive bacterial cultures or serological tests; (4) computed tomography angiography (CTA) findings revealing the aneurysm, characterized by its size, morphology (saccular, fusiform, or irregular), presence of perianeurysmal soft tissue masses, abscesses, bubble signs, and liquid-attenuated dark areas. Patients with a clinical impression of infectious aortic aneurysm yet lacking bacteriological verification of Brucella infection or any relevant bacteriological examination were excluded from the study. The retrospective analysis encompassed a thorough examination of the medical records and associated data for each participant, encompassing demographics like age and sex, occupational history, travel history, exposure history, symptomology, disease duration, rose bengal plate test (RBPT) results, serum agglutination test (SAT) titers, WBC and neutrophil (NEUT) counts, ESR, CRP, PCT levels, and the duration of follow-up.
Clinical manifestation
This study encompassed fifteen patients who had a definitively diagnosed case of Brucella aneurysm. With regard to their symptomatology, abdominal pain was the predominant complaint, observed in nine patients (60%), while upon admission, seven patients (46.7%) exhibited fever. Moreover, an urgent admission was necessitated for four patients (26.7%), stemming from either an imminent or an actual rupture of the aneurysm. A noteworthy case involved one patient who suffered from severe pain in the lower left abdomen, stemming from compression by a left iliac aneurysm; this pain significantly radiated to their left lower limb, significantly disrupting their daily activities. Importantly, none of the patients in this study had a prior history of abdominal trauma or surgical intervention. An analysis of lesion sites indicated that Brucella aneurysms predominantly impact the thoracic and abdominal aorta, as well as the iliac arteries, with specific findings revealing that twelve patients (80%) were affected in the abdominal aorta, six patients (40%) in the iliac artery, and a relatively lesser prevalence of two patients (13.3%) with involvement of the thoracic aorta. A comprehensive breakdown of the clinical profiles of these patients is provided in Table 3.
Antibiotic therapy
The current management strategy for brucellosis preponderantly hinges on antibiotics, adhering to the cornerstone principle of "early intervention, combined therapy, adequate dosing, and full treatment duration." The antibiotics chosen for administration must possess the capability to penetrate macrophages and retain their potency within an acidic cellular milieu. Crafting a tailored treatment plan necessitates a meticulous consideration of multifarious factors, encompassing the patient's disease chronicity, severity, individual physiological and pathological profiles, the specific Brucella species implicated, the route of antimicrobial administration, drug safety concerns, economic feasibility, and patient accessibility to medications.Within the realm of antimicrobial combinations, the synergistic use of doxycycline and aminoglycosides stands as the benchmark for treating uncomplicated brucellosis, attributable to its impressive clinical cure rates [11]. Further investigations have illuminated that, in comparison to the standard dual-therapy approach, a triple-therapy protocol incorporating doxycycline, rifampicin, and aminoglycoside antimicrobials exhibits superior performance in eradicating Brucella, thereby mitigating the risk of disease recurrence. This advanced regimen is especially advocated for patients presenting with complex, severe infections or life-threatening complications, as well as those exhibiting poor adherence to treatment protocols [12].
1. Systemic antibiotic regimen
Following a confirmed diagnosis of Brucella-induced aortic aneurysm, the diagnosis and treatment scheme for Brucellosis(2023 Edition) [13] recommended antibacterial therapy comprises oral administration of doxycycline hyclate tablets ( 100 mg, bid, po), in conjunction with intravenous infusions of rifampicin for injection (10 mg/kg, q12h, ivgtt) and gentamycin sulfate injection (within the dosage range of 1–1.7 mg/kg, q8h, ivgtt). Should the patient additionally manifest complications associated with brucellosis, the treatment may be augmented with intravenous ceftriaxone sodium (1 g, qd, ivgtt) or levofloxacin and sodium chloride injection (500 mg, qd, ivgtt). Prior to discharge, patients are instructed to sustain treatment through oral rifampicin capsules (600 mg, bid), continuing the same doxycycline hydrochloride dosage regimen, gentamicin sulfate tablets (240 mg, qid, po), and cefixime capsules (200 mg, bid, po).
2. Drug flushing technique of covered stent
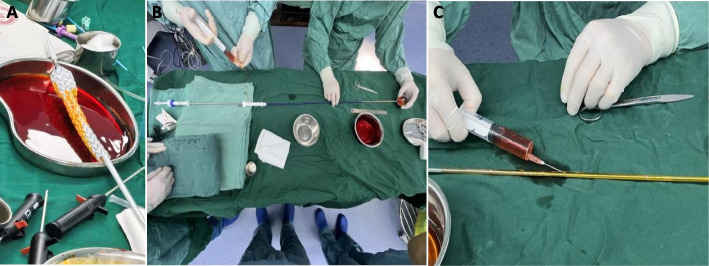
All patients have unanimously opted for the implantation of the stent-graft. a solution comprising 500 ml of normal saline blended with 2.4 g of rifampicin for injection was employed to soak the stent-graft. Nevertheless, this approach elicited an unintended consequence: the membrane tissue of the stent-graft expanded upon hydration, rendering retraction into the delivery system difficult and potentially damaging the stent structure or membrane region during repeated maneuvers, which could subsequently give rise to complications, notably endoleaks in later stages.In response, we refined the flushing procedure by administering the same proportioned rifampicin solution through a 20 ml syringe directly into the delivery system via the venting lateral hole of the stent-graft, subjecting it to a sustained flushing process for 20 min (as depicted in Fig. 1). For smaller stent-grafts deployed in the iliac artery, which lack these venting lateral holes, we innovated by puncturing the exposed portion of the delivery system with a hollow needle devoid of medication, thereby ensuring adequate drug flushing and maintaining therapeutic efficacy.
Fig. 1.
The covered stent was soaked in rifampicin liquid before implantation (A) Flushing the covered stent with rifampicin through the vent hole of the stent (B) Flushing covered stent using a syringe to puncture the bare area of the stent (C)
Endovascular therapy
All patients underwent surgical interventions under general anesthesia, with one individual receiving a hybrid approach while the remaining fourteen underwent comprehensive endovascular therapy. The surgical procedures exclusively utilized femoral artery puncture as the access point. Once successful percutaneous femoral artery puncture was achieved, a Perclose ProGlide Suture-Mediated Closure System (Abbott, Chicago, USA) was implanted, accompanied by intravenous administration of low-molecular-weight heparin (100 units/kg). The covered stents, positioned via a stiff guidewire through the established femoral artery pathway, encompassed multiple types: Endurant (Medtronic, Minneapolis, MN), Ankura (Lifetech, Shenzhen, China), Minos (Endovastec, Shanghai, China), Excluder (Gore, Newark, Delaware), and the Stent-Graft System of Shape Memory Alloy (Grimed, Beijing, China). Graft stent sizes were meticulously selected, exceeding the anchor zone dimensions by 10–20%, as determined intraoperatively through aortography. The immediate success of the endovascular procedures was verified by aortography, which confirmed graft patency and complete sealing of intimal leaks. Lastly, the femoral artery puncture site was meticulously closed percutaneously.
Statistical analysis
All statistical indicators were tested for normality. Counts are expressed as percentages and measures as .
Result
Among the treated cases, four hailed from the Inner Mongolia Autonomous Region in northern China, one originated in Gansu Province located in the northwest, and a further ten belonged to the Ningxia Hui Autonomous Region, also situated in northwest China. The mean age of disease onset across patients stood at 62.6 ± 8.1 years, spanning a range from 47 to 75 years, with male patients notably outnumbering females in their incidence. Of the 15 patients studied, diagnoses encompassed six simple AAAs, two iliac aneurysms, four instances of abdominal aortic aneurysm accompanied by iliac aneurysm, a singular case of two distinct pseudoaneurysms within the abdominal aortic segment, a complex scenario affecting the thoracic aorta, abdominal aorta, and iliac artery simultaneously, and a solitary case of thoracic aortic aneurysm. Notably, four of these cases—comprising one iliac aneurysm, one thoracic aneurysm, and two abdominal aortic aneurysms—presented with imminent rupture or actual rupture emergencies. The average diameter of these aneurysms was measured as 54.0 ± 18.8 mm, with diameters varying from 32.5 to 101 mm. Upon admission, laboratory assessments revealed elevated mean levels of inflammatory markers, specifically CRP at 67.2 mg/L, ESR at 43.9 mm/H, and NEUT at 69.6%. Subsequent to admission, blood cultures were conducted for all patients using bilateral limb samples collected over three consecutive days, with Brucella successfully isolated in six cases. The remaining nine cases were confirmed through a combination of the Rose Bengal Plate Test and Brucella serum agglutination test.
Two patients diagnosed with thoracic aortic aneurysms had covered stents placed within the thoracic aorta, while 13 patients received abdominal aorta-iliac artery stents. Notably, in three cases involving the internal iliac artery, concurrent embolization of the affected artery with coils was performed. In Cases 2, 3, 6, and 15, surgical emergencies arose from aneurysmal ruptures, with no preceding standard administration of the combined antibacterial treatment regimen consisting of doxycycline hyclate tablets, rifampicin for injection, and gentamycin sulfate injection. Conversely, the remaining patient cohort received this triple therapy regularly for 7 to 10 days prior to undergoing endovascular stent-graft repair. Notably, both Cases 1 and 12, besides aortic infection, presented radiographic indications of spinal involvement and potential neurological infection hazards, necessitating the introduction of ceftriaxone sodium for injection-a blood–brain barrier permeable agentto the-ongoing triple therapy, escalating it to a quadruple antibiotic regimen. Specifically, Case 1 underwent inferior vena cava filter implantation promptly upon the initial detection of pulmonary and left lower extremity venous thrombosis. Across all patients, their respective triple or quadruple antibacterial therapy protocols were maintained post-operatively until hospital discharge. After discharge from the hospital, the patient was asked to continue to take three or four of rifampicin capsules (600 mg, bid,po), doxycycline hyclate tablets ( 100 mg, bid, po), gentamicin sulfate tablets (240 mg, qid, po) and cefixime capsules (200 mg, bid, po) regularly according to his own needs, and to undergo regular checkups. The devised follow-up strategy encompasses:1. Conducting RBPT and SAT titers at 1, 3, 6, 9, and 12 months post-operatively to monitor brucellosis control, with treatment cessation contingent upon concurrent negative results from both tests or SAT titers of less than 1:50 in two consecutive tests.2. Reassessing Thoracoabdominal aortic CTA at 1, 6, 12 months post-operatively and followed by annual evaluations thereafter.
In Case 1 Case6 and Case 12, multifaceted system engagement necessitated continuation of oral quadruple antibiotics up to the 12th postoperative month, notwithstanding a favorable 9-month post-surgery review meeting the criteria for medication cessation. For other patients, the gradual cessation of triple antibiotics was initiated between the 6th and 9th month post-surgery, contingent upon review outcomes. Subsequent evaluations confirmed no instances of endoleaks, thrombosis formation, infection re-examination, stent fractures, stent displacement,or endovascular stent graft-related complications across all patients.Three patients suffered from complications in other systems after the surgery and underwent secondary surgical interventions. and they all remained in good health, as verified through recent telephone and in-person follow-ups.
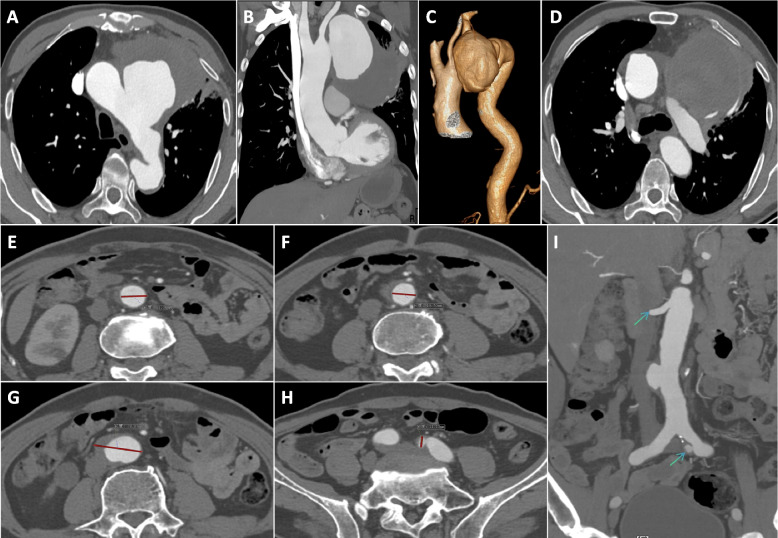
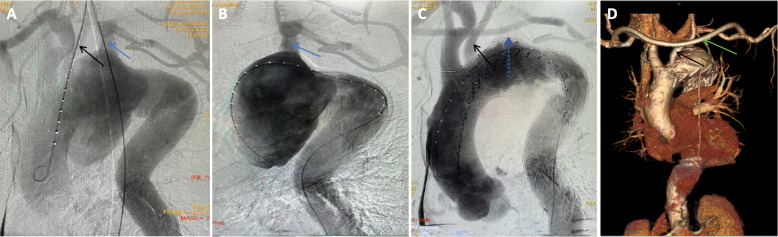
Case 12 necessitated prompt transfer to the spinal surgery department on day 3 post-operation for percutaneous, transforaminal endoscopic excision and drainage, stemming from persistent and recurrent low back pain. In Case 1, a second surgery was performed three months later to excise the inferior vena cava filter. Case 3 underwent readmission six months post-discharge for PCI, necessitated by an acute myocardial infarction. Case 6 presented with chest pain, hematemesis, and unstable vital signs upon admission; preceding this, family members reported recurrent low-grade fever. A CTA of the thoracoabdominal aorta unveiled a ruptured thoracic aortic pseudoaneurysm, accompanied by abdominal aortic and left iliac artery pseudoaneurysm formations (depicted in Fig. 2). Upon meticulous imaging analysis, a hybrid therapeutic approach was devised (depicted in Fig. 3), encompassing an artificial vessel bypass from the right to left axillary artery, endovascular repair with a drug-coated balloon-assisted thoracic aortic stent graft, left common carotid artery fenestration and reconstruction, as well as occlusion of the left subclavian artery ostium with a patent ductus arteriosus (PDA) occluder. Postoperative follow-ups at 6 months, 1 year, and 2 years demonstrated successful exclusion of the thoracic aortic aneurysm, patent artificial bypass, and no ischemic events in the pertinent vascular territories. However, abdominal aortic aneurysm diameter surpassed 40 mm and further expanded to over 42 mm with irregular contours, while the left iliac artery pseudoaneurysm exceeded 13 mm.There is a certain risk of rupture for abdominal aortic aneurysm and iliac artery aneurysm. It is recommended that patients and their families conduct regular follow-up with color Doppler ultrasound and aortic CTA for monitoring.Given that the patient and their family have previously experienced the critical situation of emergency rescue due to aortic rupture and have endured significant psychological and mental stress, at the strong request of the patient and their family, a second endovascular repair with covered stent was performed for the abdominal aorta and iliac artery. Since the patient's left limb involves artificial vascular bypass and iliac artery stent implantation, it is advised that the patient should take a long-term combination of aspirin and statins to maintain the treatment efficacy (depicted in Fig. 4).
Fig. 2.
Brucellosis aortic aneurysm(Case 6),Giant pseudoaneurysm of the thoracic aorta, threatened rupture (A-D). The proximal and distal normal vessel diameters of pseudoaneurysms of the abdominal aorta ranged from 19.7 to 21.3 mm (E–F). The maximum diameter of pseudoaneurysm was 38.8 mm in the abdominal aorta and 11.1 mm in the left iliac artery (G-I)
Fig. 3.
Brucellosis aortic aneurysm(Case 6), Pseudoaneurysm of thoracic aorta involved left common carotid artery and left subclavian artery (A-B), Thoracic aortic stent graft fenestration with left common carotid artery stent reconstruction, the left subclavian artery opening was sealed with a PDA occluder(dotted arrow) (C), Review result (D)
Fig. 4.
Review of patients with brucellosis aortic aneurysm 2 years after operation (Case 6), Pseudoaneurysm of the abdominal aorta with a maximum diameter of more than 40.2 mm and irregular morphology (A, C, E), Pseudoaneurysm of the left iliac artery up to 13.9 mm in diameter (B-D)
Discussion
Brucella-related aortic aneurysm constitutes a cardiovascular sequela of Brucella infection, characterized by a low incidence profile yet harboring a high mortality threat. Worldwide, among approximately 500,000 brucellosis patients annually, a mortality rate of roughly 1% is recorded, with a staggering 80% of these fatalities stemming from cardiovascular sequelae attributed to Brucella [14–17]. The World Health Organization designates brucellosis as one of the neglected zoonotic diseases on a global scale, observing commendable containment efforts in developed nations juxtaposed against pronounced prevalence in certain developing countries. Recently, China's thriving animal husbandry sector, alongside the intensified movement of livestock and their byproducts, has fueled the expansion of brucellosis' epidemic boundaries. This has prompted a resurgence of the disease in certain regions, while international travel, migration patterns, and food trade routes have facilitated its reemergence in developed nations as well [18, 19]. Alarmingly, the lack of an effective preventive vaccine for human Brucella infection persists, underscoring the urgency of the situation. A comprehensive literature review conducted by Willems et al., encompassing PubMed, Science Network, and Alternative Medicine Search databases, has yielded a mere 71 documented cases of diverse Brucella-associated thoracoabdominal aortic aneurysms spanning seven decades. This figure is likely a gross underestimation, overshadowed by scientific and financial constraints, limited publication avenues, and other multifaceted impediments.
Regarding Brucella-associated aneurysms, irrespective of rupture status, the exclusive reliance on pharmacological management while disregarding surgical intervention is considered a fatal oversight [11, 12]. The converged approach of surgery and antibiotic therapy has emerged as the benchmark for addressing these infectious aneurysms. Traditional surgical strategies, once hailed as the golden standard for aortic infections [10, 20], encompass intricate maneuvers like excision of infected aneurysms, local debridement, extra-anatomic bypass grafting, or in-situ reconstruction utilizing a graft, all aimed at eliminating the infectious source and affected tissue. However, these procedures are technically intricate, demanding, and fraught with elevated surgical risks, complication rates, and mortality [21–24]. Emergency open surgery for ruptured infectious aortic aneurysms poses formidable technical hurdles, often necessitating interdisciplinary teamwork. For medically underserved regions, frequently burdened by Brucellosis, performing these complex surgeries constitutes colossal challenges. In 1998, Semba et al. pioneered the adoption of endovascular repair techniques for infectious aneurysms, which have gained momentum in recent times due to their benefits of minimal invasiveness, elimination of bypass grafting needs, lesser impact on respiratory function, and reduced blood transfusion requirements [25]. This methodology is particularly favored as the frontline treatment option for elderly and critically ill patients [26–28]. Nevertheless, endovascular aneurysm repair (EVAR) confronts hurdles, including an inability to comprehensively eradicate local infection sources and the necessity to implant foreign materials within infected sites, potentially precipitating persistent, unmanageable infections or even graft infections, thereby prolonging illness and significantly augmenting patient mortality [14, 29]. Notably, graft infection is intimately linked to continued aneurysm expansion and rupture, drastically elevating mortality rates. According to the 2022 ACC/AHA Guidelines for the Diagnosis and Management of Aortic Diseases, EVAR is preferred for ruptured infectious aneurysms [30]. Conversely, the 2019 guidelines from the European Society for Vascular Surgery (ESVS) advise EVAR solely as an alternative to open surgical repair (OSR) in managing mycotic aortic aneurysms (MAA) [31]. Acknowledging the unique patient demographics in our region, we have devised a novel endovascular repair methodology integrating covered stent flushing techniques. To this purpose, we have conducted a retrospective analysis of baseline data and post-treatment follow-up information from 15 patients with Brucella aortic aneurysms, who underwent endovascular repair using covered stents coupled with drug flushing techniques in our hospital between January 2016 and January 2024. Our endeavor seeks to offer fresh therapeutic perspectives and references that may enrich clinical practice.
Brucella exhibits a broad parasitic range within animals, including sheep, cattle, dogs, and their byproducts, primarily disseminating through direct contact. Its extensive susceptibility in humans and subsequent epidemic potential stem from the virulence factor urease, empowering it to breach oral defenses and withstand gastric acid eradication. The bacterium's vesicle architecture fosters an acidic niche that evades immune surveillance while mitigating antibiotic efficacy. Additionally, its limited capacity to activate the complement system and B-cells elicits a moderated immune response, enabling it to infiltrate, persist within, and proliferate long-term within target cells [32–34]. In the absence of an efficacious vaccine, the cornerstone of brucellosis management remains antimicrobial therapy, emphasizing early intervention, combination therapy, precise dosing, and completion of the treatment cycle. Selecting antibiotics that retain activity within macrophages and acidic intracellular milieus (e.g., Doxycycline, Gentamicin, Rifampicin) is paramount for eradicating Brucella, regardless of the primary, chronic, or recurrent nature of the infection. All cases in this study adhered to rigorous and consistent antimicrobial treatment protocols. Capitalizing on rifampicin's potent local and systemic antibacterial properties, conventional open surgical procedures often involve soaking grafts destined for in situ implantation in rifampicin to amplify local antibacterial effectiveness [35]. Analogously, in our investigation, rifampicin soaking was adopted for stent-grafts employed in endovascular repair of infected aneurysms. Nevertheless, full stent soaking could induce membrane tissue distension, complicating stent delivery system retrieval and risking structural damage or stent-graft membrane disruption, ultimately predisposing to endoleaks. To mitigate these challenges, we refined our technique: administering the prepared medication via a 20 ml syringe through the stent's ventilation side holes directly into the delivery system, facilitating a continuous 20-min stent-graft flushing. For thinner iliac artery stents devoid of side holes, we punctured the unprotected region of the delivery system with a sterile needle to facilitate drug infusion. This approach not only simplifies and expedites the process but also effectively safeguards against stent structural damage.
In this research endeavor, all patients underwent a comprehensive treatment plan encompassing endovascular stent-graft technology, which featured drug-coated surfaces, in conjunction with systemic antibiotic therapy. Subsequent to the endovascular repair surgery and adherence to a postoperative standard antibiotic regimen, no instances of infection deterioration, aortic region reinfection, or relentless aneurysm expansion were observed. Notably, one patient necessitated spinal lesion debridement for recurrent low back pain post-procedure. Another case, complicated by pulmonary artery and lower extremity venous thrombosis, underwent preoperative inferior vena cava filter placement, followed by its retrieval three months later, upon confirmation of thrombus resolution during follow-up. Additionally, a patient was readmitted six months post-discharge for percutaneous coronary intervention (PCI) stemming from an acute myocardial infarction. Only a single patient required a secondary endovascular stent-graft repair, necessitated by progressive aneurysm enlargement at the site of the initial infection. This aneurysmal expansion was hypothesized to arise from the primary Brucella infection of the aorta, unrelated to its recurrence or aggravation. Despite Brucella eradication, the pre-existing aneurysm modified local hemodynamics, with altered shear stress inside the aneurysm lumen contributing to its gradual expansion. Beyond standard antibiotic administration before and after surgery, the selection of the graft during the surgical intervention holds paramount importance. While current alloy graft options exhibit comparable infection susceptibility, those with superior isolation capabilities and minimal leakage rates should be favored. While this research approach has yielded encouraging outcomes, it acknowledges limitations, and the emergence of antibacterial coating grafts heralds a promising new avenue for future investigation.
Summary
Brucellosis manifests a widespread pandemic pattern, wherein its affiliated aortic aneurysmal condition, despite its infrequent occurrence, entails a formidable mortality hazard. Heightened recognition capabilities, expedited diagnosis, prompt intervention measures, and refined care standards are paramount in differentiating patients presenting with fever of indeterminate etiology within endemic regions. Distinctive features observed in patients with brucellar aortic aneurysms encompass: (1) Predominantly, middle-aged to elderly male farmers, with heightened vulnerability among those with prior animal exposure, particularly cattle, sheep, or their derivatives; (2) Characteristic symptoms embracing lumbar and abdominal discomfort, coupled with fever; (3) Lesion predilection within the infrarenal segment of the abdominal aorta, frequently encroaching upon the iliac artery; (4) The advocacy for extended antibiotic therapy regimens tailored to individuals with multi-organ and multi-systemic involvement.
Limitations
Our reported instances of brucellar aneurysm could be encumbered by selection bias, evident in a notably higher survival rate post-treatment, in contrast to aneurysms instigated by alternate pathogens. This disparity could stem from: 1. The availability of potent drugs like doxycycline, rifampicin, and gentamicin, demonstrating efficacy against diverse brucella strains; 2. Brucella's inherently lesser virulence compared to other microbial agents; 3. A relatively low propensity for mutation and drug resistance exhibited by Brucella, contrasting with other bacterial species.4.Lack of long-term follow-up data on adverse drug reactions, such as the rate of antibiotic-resistant bacterial colonization after treatment.
Conclusion
Brucellosis manifests a widespread pandemic pattern, wherein its affiliated aortic aneurysmal condition, despite its infrequent occurrence, entails a formidable mortality hazard. Heightened recognition capabilities, expedited diagnosis, prompt intervention measures, and refined care standards are paramount in differentiating patients presenting with fever of indeterminate etiology within endemic regions. Distinctive features observed in patients with brucellar aortic aneurysms encompass: (1) Predominantly, middle-aged to elderly male farmers, with heightened vulnerability among those with prior animal exposure, particularly cattle, sheep, or their derivatives; (2) Characteristic symptoms embracing lumbar and abdominal discomfort, coupled with fever; (3) Lesion predilection within the infrarenal segment of the abdominal aorta, frequently encroaching upon the iliac artery; (4) The advocacy for extended antibiotic therapy regimens tailored to individuals with multi-organ and multi-systemic involvement.
Acknowledgements
Not applicable.
Abbreviations
- BIA
Brucellosis-Induced aortic Aneurysm
- CTATA
Computed Tomography Angiography imaging data of the Thoracoabdominal Aorta
- CTA
Computed Tomography Angiography
- AAAs
Abdominal Aortic Aneurysms
- EVAR
Endovascular Aneurysm Repair
- PCI
Percutaneous Coronary Intervention
- WBC
White Blood Cell
- CRP
C-Reactive Protein
- ESR
Erythrocyte Sedimentation Rate
- PCT
Procalcitonin
- RBPT
Rose Bengal Plate Test
- SAT
Serum Agglutination Test
- NEUT
Neutrophil
Authors’ contributions
MLL prepared the manuscript draft, YHB & WYJ contributed to the patient’s history, follow-up, electrocardiograph, ZH & LC performed the histological examination, JFD did the surgical excision, LYJ contributed to the writing and the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Ningxia Hui Autonomous Region Key R&D Program [grant number 2022BEG02030] and the Ningxia Hui Autonomous Region Central Guided Local Local Science and Technology Development Special Project [grant number 2023FRD05007].
Data availability
The data that support the findings of this study are available from the corresponding author, [Youjin Li, Email: Nxrmyymll@sina.com], upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of The People's Hospital of NingXia Hui Autonomous Region and all the subjects provided their written informed consent before participation.All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deng Y, Liu X, Duan K, Peng Q. Research Progress on Brucellosis. Curr Med Chem. 2019;26(30):5598–608. [DOI] [PubMed] [Google Scholar]
- 2.Jennings GJ, Hajjeh RA, Girgis FY, et al. Brucellosis as a cause of acute febrile illness in Egypt. Trans R Soc Trop Med Hyg. 2007;101(7):707–13. [DOI] [PubMed] [Google Scholar]
- 3.X H,Wang,H,Jiang.Global prevalence of human brucellosis.Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi,2020,41(10):1717–1722. [DOI] [PubMed]
- 4.T,Liu,Y Wu,Y Q,Tong etal.epidemic characteristics and trend of brucellosis in china from 2004 to 2018.Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi,2024,43(03):190–196.
- 5.Reguera JM, Alarcón A, Miralles F, Pachón J, Juárez C, Colmenero JD. Brucella endocarditis: clinical, diagnostic, and therapeutic approach. Eur J Clin Microbiol Infect Dis. 2003;22(11):647–50. [DOI] [PubMed] [Google Scholar]
- 6.Kakkos SK, Papadoulas S, Lampropoulos G, Marangos M, Kalogeropoulou C, Tsolakis IA. Aorto-iliac aneurysm infected by Brucella: distinctive presentation patterns of a rare entity. Vascular. 2013;21(5):307–15. [DOI] [PubMed] [Google Scholar]
- 7.Cascio A, De Caridi G, Lentini S, et al. Involvement of the aorta in brucellosis: the forgotten, life-threatening complication A systematic review. Vector Borne Zoonotic Dis. 2012;12(10):827–40. [DOI] [PubMed] [Google Scholar]
- 8.Herrick JA, Lederman RJ, Sullivan B, Powers JH, Palmore TN. Brucella arteritis: clinical manifestations, treatment, and prognosis. Lancet Infect Dis. 2014;14(6):520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willems SA, Buntinx M, Gelinck LBS, van Schaik J, Eefting D. Ruptured Aneurysm of the Common Iliac Artery Caused by Brucella melitensis: A Case Report. EJVES Vasc Forum. 2021;52:26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willems SA, Brouwers JJWM, Eefting D. Aortic and Iliac Involvement in Brucellosis - A Rare but Life Threatening Manifestation: A Review of the Literature. Eur J Vasc Endovasc Surg. 2022;63(5):743–50. [DOI] [PubMed] [Google Scholar]
- 11.Solera J. Update on brucellosis: therapeutic challenges.Int J Antimicrob Agents. 2010;36 Suppl 1:S18-S20. [DOI] [PubMed]
- 12.Vrioni G, Bourdakis A, Pappas G, et al. Administration of a triple versus a standard double antimicrobial regimen for human brucellosis more efficiently eliminates bacterial DNA load. Antimicrob Agents Chemother. 2014;58(12):7541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.General Office of National Health Commission General Department of State Administration of Traditional Chinese Medicine. Diagnosis and treatment scheme for Brucellosis(2023 Edition). Chinese J Infection Control, 2024, 23(5): 661–664.
- 14.Xiufeng L, Tan W, Yuanzhi W, et al. Short- and long-term follow-up outcomes of patients with Brucella endocarditis: a systematic review of 207 Brucella endocarditis Cases. Bioengineered. 2021;12(1):5162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min J ,Zixu F ,Ruifang G , et al.Research progress on complications of Brucellosis. Frontiers Cellular Infection Microbiology, 2023;131136674–1136674. [DOI] [PMC free article] [PubMed]
- 16.Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6(12):e1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ece İ, Epçaçan S, Bayhan Gİ, Türe M. Diastolic dysfunction in patients with brucellosis despite the absence of infective endocarditis. Cardiol Young. 2020;30(12):1840–3. [DOI] [PubMed] [Google Scholar]
- 18.Mableson HE, Okello A, Picozzi K, Welburn SC. Neglected zoonotic diseases-the long and winding road to advocacy. PLoS Negl Trop Dis. 2014;8(6):e2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facciolà A, Palamara MAR, D’Andrea G, et al. Brucellosis is a public health problem in southern Italy: Burden and epidemiological trend of human and animal disease. J Infect Public Health. 2018;11(6):861–6. [DOI] [PubMed] [Google Scholar]
- 20.Chakfé N, Diener H, Lejay A, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections [published correction appears in Eur J Vasc Endovasc Surg. 2020;60(6):958. [DOI] [PubMed]
- 21.Hsu RB, Chen RJ, Wang SS, Chu SH. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004;40(1):30–5. [DOI] [PubMed] [Google Scholar]
- 22.Fillmore AJ, Valentine RJ. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg. 2003;196(3):435–41. [DOI] [PubMed] [Google Scholar]
- 23.Bacourt F, Koskas F. Axillobifemoral bypass and aortic exclusion for vascular septic lesions: a multicenter retrospective study of 98 cases. French University Association for Research in Surgery. Ann Vasc Surg. 1992;6(2):119–26. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Kwon TW, Park SJ, et al. The Results of In Situ Prosthetic Graft Replacement for Infected Aortic Disease. World J Surg. 2018;42(9):3035–41. [DOI] [PubMed] [Google Scholar]
- 25.Chan YC, Morales JP, Taylor PR. The management of mycotic aortic aneurysms: is there a role for endoluminal treatment? Acta Chir Belg. 2005;105(6):580–7. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, Yang Y, Liu H, Sun X, Li Y, Guo M. Brucella-infected abdominal aortic aneurysm: management strategies for an uncommon aneurysm. Front Med (Lausanne). 2023;10:1271217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dang Q, Statius van Eps RG, Wever JJ,et al. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair in The Netherlands. J Vasc Surg. 2020;72(2):531–540. [DOI] [PubMed]
- 28.Li X, Li X, Cheng Z. Brucellosis involving the aorta and iliac arteries: a systematic review of 130 cases. Front Bioeng Biotechnol. 2023;11:1326246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry J, Alslaim H, Agarwal G. Brucella aortitis managed with debridement, extra-anatomical bypass, and long-term antimicrobial therapy. Vascular. 2023;31(1):178–81. [DOI] [PubMed] [Google Scholar]
- 30.Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146(24):e334-e482. [DOI] [PMC free article] [PubMed]
- 31.Wanhainen A, Verzini F, Van Herzeele I, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms [published correction appears in Eur J Vasc Endovasc Surg. 2020;59(3):494. [DOI] [PubMed]
- 32.Glowacka P, Zakowska D, Naylor K, Niemcewicz M, Bielawska-Drózd A. Brucella - Virulence Factors, Pathogenesis and Treatment. Pol. J Microbiol. 2018;67(2):151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roop RM 2nd, Barton IS, Hopersberger D, Martin DW. Uncovering the Hidden Credentials of Brucella Virulence. Microbiol Mol Biol Rev. 2021;85(1):e00021-e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byndloss MX, Tsolis RM. Brucella spp. Virulence Factors and Immunity. Annu Rev Anim Biosci. 2016;4:111–27. [DOI] [PubMed] [Google Scholar]
- 35.Müller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33(1):106–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Youjin Li, Email: Nxrmyymll@sina.com], upon reasonable request.