Abstract
Radial Extracorporeal Shock Wave Therapy (rESWT) is applied as a conservative treatment modality in orthopedics, yet its effectiveness in addressing aseptic loosening of cementless joint prostheses remains unclear. Through animal experimentation, we have revealed that in a titanium particle-induced osteolysis rat model, rESWT intervention significantly increased periprosthetic bone density compared to untreated controls, concurrently reducing osteolytic lesion area and lowering serum IL-1β levels. Histological analyses demonstrated a relative decrease in osteoclast counts within the treatment group versus non-treated controls. These findings indicate that rESWT, through mechanisms involving anti-inflammatory actions and suppression of osteoclastogenesis, may serve as a non-invasive therapeutic strategy for preventing and managing periprosthetic bone loss, demonstrating clinical potential to delay or eliminate the necessity for revision surgeries.
Keywords: Extracorporeal shock wave therapy, Wear particles, Osteolysis, Osteoclasts, Inflammatory factors
Introduction
Osteoarthritis is a common cause of disability worldwide. Local cartilage damage and inflammatory changes gradually lead to joint pain, movement disorders and loss of function [1]. Total joint replacement (TJA) is a well-established intervention that effectively alleviates pain and restores motor function in patients suffering from osteoarthritis [2]. However, research indicates that the rate of prosthetic revision can reach 10–15% within a decade following TJA [3–4]. Post-implantation, joint prostheses are subject to wear, resulting in the release of micron-sized wear particles that incite chronic inflammatory responses. This process can lead to aseptic loosening of the prosthesis due to osteolysis, which is a significant contributor to the failure of total joint replacements [5–7]. The disruption of the delicate equilibrium between osteogenesis and the osteoblastic process is critical in the pathogenesis of wear particle-induced osteolysis. Wear particles have been shown to stimulate the release of pro-inflammatory cytokines from immune cells, such as IL-1β and TNF-α, while concurrently diminishing the expression of osteoprotegerin (OPG) in osteoblasts. This cascade of events promotes the expression of receptor activator of nuclear factor κB ligand (RANKL) and nuclear factor κB (NF-κB), which, through the activation of nuclear factor of activated T cells (NFAT), enhances the expression of osteoclast-related genes. This ultimately triggers the recruitment and maturation of osteoclast precursors, thereby regulating osteoclast differentiation and proliferation, which culminates in osteolysis [7–14]. Currently, there are no effective preventive treatments available, with the only recourse being revision surgery in the advanced stages of the condition. However, revision surgery is often complex, invasive, costly, and associated with significant postoperative risks and suboptimal long-term outcomes. Consequently, there remains a pressing clinical need to investigate novel, cost-effective diagnostic and therapeutic strategies aimed at preventing prosthetic aseptic loosening.
Extracorporeal shock wave therapy (ESWT), initially developed for the treatment of urinary calculi, has been repurposed as a non-invasive modality for addressing musculoskeletal disorders [15]. Evidence suggests that ESWT is notably effective in treating various bone and soft tissue conditions, including tendinitis, lateral epicondylitis, complications related to fracture healing, bone defects, and osteonecrosis of the femoral head [16–19]. ESWT has the capacity to activate bone marrow stem cells (BMSCs) and facilitate their differentiation into osteoblasts, while also promoting neoangiogenesis, enhancing callus formation, and accelerating fracture healing [20]. Nevertheless, to date, no studies have definitively established whether ESWT can mitigate periprosthetic osteolysis induced by wear particles or reduce the risk of prosthesis loosening following arthroplasty. In this investigation, we developed a model of wear particle-induced osteolysis to examine the effects of ESWT on inflammatory factor levels, periprosthetic osteolysis, and the activity and function of bone cells in a rat model. This study aims to elucidate the inhibitory effects and potential mechanisms of ESWT on periprosthetic osteolysis, thereby providing a theoretical and experimental foundation for its clinical application.
Methods
Experimental animals and Materials
A total of thirty healthy male Sprague-Dawley (SD) rats, approximately 6–8 weeks old, with a body weight of 300 ± 20 g, were utilized for this study. All animal experiments received approval from the Laboratory Animal Ethics Committee of the First Affiliated Hospital of Harbin Medical University. The care and treatment of the experimental animals adhered strictly to the Regulations on the Management of Experimental Animals established by Harbin Medical University.
The relevant equipment and materials are listed in Table 1.
Table 1.
Experimental equipment
| Name of experimental instruments and consumables | manufacturer |
|---|---|
| The extracorporeal shock-wave therapy apparatus(XY-K-MEDICAL-A) | Xiangyu Medical, China |
| automatic tissue dehydration apparatus | Junjie, China |
| Paraffin embedding machine | Junjie, China |
| Biological tissue stall chip baking machine | Junjie, China |
| Paraffin slicing machine | Leica Biosystems, China |
| Electric heating constant temperature blast drying box | Leibo Terry, China |
| microscope | Nikon, Japan |
| Microscope camera system | Nikon, Japan |
| Small animal live imaging system | KUBTEC, USA |
| superclean bench | Thermofisher Scientific, USA |
| refrigerated centrifuge | Thermofisher Scientific, USA |
| enzyme-labeled instrument | Bio-Rad, China |
| Ultra fine titanium particles | Jinchun Metal, China |
| 0.9% saline | SJZ No.4 Pharmaceutical, China |
| pentobarbital sodium | Sanjing Pharmaceutical, China |
| carprofen | Aladdin, China |
| Dimethylsulfoxide (DMSO) | Solaibao, China |
| polyethylene glycol 300(PEG300) | Solaibao, China |
| Tween 80 | Solaibao, China |
| Sodium penicillin for injection | North China Pharmaceutical, China |
| phosphate buffer | Solaibao, China |
| absolute ethyl alcohol | Xilong Scientific, China |
| dimethylbenzene | Xilong Scientific, China |
| 4% paraformaldehyde | Solaibao, China |
| EDTA decalcifying Fluid | Solaibao, China |
| Hematoxylin | Solaibao, China |
| Eosin Y water soluble | Xiya Reagent, China |
| The TRAP staining kit | Sevierbio, China |
| Rat IL -1β ELISA Kit | Lianke Biotech, China |
Titanium particle Preparation
The titanium particles were shaken and soaked overnight in 75% ethanol to remove endotoxins. After that, they were dried through a freezing method. An endotoxin detection kit was then used to ensure there was no endotoxin contamination. The titanium particles were disinfected by exposure to γ - rays (2.5 MR) and stored at -20 °C. Prior to use, 7.5 mg of titanium particles were weighed out and added to 1.5 mL of PBS to form a suspension.
Model construction and experimental grouping for femoral wear particle-induced osteolysis
The thirty SD rats were randomly assigned to three groups(Animals were randomly assigned to three groups (n = 10/group) using block randomization, with operators blinded to group allocation during outcome assessments): a blank control group, a model group, and an extracorporeal shock wave therapy (ESWT) treatment group. (Table 2) Construct the animal model according to the modeling method described in the reference [21]. Prior to the procedures, the rats were weighed and anesthetized with an injection of 2.5% pentobarbital sodium administered via the tail vein at a dosage of 1 ml/kg. A distal femoral periprosthetic osteolysis model was constructed following established methodologies. The right knee joint of rats was selected for skin preparation, and then POvidone iodine was selected for disinfection, and the surgical field of the knee joint was fully exposed after single placement. The patella was gently displaced to locate the medial space of the patellofemoral joint. The knee joint was flexed appropriately, and an incision of suitable length was made to separate the joint capsule, surrounding tissue, and skin, thereby accessing the joint cavity.The patellar eversion was pushed out and dislocated, and then the distal femoral fossa and other fields were fully exposed. A needle was inserted into the femoral intercondylar socket, positioned 5 mm from the dorsal aspect of the femur. Following proper positioning, a bone tunnel(with a diameter of 1.5 mm and a length of 1.5 cm) was drilled using an electric drill to ensure alignment with the long axis of the femur. (Fig. 1) After achieving hemostasis, 50 µL of titanium particle suspension was injected, and pure titanium rods(with a diameter of 1.2 mm and a length of 1.5 cm) were placed to seal the bone tunnel, followed by hemostasis, reposition the patella, and suture the joint capsule, tissues, and skin layer by layer. Postoperatively, 2 mg/kg of carprofen and 6000 U/kg of penicillin were administered for three consecutive days to mitigate the risk of infection. On postoperative days 4, 8, and 12, 0.1 ml of titanium particle suspension was injected into the knee cavity of all rats, with the exception of those in the control group, which received 0.1 ml of isotonic saline. Four weeks post-intervention, blood samples were collected from the main abdominal artery, and bone tissue was harvested from the distal femur for subsequent analyses.
Table 2.
Animal grouping
| Group | Intervention measures | Number of animals |
|---|---|---|
| Blank control group | Distal femur implantation of titanium rod + injection of normal saline | 10 |
| Model group | Distal femur implantation of titanium rod + injection of titanium particle suspension | 10 |
| ESWT treatment grou | Distal femur implantation of titanium rod + injection of titanium particle suspension + ESWT treatment | 10 |
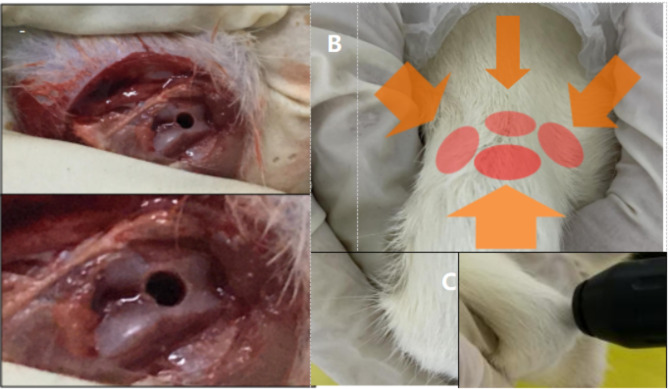
Fig. 1.
A: Photo of titanium rod implantation before the osteolytic rat model was constructed; B: Shock wave treatment site; C: Shock wave treatment position
Extracorporeal shock wave therapy
Two weeks following the successful establishment of the osteolysis model, the rats were secured in a specialized frame to extend the right hind limb, thereby exposing the knee joint and surrounding tissues, and then apply the coupling agent. Extracorporeal shock wave therapy was administered as a form of intervention.Therapeutic parameters were established with reference to the Chinese Guidelines for Extracorporeal Shock Wave Therapy in Musculoskeletal Disorders (2023 Edition) and preliminary experiments. This process revealed that the 0.2 mJ/mm² protocol achieved optimal balance between osteogenic response and inflammatory suppression without causing tissue damage, thereby confirming the final treatment parameters. The treatment utilized a D15 probe (deep, with a diameter of 15 mm, tolerance ± 15%, energy density of 0.2 mJ/mm², tolerance ± 20%, penetration depth of 7 mm, tolerance ± 20%, and pulse width of 200 µs, tolerance ± 10%). The treatment pressure for the extracorporeal shock wave was set at 1 bar, with a frequency of 5 Hz and a total of 1000 pulses per treatment session. The treatment site was alternated after every 250 shocks to prevent the occurrence of local hematomas or skin damage.During treatment, attention should be given to avoid important nerves and blood vessels around the knee joint. The rats were treated once a day, and the entire treatment lasted 10–12 min with intervention for 4 weeks (Fig. 1).
Sample collection and processing
Four weeks after shock wave intervention, abdominal aortic blood and distal femoral bone tissues of rats were collected for subsequent experiments. First, rats were anesthetized by intraperitoneal injection of 2.5% pentobarbital sodium (1 ml/kg). Then, the rats were fixed, laparotomy was performed to expose the aorta, and 3–5 ml of arterial blood was collected into an EDTA anticoagulation tube. After setting the centrifuge parameters, the abdominal aortic blood was centrifuged, and the supernatant was aspirated and stored in a − 80 °C refrigerator. When using, repeated freeze - thaw cycles should be avoided to prevent the inactivation of active substances and reduce experimental result errors.
Rats were euthanized. First, press the rat’s head, lift its tail, and quickly and forcefully pull it in a direction away from the center of the body to dislocate the cervical vertebra. After confirming the death of the rat, the entire femur was completely removed with tissue scissors. Pay attention to maintaining the integrity of the distal femoral bone tissue where the titanium rod was implanted. The specimen was immediately subjected to high - resolution CT examination. After the examination, the muscles and soft tissues of the distal femur were removed, and the femur was cut along the long axis of the femur. After removing the implanted titanium rod, the tissue around the implant within 5 mm was sampled again, and then placed in paraformaldehyde for fixation for 48 h for subsequent staining.
High-Resolution computed tomography (CT) analysis
Following the fixation of femoral tissue from each rat in 4% paraformaldehyde for a duration of 48 h, high-resolution CT imaging was conducted under the parameters of 120 kV voltage, 128 mA current, and a slice thickness of 0.7 mm. The spatial distribution of femoral cancellous bone and osteolytic changes were examined, and a region of interest (ROI) was delineated for subsequent evaluation of bone mineral density, which was analyzed using Image-Pro Plus 5.0 software.
Hematoxylin and Eosin (HE) staining
The femurs from each experimental group were similarly fixed in 4% paraformaldehyde for 48 h, followed by decalcification using EDTA, which allowed for easy penetration into the bone tissue over approximately eight weeks. The samples underwent dehydration, paraffin embedding, and sectioning at a thickness of 4 μm, after which they were subjected to HE staining. Osteolytic areas were assessed in four to five fields, and the extent of osteolysis was quantified using Image-Pro Plus 5.0 software.
Tartrate-resistant acid phosphatase (TRAP) staining
The femurs from each group were fixed in 4% paraformaldehyde for 48 h, followed by EDTA decalcification, which facilitated easy puncture into the bone tissue over a period of approximately eight weeks. The samples were then dehydrated, embedded in paraffin, sectioned to a thickness of 4 μm, and stained for TRAP. Osteolysis was evaluated in four to five fields, and the number of osteoclasts was analyzed and quantified using Image-Pro Plus 5.0 software.
Enzyme-linked immunosorbent assay (ELISA)
Blood serum samples were collected to quantify the concentration of interleukin-1 beta (IL-1β) using a Rat IL-1β ELISA Kit, following the manufacturer’s instructions. The optical density (OD) was measured at 450 nm, and a fitted curve was generated from standard concentrations to calculate the concentration of the test samples based on the established formula.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 6.0 software. Results are presented as mean ± standard deviation (x ± s). Comparisons among three groups were performed using one-way ANOVA, while pairwise comparisons were executed using t-tests, with a significance threshold set at P < 0.05 (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Results
Effects of ESWT on bone cortical thickness and mineral density
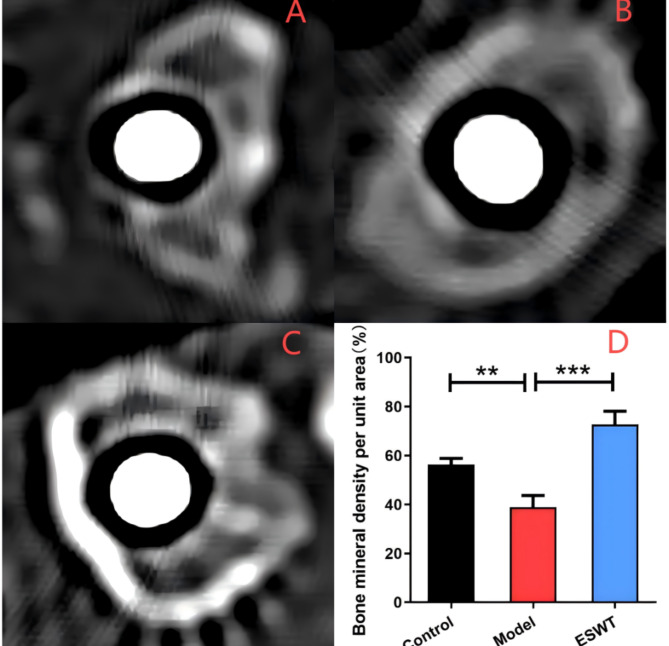
Following a 4-week treatment period, imaging data were obtained around the distal femur prosthesis using high-resolution computed tomography (CT) (Fig. 2). The findings indicated that the bone surrounding the distal femur remained continuous and intact, exhibiting no significant inflammatory responses or signs of osteolysis. In comparison to the blank control group, the model group demonstrated reduced bone density in areas of disrupted bone continuity and osteolysis (P < 0.01), suggesting a detrimental effect of titanium (Ti) particles on the bone adjacent to the implanted prosthesis. Conversely, in the ESWT treatment group, the bone around the prosthesis was continuous and intact, and the bone cortex was relatively thick. Compared with the model group, the ESWT treatment group had a significantly higher bone density (P < 0.001), and no obvious osteolysis was observed.
Fig. 2.
High-resolution CT analysis of the periprosthetic BMD in each rat group. A: blank control group; B: model group; C: ESWT treatment group; D: Bone mineral density in each group per unit area (**, P < 0.01;***, P < 0.001)
IL-1β expression can be suppressed by ESWT
Compared with that in the blank control group, the level of IL-1β in the model group was significantly greater (P < 0.001), and compared with that in the model group, the level of IL-1β in the ESWT group was significantly lower (P < 0.001, Fig. 3).
Fig. 3.
IL-1β levels in the serum of rats determined via ELISA (* * *, P < 0.001)
Inhibition of osteolysis and osteoclastogenesis by ESWT
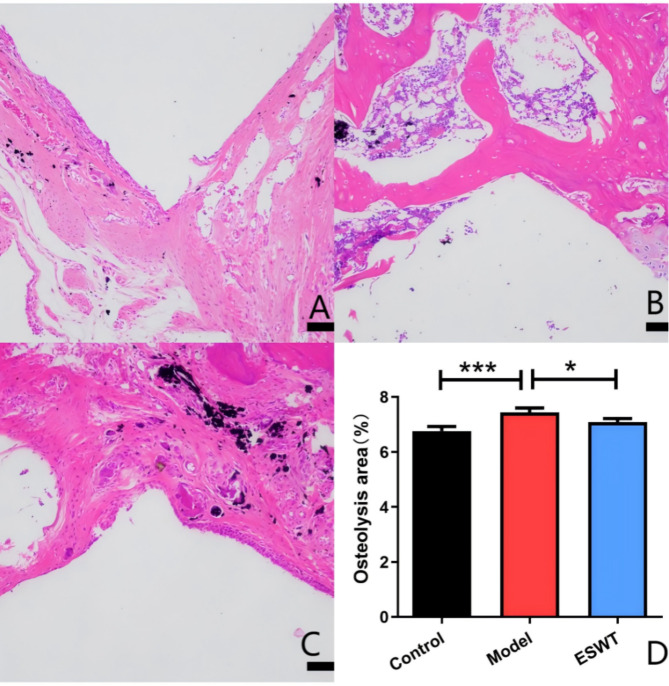
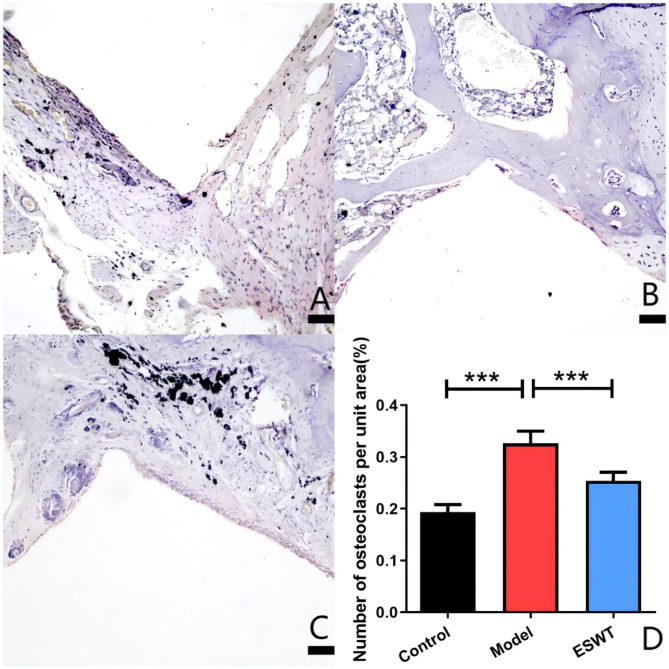
In the model group, there was partial continuity of direct contact between the prosthesis and bone, with visible black titanium particles in the surrounding bone. This area exhibited extensive osteolytic cavities that were populated by numerous inflammatory macrophages and osteoclasts (P < 0.001), leading to a significant increase in the osteolysis area (P < 0.001). In contrast, the ESWT-treated group displayed complete and continuous direct contact between the prosthesis and bone, with only minor osteolytic cavities surrounding the titanium particles. This group also showed a reduced presence of inflammatory cells and a significant decrease in the number of osteoclasts (P < 0.001), as well as a reduction in osteolysis (P < 0.05) (Figs. 4 and 5).
Fig. 4.
The area of periprosthetic osteolysis in each rat group was analyzed via HE staining. A: blank control group; B: model group; C: ESWT treatment group; D: osteolysis area of each group per unit area (*P < 0.05; **P < 0.01; ***P < 0.001)
Fig. 5.
TRAP staining analysis. A: blank control group; B: model group; C: ESWT treatment group; D: number of osteoclasts per unit area in each group per unit area (*P < 0.05; **P < 0.01; ***P < 0.001)
Discussion
Joint arthroplasty is a key approach for joint injury and reconstruction, and despite the increasing popularity of its clinical use, postoperative complications such as infections, aseptic loosening and peripheral fractures still need to be focused on [22]. A retrospective study revealed that in 23,269 patients who underwent hip revision, more than 50% of the secondary surgeries involved aseptic loosening [23].
The bioactive particles released by micromovement and wear between artificial joint components can promote osteoclast maturation and differentiation, lead to osteolysis, and affect the metabolism of osteoclasts and osteoblasts by triggering an inflammatory response. For example, bone metabolism is affected by the ability of macrophages, fibroblasts, osteoblasts and osteoclasts to synthesize and release chemokines and cytokines, including IL-1β blasts and TNF-α [7, 24]. Bone metabolic homeostasis is dependent on the balance between osteoclasts and osteoblasts. After joint prosthesis replacement, wear particles disrupt this balance, leading to increased bone resorption and decreased bone formation, the main cause of aseptic loosening of the prosthesis [25]. Reducing osteoclast formation and activity in periprosthetic bone tissue through treatment and maintaining local bone metabolic balance may be a key strategies for preventing artificial joint loosening. In previous studies, we reported that inhibiting TNF-α and enhancing OPG could effectively inhibit the periprosthetic osteolysis induced by Ti particles [26–28]. In addition, in the presence of Ti particles, the expression of NFATc1 in periprosthetic tissue increased significantly, and inhibiting RANKL could effectively inhibit the expression of NFATc1 and the proliferation and differentiation of osteoclasts [29], indicating that the regulation of the RANKL/RANK/OPG/NFATc1 pathway can effectively prevent wear particle-induced osteolysis and further prove the importance of regulating bone metabolic homeostasis for the prevention of aseptic loosening of the prosthesis.
As the drug for the treatment of osteoporosis, bisphosphonates can inhibit osteoclast activity, reduce bone resorption, increase bone density, reduce osteoblast apoptosis and promote bone formation, with the potential to prevent or reduce osteolysis [30]. However, Bisphosphonate drugs have relatively poor medium - and long - term efficacy [31], and they carry the risks of increasing the likelihood of atrial fibrillation and bone fragility [32]. When combined with anti-inflammatory drugs, a high blood concentration is needed to maintain the local effective concentration, causing serious side effects and delaying its application in clinical practice. Gene therapy has also made some advances in periprosthetic osteolysis therapy, but it is still in its early stage and cannot be used clinically in the short term [33]. Therefore, finding safe and economical nonsurgical nondrug treatments to intervene in and treat periprosthetic osteolysis remains an urgent clinical problem.
With the increasing application of ESWT in the medical field, researchers have explored the site of action and treatment principles, and increasing evidence has revealed the positive influence of ESWT on the balance between osteogenesis and osteolysis of bone tissue. ESWT can promote the differentiation of BMSCs into osteoblasts through the MAPK signaling pathway and can inhibit the proliferation of osteoclasts and reduce bone resorption [34–36]. Moreover, the expression of ERK and P38 was significantly increased in some bone defect areas, and an obvious osteogenesis process occurred, which promoted bone formation [37]. Studies have shown that high-energy extracorporeal shock wave therapy (ESWT) can be used at the interface between muscle and bone. Due to differential acoustic impedance, energy pulses act on bone tissue, stimulate the biosynthetic response of cancellous bone and cortical bone, activate mesenchymal stem cells, enable them to transform into osteoblasts, and accelerate the growth of bone tissue [38]. On the other hand, appropriate-strength ESWT (0.08 mJ/mm2, 500 times) inhibited the transcriptional activity of NFATc1 and the differentiation of mature osteoclasts and bone resorption through the regulation of c-fos gene expression [39]. Of course, extracorporeal shock wave is not completely without side effects. Any treatment method needs to be applied within an appropriate range. Moreover, different treatment energy flux densities and frequencies of shock waves can also have a significant impact on the treatment effect. In an animal experiment, Martini et al. [40]. measured the cell number and cell viability at 24 h and 48 h after using different energy flux levels. It was found that compared with the control group, the high - energy flux level group showed cell damage and a general decrease in cell metabolism, while the control group showed an increase in cell metabolism. Therefore, for various diseases in different parts of the body during shock wave treatment, appropriate parameters should be selected.
The present study first confirmed via high-resolution CT analysis that ESWT could decrease the occurrence of titanium particle-induced periprosthetic osteolysis around the distal femur in rats. Moreover, ESWT also increased the periprosthetic BMD in the osteolysis model, which is consistent with the conclusion of Shi et al. [41], who reported that high-energy ESWT (0.28 mJ/mm2, 4 Hz, 4000 times) was better at improving the local BMD, demonstrating the potential of ESWT in treating periprosthetic osteolysis. ESWT helps reduce the serum IL-1β levels caused by wear particles and suppresses systemic inflammation. As observed by staining, ESWT reduced the number of osteoclasts and macrophages within the area of osteolysis, probably because it reduced the level of inflammation and inhibited the differentiation of osteoclasts, thereby inhibiting osteolysis. ESWT may also act directly on bone marrow-derived macrophages to prevent their differentiation into osteoclasts. However, this study has several limitations: Only titanium particles were studied, while the wear particles generated by prostheses in clinical settings also include polyethylene, ceramic particles, etc.; the therapeutic effect of ESWT on titanium particle-induced osteolysis was preliminarily explored, but the osteogenic effect was not verified at the protein level, and the ability of ESWT to inhibit the osteoclastic differentiation of BMSCs was not evaluated. Although the inhibitory effect and underlying molecular mechanism of ESWT on osteolysis at the in vivo level have been defined, the influence and regulatory mechanism of ESWT on BMSCs, BMMs and osteoclasts at the cellular level still need further investigation.Meanwhile, in the experiment, we only took into account the osteolysis near the body of the prosthesis. However, in actual clinical situations, prostheses are not merely rod - shaped structures. Therefore, this experiment still has certain limitations. Moreover, subsequent investigations should systematically address these constraints to establish a robust theoretical foundation that facilitates early-stage clinical application of rESWT in preventing wear particle-induced periprosthetic osteolysis.
Conclusion
Radial Extracorporeal Shock Wave Therapy (rESWT) significantly attenuated titanium particle-induced periprosthetic osteolysis in a rat model, as evidenced by reduced serum IL-1β levels and decreased osteoclast density. These findings highlight rESWT’s dual mechanism of suppressing inflammatory cascades and osteoclastogenesis, providing a non-invasive therapeutic strategy to prolong prosthetic implant longevity. In clinical practice, rESWT could be integrated into postoperative management protocols to mitigate risks of aseptic loosening, thereby potentially reducing revision surgery rates.
Acknowledgements
Not applicable.
Author contributions
Fengnian Zhao put forward the research conception, designed the experimental scheme, investigated and collected the experimental data, carried out data analysis, and accomplished the writing of the manuscript. Yufei Chen collected and analyzed research data and was involved in manuscript writing.Ao Dong collects and analyzes research data and is involved in manuscript writing. Keguan Song reviewed, edited, and supervised manuscripts.
Funding
The authors reported there is no funding associated with the work featured in this article.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All the animal experiments were approved by the Laboratory Animal Ethics Committee of the First Affiliated Hospital of Harbin Medical University. All experimental animals were treated and cared for in strict accordance with the Regulations on the Management of Experimental Animals of Harbin Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krakowski P, Rejniak A, Sobczyk J, Karpiński R. Cartilage integrity: A review of mechanical and frictional properties and repair approaches in osteoarthritis. Healthc (Basel). 2024;12(16):1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilczyński M, Bieniek M, Krakowski P, Karpiński R. Cemented vs. Cementless fixation in primary knee replacement: A narrative review. Mater (Basel). 2024;17(5):1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukamoto M, Ohnishi H, Mori T, Kawasaki M, Uchida S, Sakai A. Fifteen-YearComparison of wear and osteolysis analysis for Cross-Linked or conventional polyethylene in cementless total hip arthroplasty for hip Dysplasia-A retrospective cohort study. JArthroplasty. 2017;32(1):161–e1651. [DOI] [PubMed] [Google Scholar]
- 4.Lachiewicz PF, Soileau ES. Highly Cross-linked polyethylene provides decreased osteolysis and reoperation at minimum 10-Year Follow-Up. J Arthroplasty. 2016;31(9):1959–62. [DOI] [PubMed] [Google Scholar]
- 5.Li, Wenbo. Song Keguan. Associated biological mechanisms of periprosthetic osteolysis after hip replacement [J]. 2018,22(03):464–470.
- 6.Panez-Toro I, Heymann D, Gouin F, Amiaud J, Heymann MF, Córdova LA. Roles of inflammatory cell infiltrate in periprosthetic osteolysis. Front Immunol. 2023;1(14):1310262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xu S, Li K, et al. mTORC1 inhibits NF-κB/NFATc1 signaling and prevents osteoclast precursor differentiation, in vitro and in Mice[J]. J Bone Min Res. 2017;32(9):1829–40. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Xu S, Li K, Tan K, Liang K, Wang J, Shen J, Zou W, Hu L, Cai D, Ding C, Li M, Xiao G, Liu B, Liu A, Bai X. mTORC1 inhibits NF-κB/NFATc1 signaling and prevents osteoclast precursor differentiation, in vitro and in mice. J Bone Min Res. 2017;32(9):1829–40. [DOI] [PubMed]
- 9.Park JH, Lee NK, Lee SY. Current Understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40(10):706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altaf H, Revell PA. Evidence for active antigen presentation by monocyte/macrophages in response to stimulation with particles: the expression of NFκB transcription factors and costimulatory molecules. Inflammopharmacology. 2013;21(4):279–90. [DOI] [PubMed] [Google Scholar]
- 11.Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, Udagawa N, Aoki K, Suzuki H. Coupling of bone resorption and formation by RANKL reverse signaling. Nature. 2018;561(7722):195–200. [DOI] [PubMed] [Google Scholar]
- 12.Yin Z, Gong G, Liu X, Yin J. Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis. Front Immunol. 2023;14:1274679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Wang C, Qiu H, Yuan Y, Chen K, Cao Z, Xiang Tan R, Tickner J, Xu J, Zou J. Asperpyrone A attenuates RANKL-induced osteoclast formation through inhibiting NFATc1, Ca2 + signaling and oxidative stress. J Cell Mol Med. 2019;23(12):8269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Wang C, Wang G, Sun Y, Deng Z, Chen L, Chen K, Tickner J, Kenny J, Song D, Zhang Q, Wang H, Chen Z, Zhou C, He W, Xu J. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis by attenuating NFATc1 and ROS activities. Theranostics. 2019;9(16):4648–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Haojun J, Haiguang Z, Junyu, et al. Guidelines for extracorporeal shock wave therapy for bone and muscle diseases in China (2023 edition) [J]. Chin J Frontier Med (electronic edition). 2023;15(09):1–20. [Google Scholar]
- 16.Li Hui G, Guofang YH, et al. Progress in extracorporeal shock wave treatment of knee osteoarthritis [J]. Chin Health Standard Manage. 2024;15(07):195–8. [Google Scholar]
- 17.Maffulli G, Hemmings S, Maffulli N. Assessment of the effectiveness of extracorporeal shock wave therapy (ESWT) for soft tissue injuries (ASSERT): an online database protocol. Transl Med UniSa. 2014;4(8):10. [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz C, Császár NB, Milz S, Schieker M, Maffulli N, Rompe JD, Furia JP. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br Med Bull. 2015;116(1):115–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An Guoyao G, Mingxuan Z, Li, et al. Progress in the mechanism of bone nonunion [J]. Chin Contemp Med. 2023;30(25):38–42. [Google Scholar]
- 20.Rosso F, Bonasia DE, Marmotti A, Cottino U, Rossi R. Mechanical Stimulation(Pulsed Electromagnetic Fields PEMF and Extracorporeal Shock Wave TherapyESWT) and Tendon Regeneration: A Possible Alternative. Front Aging Neurosci. 2015;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jianli F, Yueyan J, Hangxing B, Wenxi D, Luwei X, Tong Peijian. Evaluation of an animal model of osteolysis [J]. Chin J Orthop Trauma. 2011;13(10):960–3. [Google Scholar]
- 22.ONeill SC, Queally JM, Devitt BM, Doran PP, OByrne JM. The role of osteoblasts in peri-prosthetic osteolysis. Bone Joint J. 2013;95–B(8):1022–6. [DOI] [PubMed] [Google Scholar]
- 23.Prieto-Alhambra D, Javaid MK, Judge A, Murray D, Carr A, Cooper C, Arden NK. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ. 2011;343:d7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Yingjun W. Lianguo. Progress on wear particles and periprosthetic osteolysis after arthroplasty [J]. 2016,29(10):968–72. [DOI] [PubMed]
- 25.Abu-Amer W, Arra M, Clohisy JCF, Abu-Amer Y, Swarnkar G. Targeting vascular endothelial growth factor ameliorates PMMA-particles induced inflammatory osteolysis in murine calvaria. Bone. 2019;123:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PENG P, LI L, et al. Lentivirus-mediated TNF-α gene Silencing and overexpression of osteoprotegerin inhibit titanium particle-induced inflammatory response and osteoclastogenesis in vitro. Mol Med Rep. 2016;13(1):1010–8. [DOI] [PubMed] [Google Scholar]
- 27.Peng,Li et al. TNF-α suppression and osteoprotegerin overexpression inhibits wear Debris-Induced inflammation and osteoclastogenesis in vitro[J]. Int J Artif Organs 2015,38:565–71. [DOI] [PubMed]
- 28.Zhang H-W et al. The role of RANKL/RANK/OPG system in the canine model of hip periprosthetic infection Osteolysis[J]. Int J Artif Organs 2016,39:619–24. [DOI] [PMC free article] [PubMed]
- 29.Zhang. Yunge, Calcineurin/NFAT signaling pathway mediates titanium particle-induced inflammation and osteoclast formation by inhibiting RANKL and M-CSF in vitro[J]. Mol Med Rep 2017,16:8223–30. [DOI] [PubMed]
- 30.Larrañaga-Vera A, Toti KS, Flatow JS, Haraczy AJ, Warnick E, Rao H, Gao ZG, Sussman SM, Mediero A, Leucht P, Jacobson KA, Cronstein BN. Novel alendronate-CGS21680 conjugate reduces bone resorption and induces new bone formation in postmenopausal osteoporosis and inflammatory osteolysis mouse models. Arthritis Res Ther. 2022;24(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson JM, Little DG. Bisphosphonates in orthopedic applications. Bone. 2011;49(1):95–102. [DOI] [PubMed] [Google Scholar]
- 32.Yuan K, Chen KC, Chan YJ, Tsai CC, Chen HH, Shih CC. Dental implant failure associated with bacterial infection and long-term bisphosphonate usage: a case report. Implant Dent. 2012;21(1):3–7. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Jiang P, Li W, Liu X, Lu Y, Huang Z, Song K. Calcineurin/NFAT signaling pathway mediates titanium particle- induced inflammation and osteoclast formation by inhibiting RANKL and M- CSF in vitro. Mol Med Rep. 2017;16(6):8223–30. [DOI] [PubMed] [Google Scholar]
- 34.Zhai L, Sun N, Zhang B, Liu ST, Zhao Z, Jin HC, Ma XL, Xing GY. Effects of focused extracorporeal shock waves on bone marrow mesenchymal stem cells in patients with avascular necrosis of the femoral head. Ultrasound Med Biol. 2016;42(3):193–62. [DOI] [PubMed] [Google Scholar]
- 35.Gao T, Yu C, Shi X, Hu Y, Chang Y, Zhang J, Wang Y, Zhai Z, Jia X, Mao Y. Artemisinic acid attenuates osteoclast formation and titanium particle-induced osteolysis via Inhibition of RANKL-induced ROS accumulation and MAPK and NF-κB signaling pathways. Front Pharmacol. 2024;1:15:1345380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Keqiang L, Yi W, Baogang, et al. Expression of c-fos, c-jun during transformation of BMSCs to osteoblasts [J]. Shandong Med. 2008;4(48):6364. [Google Scholar]
- 37.Chen YJ, Kuo YR, Yang KD, Wang CJ, Sheen Chen SM, Huang HC, Yang YJ, Yi-Chih S, Wang FS. Activation of extracellular signal-regulated kinase (ERK) and p38 kinase in shock wave-promoted bone formation of segmental defect in rats. Bone. 2004;34(3):466–77. [DOI] [PubMed] [Google Scholar]
- 38.Xia W, Mørch CD, Matre D, Andersen OK. Exploration of conditioned pain modulation effect on long-term potentiation-like pain amplification in humans. Eur J Pain. 2017;21(4):645–57. [DOI] [PubMed] [Google Scholar]
- 39.Huan G, Ming L, Shuitao L et al. Effect of extracorporeal shock wave on bone marrow-derived macrophages into osteocllasts and bone resorption activity [J]. 2017,9(02):20–4.
- 40.Martini L, Fini M, Giavaresi G, et al. Primary osteoblasts response to shock wave therapy using different parameters [J]. Artif Cells Blood Substit Immobil Biotechnol. 2003;31(4):449–66. [DOI] [PubMed] [Google Scholar]
- 41.Shi L, Gao F, Sun W, Wang B, Guo W, Cheng L, Li Z, Wang W. Short-term effects of extracorporeal shock wave therapy on bone mineral density in postmenopausal osteoporotic patients. Osteoporos Int. 2017;28(10):2945–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.