Abstract
Background
Primary liver cancer is a highly aggressive neoplasm with high incidence and mortality. Due to the high ability to metastasis, the 5-year survival rate of patients with primary liver cancer is poor.
Aim
To investigate the clinical value of systemic immunoinflammatory index (SII) in predicting recurrence and metastasis after interventional therapy in patients with primary liver cancer.
Methods
Total 186 patients with primary liver cancer were included and underwent Transcatheter arterial chemoembolization (TACE), and followed up for 3 years. Then, patients were divided into 110 cases in the recurrent metastasis group and 76 cases in the non-recurrent metastasis group according to presence or absence of recurrence and metastasis. Baseline data, SII and alpha-fetoprotein (AFP) levels were compared. Cox proportional hazards regression analysis was used to analyze factors affecting recurrence and metastasis. ROC curve was used to analyze SII and AFP levels in predicting recurrence and metastasis after interventional therapy in patients. Kaplan-Meier survival curves were used to evaluate the survival of patients.
Results
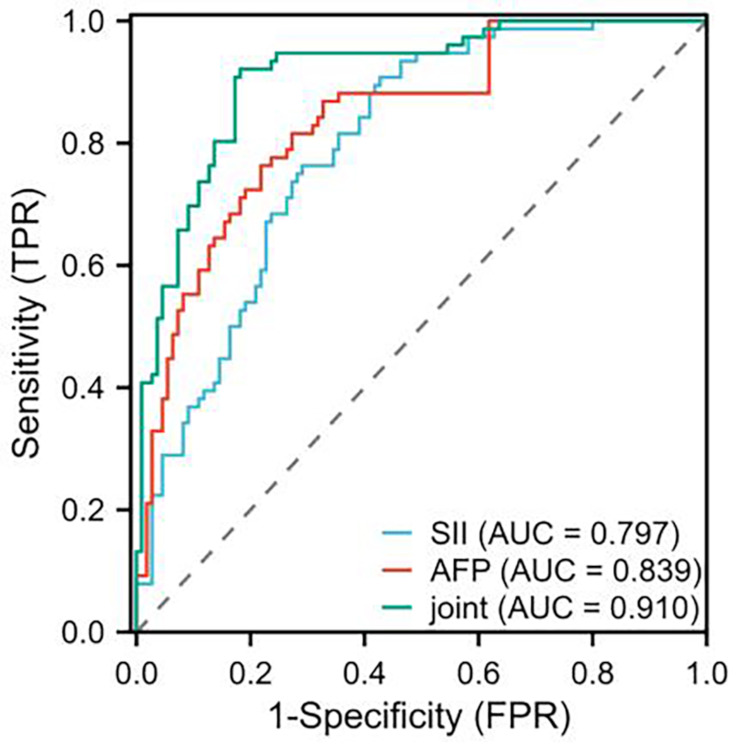
The SII index and AFP levels in the recurrence and metastasis group were higher than those in the non-recurrence and metastasis group (P < 0.001). Cox proportional hazards regression analysis confirmed tumor size ≥ 5 cm, presence of vascular tumor thrombus, presence of vascular invasion, no tumor capsule, SII index, AFP Levels were closely related to the recurrence and metastasis of patients with primary liver cancer (P < 0.05). ROC curve analysis showed that AUC of SII and AFP predicted recurrence and metastasis after intervention were 0.797 and 0.839, respectively, and the jointed AUC was 0.910. After a 3-years of follow-up, the overall survival rate of the 186 patients was 45.70% (85/186). Kaplan-Meier survival curve analysis showed that patients with high SII levels had shorter survival time than that of patients with low SII levels (P < 0.05).
Conclusion
Preoperative SII was closely associated with early recurrence and metastasis, and combined with AFP may have higher value in predicting recurrence and metastasis after interventional therapy in patients with primary liver cancer.
Keywords: SII, Primary liver cancer, TACE, Recurrence, Metastasis
Introduction
Primary liver cancer is a common cancer that originates in the liver, and it can be divided into three categories according to pathology, including hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and mixed hepatocellular carcinoma-cholangiocarcinoma. Among them, hepatocellular carcinoma (HCC) ranks as the most prevalent cancers worldwide, accounting for approximately 75-85% of primary liver malignancies [1]. Primary liver cancer is known as the six most commonly diagnosed cancer and the third leading cause of cancer death around the world [2]. Epidemiological report by World Health Organization (WHO), Global Burden Disease (GBD) and Global Cancer Observatory (GCO) confirms approximately 906,000 new cases of liver cancer and 830, 000 deaths worldwide in 2020 [2]. It is generally believed that the incidence of primary liver cancer is a complicated pathological process that involves in multiple risk factors, such as chronic viral hepatitis, alcohol consumption, and improper diet [3]. Thus, early diagnosis and treatment are of great significance to improve the survival rate and quality of life of patients with primary liver cancer.
Currently, many clinical therapeutic strategies are applied to patients with primary liver cancer; however, most patients are unable to receive radical treatment at the time of diagnosis, which leads to the poor overall therapeutic efficacy [4]. Moreover, the traditional surgical resection and liver transplantation result in greater damage to patients with primary liver cancer and are prone to recurrence after surgery. Intriguingly, interventional therapy has drawn increasing interesting in the treatment of liver cancer due to the characteristics of less invasiveness, short treatment time, fast recovery and high reproducibility. Transcatheter arterial chemoembolization (TACE) represents a popular interventional approach for liver cancer patients by blocking the hepatic artery for inhibiting tumor blood supply [5]. Based on the advantages in less invasive, clear effects, and wide range of applications, TACE has become the first-line therapy for unresectable liver cancer and main palliative treatment for advanced liver carcinoma patients. However, due to the high degree of malignancy in patients with liver cancer, the recurrence and metastasis rates in patients after TACE or radiofrequency ablation can be as high as 20% [6]. Therefore, accurately identifying the risk of recurrence and metastasis in liver cancer patients after TACE is important to improve prognosis of patients.
Blood biomarkers can accurately reflect tumor biology information due to multiple advantages, such as convenient collection, low cost, and standard detection methods. Alpha-fetoprotein (AFP), a glycoprotein belonging to albumin family, is mainly synthesized by fetal liver cells and yolk sac. High AFP is confirmed in many carcinomas including liver cancer and can be recognized as a positive indicator for cancers [7]. Noticeably, a novel inflammation-related index called systemic inflammatory response index (SII) is a new inflammation-based evaluation system that can be calculated through peripheral blood platelet, neutrophil and lymphocyte, and can reflect body’s local immune and systemic inflammatory response [8]. From the perspective of immune inflammation, neutrophils and lymphocytes play a key role in the body’s immune response [8–10]. Neutrophils are an important component of innate immunity and can be recruited by cancer cells to release pro-inflammatory cytokines, angiogenic factors to promote tumor cell proliferation, invasion, and metastasis. Lymphocytes are the core of adaptive immunity, among which CD4+T cells assist in immune response and CD8+T cells directly kill tumor cells. The decrease in lymphocytes means that the body’s immune surveillance and anti-tumor ability are reduced [9, 10]. Platelets not only participate in the coagulation process, but also release various growth factors and cytokines to affect the survival, migration, and angiogenesis of tumor. Therefore, SII integrates information from platelet, neutrophil and lymphocyte and can comprehensively reflect the relationship between immune inflammation and tumor microenvironment in the body. SII can act as a useful predictor of poor prognosis during malignancy treatment [9]. It is a fact that immune cells and inflammation constitute important components of tumor microenvironment and are associated with progression of cancer [10, 11]. Cancer-associated SII are related to alterations in the distribution of circulating blood cells, and patients with elevated SII often have neutrophilia, thrombocytosis, and a relative reduction in lymphocytes [12]. Moreover, increased neutrophils and angiogenesis growth factors, chemokines and proteases after systemic inflammatory response may contribute to tumor growth, metastasis and poor prognosis in gastric cancer patients [13]. Additionally, lymphocytopenia reveals suppressive state of host immune system [14]. Several studies highlight that SII is associated with prognosis of multiple cancers [15, 16]. Intriguingly, a previous study revealed that SII was a potential risk factor for poor prognosis in patients with hepatocellular carcinoma after liver transplantation and could act as a prognostic marker for patients [17]. However, there are the limited researches on the relationship between SII and recurrence and metastasis of primary liver cancer. Thus, this study sought to systematically explore the value of SII in predicting the recurrence and metastasis of patients with primary liver cancer after interventional therapy by enrolling a relatively large sample size of patients with primary liver cancer and using uniform detection methods and follow-up procedures. Moreover, this study also investigated the combined values of SII and AFP to predict the recurrence and metastasis of patients, which may provide new ideas and basis for more accurately predicting the risk of recurrence and metastasis in patients with primary liver cancer after interventional treatment in clinical practice.
Materials and methods
General information
Total of 186 patients with primary liver cancer who treated in our hospital from January 2019 to May 2021 were enrolled. Inclusion criteria: (1) All patients met the diagnostic criteria for primary liver cancer established by the World Health Organization [18], and all of them were hepatocellular carcinoma; (2) All patients who were not suitable for radical surgery due to tumor size, location, number, and physical conditions (liver function, complications, etc.) and met the indications for interventional surgery underwent TACE treatment; (3) Preoperative imaging examination showed that the tumor had no distant metastasis and lymph nodes metastasis; (4) All tumors were primary; (5) Patients were followed up for more than 1 year after surgery; (6) There was no special treatment to inhibit or promote bone marrow growth before TACE treatment; (7) The clinical records and related examination data were complete. Exclusion criteria: (1) Patients with non-primary liver cancer diagnosed by clinical pathology; (2) Patients combined with autoimmune system diseases, hematological diseases and other diseases affecting the efficacy of TACE surgery; (3) Patients combined with cardiovascular and cerebrovascular diseases, kidney diseases, respiratory system diseases, and endocrine system diseases; (4) Portal vein was completely blocked by tumor thrombus and there was little collateral blood vessel formation; (5) Patients with multiple organ failure.
Preoperative imaging assessment and evaluation of metastasis
Preoperative imaging examinations indicated that there were no distant metastasis or lymph node metastasis of tumor. The preoperative imaging examinations mainly performed by 64-slice spiral CT scan and magnetic resonance imaging (MRI). The CT assay had high resolution, a slice thickness of up to 1 mm, and clearly showed the anatomical structure of liver and surrounding tissues. The above CT had a high sensitivity for lymph nodes with a diameter of ≥ 5 mm and could effectively identify enlarged lymph nodes. For distant metastasis, this CT could effectively identify the metastatic foci with a diameter of > 3 mm and detect the common metastatic sites, such as the lungs and bones. The criteria for metastasis: CT images showed lymph nodes with a short diameter greater than 10 mm, irregular morphology, uneven enhancement, or abnormal space occupying lesions found in other organs outside the liver (such as specific enhancement patterns and density/signal characteristics). MRI examination was conducted by a 3.0-Tesla Siemens MAGNETOM Skyra with a 64-channel head coil using a T1-weighted MR images with a voxel size of 1.0 mm × 1.0 mm × 2.0 mm and T2-weighted imaging with a voxel size of 1.0 mm × 1.0 mm × 5.0 mm. The MRI had a high sensitivity for lymph nodes with a diameter of ≥ 5 mm and could effectively identify enlarged lymph nodes. For distant metastasis, MRI could effectively identify soft tissue resolution, especially in brain and bone metastases, and detect micro-metastatic foci with a diameter of ≥ 2 mm in brain. The criteria for metastasis: new lesions showed low signal on T1-weighted images and high signal on T2-weighted images, contrast-enhanced images presented a typical “fast-in-fast-out” enhancement feature of liver cancer. If lesions with signals consistent with the primary lesion appear in distant organs (such as lungs, bones, and brain) and other diseases are excluded, the lesion was considered to be a potential metastasis.
TACE therapy
Patients is placed in a supine position, the bilateral groin area was routinely disinfected with iodophor. Subcutaneous local anesthesia is performed on the right femoral artery, and the right femoral artery was punctured using seldiner technique under the support of digital subtraction vascular imaging technology equipment. A short 5 F RH arterial sheath was placed via femoral artery and a 5 F RH catheter was inserted along the sheath from the celiac trunk of abdominal aorta into hepatic artery. Then, hepatic arteriography was performed in anteroposterior and right anterior oblique 30° to identify focal location, and microguidewire and microcatheter were subsequently superselected to tumor-bearing artery to confirm the extent of the tumor. The antineoplastic drugs and embolic agents were injected into tumor location via the microcatheter, including ariamycin (ADM), cisplatin (CDDP), carboplatin (CBP), mitomycin C (MMC), Cell cycle-specific drug 5-fluorouracil (5-FU). The above drugs were embedded in gelatin sponge embolization, blank embolization microspheres or drug-loaded microspheres in combination of 2 to 3 types. Lipiodol embolization: The patients were embolized with superemulsified lipiodol and anti-tumor drugs [superemulsified lipiodol and lorboplatin raltitrexed suspension emulsion combined with blank embolization microspheres (100 ∼ 300 μm, 300 ∼ 500 μm) or drug-loaded microspheres and epirubicin] until tumor staining basically disappeared. Subsequently, microcatheter was withdrawn, the RH arterial sheath was retreated to the proper hepatic artery and the diluted raltitrexed/epirubicin were slowly injected. After the operation, the catheter sheath was then removed after operation and right femoral artery puncture point was bandaged to stop bleeding.
Observation indicators
Clinical efficacy: According to tumor efficacy evaluation standard based on the results of magnetic resonance imaging (MRI), the clinical effects were evaluated at 2 months after TACE treatment and divided into progressive disease (PD) (the sum of the maximum diameters of target lesions increased ≥ 20% compared with that before treatment), partial response (PR) (the sum of the maximum diameters of target lesions decreased ≥ 30% compared with that before treatment), stable disease (SD) (the change of target lesions between PD and PR), complete response (CR) (the complete disappearance of target lesions). The objective response rate (ORR) was defined as (CR + PR)/number of evaluable cases × 100%, while the disease control rate (DCR) was defined as (CR + PR + SD)/number of evaluable cases × 100%.
Baseline data: Clinical and pathological data of all patients were collected, including age, gender, body mass index (BMI), hepatitis B, tumor size, Child-Pugh grade, number of lesions, vascular tumor thrombus, vascular invasion, and tumor capsule.
Detection of SII: Approximately 2 mL of fasting peripheral venous blood from patients was collected and the blood routine test was performed using a Bc-6800 Mindray Auto Hematology Analyzer. All protocols were performed according to the instructions of a commercial kits. The main examination indexes were analyzed, including neutrophil count, lymphocyte count, platelet count. SII= (platelet count ×neutrophil count)/lymphocyte count.
Determination of AFP: Fasting peripheral venous blood (4 mL) was collected and placed at room temperature for 15 to 30 min. Then, blood samples were centrifuged at 3000 r/min for 15 min to separate serum. The levels of AFP in serum were then determined using a commercial AFP kit (Shenzhen Haodi Huatuo Biotechnology Co., Ltd., Shenzhen, China) under an automatic chemiluminescent immunoassay analyzer. All specimens were detected within 4 h. Follow-up and groups: All patients were followed up for 3 years via telephone and revisit in our hospital at the end of the operation. The end point of follow-up was the death of patient or the end of follow-up, and the end time of follow-up was May 31, 2024. During the follow-up period, patients received regular imaging examinations to monitor for recurrence and metastasis. The specific time intervals were as follows: in the first year after surgery, liver ultrasound was performed every 3 months and CT or MRI was performed every 6 months; in the second year after surgery, CT or MRI was performed every 6 months. If patients experienced worsening pain in liver, abdominal mass, weight loss, fatigue, jaundice, or progressive elevation of biochemical indicators like AFP (AFP exceeded the normal reference value and showed a continuous upward trend), CT or MRI should be performed promptly to determine whether recurrence or metastasis occurred. The recurrence and metastasis of patients were defined by imaging examinations or positive results of puncture pathological examination and counted. Recurrence was defined as the reappearance of lesions similar to the primary lesion in the same organ or tissue after the primary lesion had disappeared or been controlled after TACE treatment. Metastasis was defined as the appearance of lesions in other organs or tissues after TACE treatment, and pathologically confirmed to be hepatocellular carcinoma cells. The ‘recurrence and metastasis’ was used as a composite endpoint, meaning that if a patient experienced either recurrence or metastasis, it is considered as an endpoint event. All patients were divided into a recurrence and metastasis group (110 cases) and a non-recurrence and metastasis group (76 cases) according to the presence or absence of tumor recurrence and metastasis.
During the follow-up period, we established a standardized blood sample collection procedure to calculate the average SII index and AFP level for each patient. Following surgery, fasting peripheral venous blood samples were collected from patients at the 1 month, 3 months, 6 months, 9 months, 12 months, and every 6 months thereafter. The average SII index and AFP level of each patient represented the arithmetic mean of the measured values at each time point. Collecting blood samples multiple times and calculating the average values can reduce the fluctuations in indicators caused by factors such as inflammation, infection, or treatment-related influences to a certain extent, making the data more stable and representative, which will provide a reliable data foundation for subsequent statistical analysis.
Statistical analysis
Statistical analysis was performed using SPSS22.0 software. Measurement data are shown as mean ± standard deviation (mean ± SD) and statistical comparison between groups was performed using t test. Count data were expressed as % and analyzed using the χ2 test. Cox proportional hazards regression analysis was applied to analyze the factors affecting recurrence and metastasis of patients with primary liver cancer after interventional therapy. The values of SII and AFP levels in predicting recurrence and metastasis of patients were analyzed using a ROC curve assay. Kaplan-Meier survival curves with log-rank tests were used to evaluate the survival of patients. P < 0.05 was defined as a statistical significance.
Results
Therapeutic effects of TACE
After TACE treatment, among the 186 cases, there were 37 cases of PD (19.89%), 49 cases of SD (26.34%), 70 cases of PR (37.63%), 30 cases of CR (16.13%). Moreover, the ORR was 53.76% and DCR was 80.11%.
Comparison of baseline data
In the recurrence and metastasis groups, there were 45 patients with intrahepatic recurrence, 15 patients with extrahepatic recurrence, 30 patients with intrahepatic and extrahepatic metastasis, and 20 patients with extrahepatic metastasis. In no-recurrence and metastasis groups, patients did not develop recurrences or metastases. There was obvious statistical difference in the recurrence and metastasis group and the non-recurrence and metastasis group, including tumor size, vascular tumor thrombus, vascular invasion, and tumor capsule (P < 0.05). No dramatic difference was observed in two groups, including age, gender, and BMI, Child-Pugh grade, and number of lesions had (P > 0.05). See Table 1.
Table 1.
Comparison of baseline data ( , %)
, %)
| Baseline data | recurrence and metastasis group (n = 110) | non-recurrence and metastasis group (n = 76) | t/χ2 | P | |
|---|---|---|---|---|---|
| Age (years) | ≤ 50 | 53(48.18) | 46(60.53) | 2.751 | 0.097 |
| >50 | 57(51.82) | 30(39.47) | |||
| Gender (%) | Male | 61(55.45) | 37(48.68) | 0.827 | 0.363 |
| Female | 49(44.55) | 39(51.32) | |||
| BMI (kg/m2) | 23.25 ± 1.87 | 23.88 ± 4.26 | 1.373 | 0.172 | |
| Hepatitis B | Negative | 45(40.91) | 33(43.42) | 0.117 | 0.733 |
| Positive | 65(59.09) | 43(56.58) | |||
| Tumor size | ≥ 5 cm | 70(63.64) | 35(46.05) | 5.653 | 0.017 |
| <5 cm | 40(36.36) | 41(53.95) | |||
| Child-Pugh grade | Grade A | 63(57.27) | 42(55.26) | 0.074 | 0.784 |
| Grade B | 47(42.73) | 34(44.74) | |||
| Number of lesions | Single focus | 58(52.73) | 35(46.05) | 0.801 | 0.371 |
| Multifocal | 52(47.27) | 41(53.95) | |||
| Vascular tumor thrombus | Yes | 50(45.45) | 23(30.26) | 4.350 | 0.037 |
| No | 60(54.55) | 53(69.74) | |||
| Vascular invasion | Yes | 44(40.00) | 18(23.68) | 5.384 | 0.020 |
| No | 66(60.00) | 58(76.32) | |||
| Tumor capsule | Complete | 58(52.73) | 55(72.37) | 7.919 | 0.019 |
| Incomplete | 42(38.18) | 15(19.74) | |||
| Absence | 10(9.09) | 6(7.89) |
Comparison of serological indicators
For the SII index and AFP levels, we calculated the average levels for each patient during the follow-up period and used these averages for follow-up statistical analysis. The SII index and AFP levels of patients in the recurrence and metastasis group were significantly higher than those in the non-recurrence and metastasis group (P < 0.001). See Table 2.
Table 2.
Comparison of serological indicators ( , %)
, %)
| Groups | Cases | SII | AFP(ng/ml) |
|---|---|---|---|
| recurrence and metastasis group | 110 | 420.76 ± 253.30 | 344.25 ± 98.33 |
| Non-recurrence and metastasis group | 76 | 283.36 ± 101.56 | 198.25 ± 72.30 |
| t | 4.483 | 11.042 | |
| P | <0.001 | <0.001 |
Cox proportional hazards regression analysis of factors affecting recurrence and metastasis of patients with primary liver cancer
Tumor size, vascular tumor thrombus, vascular invasion, tumor capsule, SII index, and AFP level were used as independent variables and assigned values. Recurrence or metastasis after interventional treatment were defined as the dependent variable, and the assignment information was shown in Table 3. Cox proportional hazards regression analysis confirmed that tumor size ≥ 5 cm, presence of vascular tumor thrombus, presence of vascular invasion, no tumor capsule, SII index, AFP Levels were closely related to the recurrence and metastasis of patients with primary liver cancer (P < 0.05). See Table 4.
Table 3.
Assignment of independent variables
| Independent variables | Assignment condition | |
|---|---|---|
| X1 | Tumor size | 0 = <5 cm, 1 = ≥ 5 cm |
| X2 | Vascular tumor thrombus | 0 = no, 1 = yes |
| X3 | Vascular invasion | 0 = no, 1 = yes |
| X4 | Tumor capsule | 0 = no, 1 = yes |
| X5 | SII index | 0=<370.26, 1 = ≥ 345.26 |
| X6 | AFP levels | 0=<274.25, 1 = ≥ 274.25 |
| Y | recurrence and metastasis | 0 = no, 1 = yes |
Table 4.
Cox proportional hazards regression analysis of factors affecting recurrence and metastasis of patients with primary liver cancer
| Index | β | SE | Wald | P value | OR | 95%CI |
|---|---|---|---|---|---|---|
| Tumor size | 1.849 | 0.755 | 5.994 | 0.014 | 6.354 | 1.446–27.924 |
| Vascular tumor thrombus | 0.103 | 0.052 | 3.900 | 0.048 | 1.108 | 1.001–1.227 |
| Vascular invasion | 0.309 | 0.145 | 4.525 | 0.033 | 1.362 | 1.025–1.810 |
| Tumor capsule | 0.282 | 0.126 | 5.032 | 0.025 | 1.326 | 1.036–1.696 |
| SII index | 2.496 | 0.818 | 9.315 | 0.002 | 12.130 | 2.442–60.240 |
| AFP levels | 0.633 | 0.250 | 6.418 | 0.011 | 1.883 | 1.154–3.071 |
ROC curve analysis the predicting value of SII and AFP levels in recurrence and metastasis in patients with primary liver cancer after interventional treatment
ROC curve analysis results revealed that the areas under the curve (AUC) of SII and AFP levels alone predicted recurrence and metastasis of liver cancer patient after intervention were 0.797 and 0.839, respectively. The AUC of combined SII and AFP was 0.910, which was higher than that of single indicator. See Table 5; Fig. 1.
Table 5.
ROC curve analysis the predicting value of SII and AFP levels in recurrence and metastasis in patients with primary liver cancer after interventional treatment
| Index | AUC | 95%CI | P | Sensitivity | Dpecificity | Cut-off value | Youden’s index |
|---|---|---|---|---|---|---|---|
| SII | 0.797 | 0.735–0.860 | 0.001 | 90.79 | 57.27 | 375.56 | 0.481 |
| AFP | 0.839 | 0.782–0.897 | 0.001 | 76.32 | 78.18 | 287.10 | 0.545 |
| Combined | 0.910 | 0.868–0.952 | 0.001 | 92.11 | 81.82 | / | 0.739 |
Fig. 1.
ROC curve analyzed the predicting value of SII and AFP levels in recurrence and metastasis in patients with primary liver cancer after interventional treatment
Kaplan-Meier survival analysis
After a 3-years of follow-up, the overall survival rate of the 186 patients was 45.70% (85/186). Patients were divided into the SII high-level group and the SII low-level group based on the cutoff value of SII by ROC curve. The Kaplan-Meier survival curve analysis showed that patients with high SII levels had shorter survival time than that of patients with low SII levels (P < 0.05). See Fig. 2.
Fig. 2.
Kaplan-Meier survival analysis of patients with primary liver cancer after TACE
Discussion
Primary liver cancer causes great threat to patients due to high incidence and mortality. Because of high ability to metastasis, the 5-year survival rate of patients with primary liver cancer is still 60% after radical resection; moreover, patients underwent local palliative treatment have higher incidence of metastasis and recurrence [19]. Even if tumor is completely resected or ablated, some cancer cells or tiny lesions are difficult to detect due to invasion of peritumoral tissue and vascular tumor thrombus formation [20]. These remaining cancer cells had high ability to proliferation and will form new tumors, leading to the recurrence of liver cancer [21]. It is known that portal vein tumor thrombus, tumor number, maximum tumor diameter, tumor differentiation degree, capsule, resection margin are closely related to the development and prognosis of liver cancer [22]. However, lacking of reliable markers in clinical practice makes it difficult to timely identify tumor recurrence and metastasis.
SII, as an indicator to reflect systemic inflammatory and immune status, has recently drawn increasing attention in tumors [23, 24]. SII is associated with prognosis of cancers, such as gastric cancer [25], colorectal cancer [26] and urinary system tumors [27]. A meta-analysis revealed that high SII levels were associated with poor progression-free survival (PFS), overall survival (OS) and tumor-specific survival (CSS) in patients with multiple urinary system tumors [27]. Furthermore, high SII not only means worse OS, but may also be associated with adverse pathological characteristics, including larger tumor size, lower differentiation, and later tumor stage [28]. Tumor cells can interfere with immune cells to escape immune cell surveillance and clearance, patients with high SII levels may have high inflammatory response and immune evasion [23]. Intriguingly, a previous study confirmed that the increased SII was a potential prognostic factor for patients with hepatocellular carcinoma after liver transplantation [17]. In this study, compared with the non-recurrence and metastasis group, higher SII index was observed in the recurrence and metastasis group, which indicates that preoperative SII index were closely related to early recurrence and metastasis in patients with primary liver cancer after surgical resection. The specific mechanisms may include the following points [29, 30]: first, high SII levels mean excessive body’s inflammatory response that is conducive to tumor growth and metastasis; second, high SII levels may weaken immune surveillance functions, making tumors cells have high ability to escape immune clearance; finally, high SII levels can further promote tumor growth and metastasis by affecting the distribution and function of immune cells within tumor microenvironment. SII can be calculated through peripheral blood platelet, neutrophil and lymphocyte. Intriguingly, neutrophils can degrade extracellular matrix components and disrupt the integrity of basement membrane by secreting matrix metalloproteinases (MMPs) to facilitate migration and invasion of tumor cells; moreover, neutrophils can also release vascular endothelial growth factor (VEGF) and other pro-angiogenic factors to promote angiogenesis to support tumor growth and metastasis [10, 13]. Platelets can release various growth factors and cytokines to promote proliferation, migration, and invasion of tumor cells, such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) [12, 16]. Lymphocytes play a key role in tumor immune surveillance. CD8 + T lymphocytes are the main effector cells of anti-tumor immunity and can recognize and kill tumor cells, and the decreases of their number and function will lead to tumor cell escape from immune clearance [14]. It is known that there is abundant infiltration of inflammatory cells in liver cancer tissues, which can secrete various cytokines and chemokines to generate an inflammatory microenvironment that is conducive to the survival, metastasis and recurrence of tumor [31]. In addition, cytokines in inflammatory microenvironment, such as IL-6 and IL-8, can activate various signaling pathways to evoke epithelial-mesenchymal transition (EMT) process that will cause tumor cells to lose polarity and obtain strong migration and invasion capabilities to facilitate tumor metastasis [31, 32]. The tumor microenvironment of liver cancer patients makes it an important basis for SII to exert its predictive value. Most patients with liver cancer have viral infections of hepatitis B or C, which will lead to long-term chronic inflammatory stimulation and cause immune dysfunction in the body. The number of neutrophils increases after inflammatory stimulation, and they can release various pro-inflammatory factors and proteases to promote cancer cell proliferation, invasion and metastasis; at the same time, the decreases of their function and number may lead to decline of the body’s immune surveillance ability and tumor cell escape from immune clearance [13, 31]. Thus, under the inflammatory microenvironment, SII can more comprehensively reflect the imbalance of immune inflammation in liver cancer patients, which is more valuable for reference than a single cell index.
AFP is a special glycoprotein synthesized by liver cells during fetal period and its content in serum of normal adults is very low; however, AFP is elevated when liver cells undergo cancerous transformation [33]. In patients with liver cancer, AFP levels are increased because liver cancer cells can reactivate gene expression during the embryonic period and produce AFP [33]. In this study, higher SII index and AFP levels were observed in the recurrence and metastasis group relative to the non-recurrence and metastasis group, suggesting that preoperative SII index and AFP levels were closely correlated with early recurrence and metastasis in patients with primary liver cancer after surgical resection. The mechanism of AFP in predicting liver cancer recurrence and metastasis mainly involves the following aspects [34, 35]: first, high levels of AFP indicate that liver cancer cells have strong capabilities to growth and invasion; second, AFP can serve as an adhesion molecule of liver cancer cells to promote cancer cell adhesion to vascular endothelial cells, thereby facilitating cell metastasis; finally, AFP may contribute to liver cancer cell invasion and metastasis by regulating degradation and remodeling of extracellular matrix.
Next, Cox proportional hazards regression model confirmed that tumor size ≥ 5 cm, presence of vascular tumor thrombus, presence of vascular invasion, absence of tumor capsule, SII index, and AFP level were closely related to the recurrence and metastasis in patients with primary liver cancer after interventional treatment. It is a fact that tumor size reflects time of cancer occurs and severity, and larger tumor diameter means the sooner relapse after tumor resection and higher recurrence rate. The reason may be that large tumors often have micro-metastasis beyond resection margin during surgical resection, which greatly reduces possibility of radical resection of tumor and makes it easy to relapse and worsen prognosis [36]. Vascular tumor thrombus refers to the presence of tumor emboli in blood vessels in resected tumor specimen, indicating that tumor has invaded blood vessels. Vascular tumor thrombus can promote local recurrence and distant metastasis, and can serve as a reliable indicator of distant metastasis and overall survival of patients with breast cancer, ovarian cancer and other tumors [37]. A complete tumor capsule indicates that the boundary between tumor and other tissues is clear, while the absence of capsule or an incomplete tumor capsule indicates outward infiltration or spread of metastasis [38]. AFP is a recognized affordable oncological marker in patients with hepatocellular carcinoma and the persistently elevated AFP is a risk factor for the progression of hepatocellular carcinoma [39]. Moreover, serum levels of AFP can be useful for predicting postoperative recurrence of patients with hepatocellular carcinoma [40]. Therefore, AFP may be the result of tumor recurrence and metastasis in liver cancer, not necessarily independent risk factors affecting the recurrence and metastasis. Thus, the findings reveal that the increased AFP levels were closely related to the recurrence and metastasis in patients with primary liver cancer. Elevation of SII means higher inflammatory response and a lower immune response of patients; thus, compared with a single indicator, SII has s higher sensitivity in assessing the development of patients [41]. Therefore, the current study suggests that SII is closely related to recurrence and metastasis in patients with primary liver cancer after resection, indicating that SII may act as an effective predictor of recurrence and metastasis in liver cancer patients. This study confirmed the existence of patients with intrahepatic recurrence and extrahepatic metastasis. Notably, the prognosis of patients with extrahepatic metastasis is often worse than that of patients with intrahepatic recurrence. For intrahepatic recurrence, tumor cells are still confined within liver and can be intervened to some extent by surgical resection, local ablation, and interventional therapy. Thus, patients with intrahepatic recurrence may still have a better therapeutic efficacy and prognosis. However, once extrahepatic metastasis occurs, it means that tumor cells have broken through the local restrictions of the liver and spread to other tissues, such as the lungs, which not only elevates the complexity of treatment but also often renders it challenging to eradicate tumor cells entirely through local treatment methods due to the widespread distribution of metastatic lesions. For patients with extrahepatic metastasis, systemic therapy serves as the standard treatment modality, such as chemotherapy, targeted therapy, and immunotherapy. However, due to the varying sensitivities of different organs to therapeutic drugs and the heterogeneity of metastatic lesions, some patients may have a poor treatment outcomes and rapid disease progression [42]. It has been reported that serum level of AFP is a useful predictor of invasion and metastasis in Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma, and AFP levels can also predict early tumor recurrence after surgical resection [43]. ROC curve analysis in this study showed that the AUC of SII and AFP levels alone in predicting recurrence and metastasis in patients with primary liver cancer after interventional treatment were 0.797 and 0.839, respectively, and the AUC of combined prediction was 0.910, suggesting that SII combined with AFP have higher diagnostic value for primary liver cancer than alone. From the perspective of feasibility, SII is a novel systemic inflammatory index that integrates peripheral blood platelet, neutrophil and lymphocyte count, can effectively reflect the immune and inflammatory status of the body. In this study, the SII index was significantly higher in the recurrence and metastasis group than in the non-recurrence and non-metastasis group, and the Cox proportional hazards regression analysis confirmed that SII was related to the recurrence and metastasis of primary liver cancer patients after interventional therapy. Thus, these findings indicate that SII may have potential application value in predicting the recurrence and metastasis of liver cancer patients after treatment.
Conclusion
Preoperative SII, as a non-invasive, low-cost, easily evaluated and repeatable parameter, was closely related to early recurrence and metastasis in patients with primary liver cancer after TACE surgery, and combined with AFP detection may help to predict recurrence and metastasis in patients with primary liver cancer after interventional therapy. However, while affirming the potential predictive value of SII, we should also fully recognize its limitations. Firstly, although SII can reflect the immune-inflammatory status of the body, it cannot directly reveal the biological characteristics of the tumor itself, such as tumor size, differentiation degree, and vascular invasion, which are also important factors affecting the recurrence and metastasis of liver cancer. Therefore, when predicting the recurrence and metastasis of liver cancer patients, SII should be combined with other tumor markers and clinical pathological features to improve the accuracy and reliability of prediction. Secondly, the levels of SII may be affected by multiple factors, such as infection, inflammatory diseases, and drug treatment, which may cause temporary increases or decreases in SII levels, thereby leading to false positives or negatively results and affecting its stability and accuracy as a predictive indicator in clinical utility [44, 45]. Therefore, in clinical application, we should combine the patient’s specific conditions and medical history to conduct a comprehensive analysis and judgment of SII levels. In future research, we will continue to explore the combination of SII and other predictive models in order to provide more powerful support for the precision treatment of primary liver cancer.
Acknowledgements
Not applicable.
Author contributions
Study conception and design: QFH; data collection: YD, ZLC, QFH, BW, TS, CJM, RH; analysis and interpretation of results: YD, ZLC, QFH, BW, TS, CJM, RH; draft manuscript preparation: YD, ZLC. All authors reviewed the results and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
Experiments involved in Human participants were approved by the Institutional Review Board of Public Health Clinical Center of Chengdu and conducted according to the Declaration of Helsinki. All patients signed the informed consent form.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ma C, Zhang Q, Greten TF. MDSCs in liver cancer: A critical tumor-promoting player and a potential therapeutic target. Cell Immunol. 2021;361:104295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, He WQ. Comparison of primary liver cancer mortality estimates from world health organization, global burden disease and global cancer observatory. Liver Int. 2022;42:2299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu T, Buhler S, Bartenschlager R. Chronic viral hepatitis and its association with liver cancer. Biol Chem. 2017;398:817–37. [DOI] [PubMed] [Google Scholar]
- 4.Liang C, He Z, Tao Q, Tang X, Jiang L, Tu X et al. From conversion to resection for unresectable hepatocellular carcinoma: A review of the latest strategies. J Clin Med. 2023; 12. [DOI] [PMC free article] [PubMed]
- 5.Shao Z, Liu X, Peng C, Wang L, Xu D. Combination of transcatheter arterial chemoembolization and portal vein embolization for patients with hepatocellular carcinoma: a review. World J Surg Oncol. 2021;19:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Fu J, Xia P, Chu C. A systematic review and meta-analysis on the efficacy and safety of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of primary liver cancer. Transl Cancer Res. 2022;11:1297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao K, Zhou X, Xiao Y, Wang Y, Wen L. Research progress in Alpha-fetoprotein-induced immunosuppression of liver Cancer. Mini Rev Med Chem. 2022;22:2237–43. [DOI] [PubMed] [Google Scholar]
- 8.Kars A, Sahin A, Kilic K, Sakat MS, Bilen A. Systemic immune inflammation index in differentiated thyroid cancers. Acta Otorhinolaryngol Ital. 2022;42:150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: A meta-analysis. Med (Baltim). 2020;99:e23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi Q, Wu JY, Qiu XM, Zhang JD, Sun ZJ, Wang W. Tumor-Associated Inflammation: The Tumor-Promoting Immunity in the Early Stages of Tumorigenesis. J Immunol Res. 2022;2022:3128933. [DOI] [PMC free article] [PubMed]
- 11.Mehla K, Hollingsworth MA. Inflammatory and immune effects on tumor progression. Trends Immunol. 2022;43:93–5. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Qu Y, Yang Y, An W, Li S, Wang B, et al. Prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index in patients with laryngeal squamous cell carcinoma. Clin Otolaryngol. 2021;46:395–405. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Ji C, Li W, Mao Z, Shi Y, Shi H, et al. Tumor-Educated neutrophils activate mesenchymal stem cells to promote gastric Cancer growth and metastasis. Front Cell Dev Biol. 2020;8:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci 2022; 23. [DOI] [PMC free article] [PubMed]
- 15.Ji Y, Wang H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J Surg Oncol. 2020;18:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walzik D, Joisten N, Zacher J, Zimmer P. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol. 2021;121:1803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu H, Zheng J, Cai J, Zeng K, Yao J, Chen L et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within Hangzhou criteria. Cell Physiol Biochem. 2018;47:293-301. [DOI] [PubMed]
- 18.Yen CC, Yen CJ, Shan YS, Lin YJ, Liu IT, Huang HY et al. Comparing the clinicopathological characteristics of combined hepatocellular-cholangiocarcinoma with those of other primary liver cancers by use of the updated World Health Organization classification. Histopathology. 2021;79:556 − 72. [DOI] [PubMed]
- 19.Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: A review. Int J Mol Sci 2021; 22. [DOI] [PMC free article] [PubMed]
- 20.Ge Y, Mu W, Ba Q, Li J, Jiang Y, Xia Q, et al. Hepatocellular carcinoma-derived exosomes in organotropic metastasis, recurrence and early diagnosis application. Cancer Lett. 2020;477:41–8. [DOI] [PubMed] [Google Scholar]
- 21.Niu ZS, Wang WH, Niu XJ. Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma. World J Gastroenterol. 2022;28:6433–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Zhao G, Dong H. Effect of Radiofrequency Ablation with Interventional Therapy of Hepatic Artery on the Recurrence of Primary Liver Cancer and the Analysis of Influencing Factors. J Oncol. 2021;2021:3392433. [DOI] [PMC free article] [PubMed]
- 23.Tian BW, Yang YF, Yang CC, Yan LJ, Ding ZN, Liu H, et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy. 2022;14:1481–96. [DOI] [PubMed] [Google Scholar]
- 24.Nost TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. 2021;36:841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Y, Zhang Z, Chen Y. Prognostic value of pretreatment systemic Immune-Inflammation index in gastric cancer: A Meta-Analysis. Front Oncol. 2021;11:537140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung SH, Hao J, Shivakumar M, Nam Y, Kim J, Kim MJ, et al. Development and validation of a novel strong prognostic index for colon cancer through a robust combination of laboratory features for systemic inflammation: a prognostic immune nutritional index. Br J Cancer. 2022;126:1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Zhu SR, Huang XP, Liu XQ, Liu JB, Tian G. Prognostic value of systemic immune-inflammation index in patients with urinary system cancers: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:1302–10. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Gu L, Chen Y, Chong Y, Wang X, Guo P, et al. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med. 2021;53:1827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susok L, Said S, Reinert D, Mansour R, Scheel CH, Becker JC, et al. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J Cancer Res Clin Oncol. 2022;148:3103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, et al. Systemic Immune-Inflammation index (SII) predicts poor survival in pancreatic Cancer patients undergoing resection. J Gastrointest Surg. 2020;24:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donne R, Lujambio A. The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Wang Z, Ding Y, Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14:1133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhao Y, Li M, Hou H, Jian Z, Li W, et al. Conversion of primary liver cancer after targeted therapy for liver cancer combined with AFP-targeted CAR T-cell therapy: a case report. Front Immunol. 2023;14:1180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JG, He XF, Huang B, Zhang HA, He YK. Rule of changes in serum GGT levels and GGT/ALT and AST/ALT ratios in primary hepatic carcinoma patients with different AFP levels. Cancer Biomark. 2018;21:743–6. [DOI] [PubMed] [Google Scholar]
- 35.Gan L, Ren S, Lang M, Li G, Fang F, Chen L, et al. Predictive value of preoperative serum AFP, CEA, and CA19-9 levels in patients with single small hepatocellular carcinoma: retrospective study. J Hepatocell Carcinoma. 2022;9:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagante F, Spolverato G, Merath K, Weiss M, Alexandrescu S, Marques HP, et al. Intrahepatic cholangiocarcinoma tumor burden: A classification and regression tree model to define prognostic groups after resection. Surgery. 2019;166:983–90. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Zhu Y, Zhao X, Li JH, Xu D, Jia HL, et al. The prognostic comparison between hepatocellular carcinoma with portal vein tumor Thrombus and bile duct Cancer Thrombus after liver resection. Cancer Manag Res. 2020;12:12077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoki T, Matsuda K, Mansour DA, Koizumi T, Goto S, Watanabe M, et al. Narrow-band imaging examination of microvascular architecture of subcapsular hepatic tumors. J Surg Res. 2021;261:51–7. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Liu K, Chen Y, Zhu MY, Li MS. The role of alpha-fetoprotein in hepatocellular carcinoma drug resistance. Curr Med Chem. 2021;28:1126–42. [DOI] [PubMed] [Google Scholar]
- 40.Morimoto O, Nagano H, Miyamoto A, Fujiwara Y, Kondo M, Yamamoto T, et al. Association between recurrence of hepatocellular carcinoma and α-fetoprotein messenger RNA levels in peripheral blood. Surg Today. 2005;35:1033–41. [DOI] [PubMed] [Google Scholar]
- 41.Nakamoto S, Ohtani Y, Sakamoto I, Hosoda A, Ihara A, Naitoh T. Systemic Immune-Inflammation index predicts tumor recurrence after radical resection for colorectal Cancer. Tohoku J Exp Med. 2023;261:229–38. [DOI] [PubMed] [Google Scholar]
- 42.Long HY, Huang TY, Xie XY, Long JT, Liu BX. Treatment strategies for hepatocellular carcinoma with extrahepatic metastasis. World J Clin Cases. 2021;9:5754–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suh SW, Choi YS. Predictors of micrometastases in patients with Barcelona clinic liver Cancer classification B hepatocellular carcinoma. Yonsei Med J. 2017;58:737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pricop M, Ancusa O, Talpos S, Urechescu H, Bumbu BA. The Predictive Value of Systemic Immune-Inflammation Index and Symptom Severity Score for Sepsis and Systemic Inflammatory Response Syndrome in Odontogenic Infections. J Pers Med. 2022;12:2026. [DOI] [PMC free article] [PubMed]
- 45.Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. 2020;24:2993–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript.