Abstract
Background
Chronic migraine (CM) patients with medication overuse headache (MOH) exhibit distinct neurobiological alterations compared to those without MOH. However, prior studies, often limited to single imaging modalities, have yielded inconsistent findings. This study employs multimodal MRI—combining structural, diffusion tensor, and functional imaging—to characterize brain abnormalities in CM patients with and without MOH, while investigating the relationship between acute analgesic use frequency and these changes.
Methods
The study employed comparative analyses to examine differences in gray matter volume, white matter integrity, and spontaneous brain activity between CM patients with (CM + MOH) and without (CM − MOH) medication overuse headache, as well as healthy controls. Additionally, brain regions associated with the frequency of acute medication use were identified and further investigated.
Results
Nineteen CM − MOH patients, twenty-five CM + MOH patients, and nineteen healthy controls were enrolled. Compared to CM − MOH patients, CM + MOH patients exhibited significantly reduced gray matter volume in the parahippocampal gyrus and middle occipital gyrus, alongside markedly lower fractional anisotropy (FA) in the left cingulum bundle. Moreover, fractional amplitude of low-frequency fluctuations (fALFF) values in the right putamen were significantly decreased and demonstrated a negative correlation with the frequency of acute pain medication use. Functional connectivity analysis further revealed significantly enhanced connectivity between the right putamen and regions such as the frontal lobe, middle cingulate gyrus, lingual gyrus, and precuneus, which positively correlated with the frequency of acute analgesic use.
Conclusion
Compared to CM − MOH patients, those with MOH exhibit distinct patterns of gray matter volume reduction in regions associated with memory and visual processing, accompanied by significant white matter disruption. Additionally, decreased spontaneous activity in the right putamen and heightened functional connectivity between the putamen and multiple brain regions are strongly correlated with the frequency of acute medication use. These results highlight the significant impact of medication overuse on brain structure and function, shedding light on the mechanisms of migraine chronification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-025-01988-3.
Keywords: Chronic migraine, Medication overuse headache, Acute analgesic use frequency, 7 Tesla MRI, Multimodal imaging
Introduction
Chronic migraine (CM), a primary headache disorder with a global prevalence of 1.4 -2.2%, predominantly affects females and is often considered to evolve from episodic migraine (EM). Among individuals with CM, those with comorbid medication-overuse headache (MOH) exhibit greater therapeutic complexity and management challenges than CM patients without MOH [1, 2]. MOH, a secondary headache disorder resulting from the prolonged overuse of acute pain medications [3], has a global prevalence of approximately 1–2% [4] and 0.6% in China [5]. Aside from its impact on individual health, MOH substantially impairs patients’ quality of life and imposes a significant burden on families and society. According to the 2015 Global Burden of Neurological Disorders study, the annual loss of disability-adjusted life years (DALYs) attributable to MOH accounted for 3.7%, ranking it sixth among all neurological diseases [6]. Furthermore, medication overuse is the most significant risk factor for migraine chronification, although its underlying mechanisms remain poorly understood.
Magnetic resonance imaging (MRI) has been extensively used to investigate structural and functional brain changes in patients with CM. However, a key limitation in existing studies is the failure to adequately distinguish between CM patients with and without MOH [7–9]. The observed changes in brain structure and function may be confounded by multiple factors, such as chronic pain and excessive medication use. Moreover, while some studies focus on CM patients with MOH, their analyses predominantly correlate neuroimaging findings with headache frequency and intensity metrics, neglecting systematic investigation of acute analgesic consumption patterns [10]. Excessive acute analgesic use may induce neuroplastic changes in both brain structure and function, potentially driving migraine chronification. Therefore, the frequency of acute analgesic use should be considered a critical clinical parameter and incorporated into neuroimaging research, in order to better understand its impact on the pathophysiological mechanisms of CM with MOH. Most prior MOH neuroimaging studies have relied on unimodal approaches [10–14], which lack the capacity to characterize multidimensional brain alterations across structural and functional domains. In contrast, multi-modal imaging combines the strengths of various imaging techniques, enabling the acquisition of more comprehensive information about brain regions and thereby offering more precise neuroimaging evidence for the pathophysiological mechanisms of MOH. Furthermore, while prior investigations have predominantly employed 3 Tesla MRI systems, 7 Tesla MRI’s enhanced spatial resolution and contrast sensitivity enable superior visualization of subtle structural and functional alterations in brain regions [15].
Building on this context, this study utilizes 7 Tesla multimodal MRI, combining structural MRI (sMRI), diffusion tensor imaging (DTI), and functional MRI (fMRI), to investigate brain structural and functional differences in female CM patients with and without MOH. We hypothesize that frequent acute analgesic use is associated with brain structure and function alterations, potentially facilitating the progression of chronic migraine through changes in gray matter volume, white matter integrity, and spontaneous neural activity in specific brain regions. This study aims to elucidate the impact of frequent analgesic use on the structural and functional alterations in brain regions of patients with CM, with or without MOH. The analysis will focus on the relationship between analgesic consumption frequency and brain changes, while excluding the effects of chronic migraine itself on brain regions.
Methods
Subjects
This study is a cross-sectional observational study designed to investigate neuroimaging differences between CM patients with and without MOH. The study population consisted of female participants divided into three groups: one group of chronic migraine without medication overuse headache (CM − MOH), one group of chronic migraine with medication overuse headache (CM + MOH), and a group of healthy controls. All participants in both the patient and control groups were matched for age and educational years to minimize potential confounding factors. Participants were recruited from the International Headache Center of the First Medical Center of the Chinese People’s Liberation Army General Hospital between July 2023 and July 2024, and diagnoses were confirmed by two senior neurologists specialized in headache management.
The inclusion criteria were as follows: (1) aged between 18 and 65 years; (2) right-handed; (3) voluntary participation with written informed consent; (4) all participants met the diagnostic criteria for chronic migraine (CM) as defined in the International Classification of Headache Disorders, 3rd edition (ICHD-3), with or without MOH; (5) all participants had chronic migraine without aura; (6) no prior use of migraine prophylactic medications; (7) no migraine attacks 24 h prior to MRI scanning.
The exclusion criteria were as follows: (1) presence of any neurological or psychiatric disorders; (2) any systemic diseases (e.g., cardiovascular diseases, tumors, diabetes); (3) other chronic pain conditions (e.g., chronic back pain); (4) women who were pregnant or menstruating; (5) inability to undergo MRI scanning (e.g., due to metal implants); (6) headache occurrence during the MRI scan. Healthy controls were recruited from primary healthcare facilities or community centers, meeting the first three inclusion criteria and exclusion criteria, and with no family history of headaches in first-degree relatives.
After eligibility assessment, all participants completed a questionnaire to collect demographic information (sex, age, body mass index, and educational years) and clinical data, including the number of monthly headache days, number of monthly migraine days, average headache duration, headache laterality (unilateral, alternating sides, or bilateral simultaneous onset), and associated symptoms (worsening with physical activity, nausea, vomiting, photophobia, and phonophobia). Additionally, data on the types and frequency of analgesic use were recorded. Participants also underwent the following assessments: the Visual Analogue Scale (VAS, range: 0–10) to evaluate average headache intensity, the Patient Health Questionnaire-9 (PHQ-9, range: 0–27) for depressive symptoms, the Generalized Anxiety Disorder-7 (GAD-7, range: 0–21) for anxiety symptoms, and the Pittsburgh Sleep Quality Index (PSQI, range: 0–21) to assess sleep quality. Higher scores on these scales indicate more severe symptoms.
All participants underwent 7T MRI scanning to obtain neuroimaging data. To minimize any potential influence on neuroimaging outcomes, all participants were instructed to refrain from using acute analgesics or consuming caffeinated beverages (e.g., coffee, tea, cola) for at least 12 h prior to the MRI examination. They were also instructed to keep their eyes closed, remain quiet, maintain head stability, and use earplugs to mitigate noise exposure. Additionally, only participants who self-reported no migraine attacks during or immediately after the MRI scan were included in the final analysis.
The study was approved by the Ethics Committee of the Chinese PLA General Hospital (S2023-459-01). All participants provided written informed consent prior to participation, in accordance with the principles of the Declaration of Helsinki.
MRI acquisition
All subjects underwent structural MRI (sMRI), functional MRI (fMRI), and diffusion tensor imaging (DTI) acquisitions using a 7 Tesla MRI scanner (Siemens, Germany). The sMRI images were obtained using a three-dimensional T1-weighted magnetization-prepared rapid gradient-echo sequence with the following parameters: repetition time (TR) = 6000 ms, echo time (TE) = 2.21 ms, field of view (FOV) = 224 × 216 × 156 mm, slice thickness = 0.75 mm, spacing = 0.375 mm, voxel size = 0.7 × 0.7 × 0.8 mm, number of slices = 300, and flip angle (FA) = 4°. For fMRI, the parameters were: ep2d-trace-tra sequence with TR = 2000 ms, TE = 24 ms, FOV = 216 × 216 × 144 mm, slice thickness = 1.8 mm, spacing = 0 mm, voxel size = 1.8 × 1.8 × 1.8 mm, number of slices = 80, and FA = 90°. DTI parameters included TR = 5120 ms, TE = 59 ms, FOV = 224 × 224 × 128 mm, slice thickness = 1.6 mm, spacing = 0 mm, voxel size = 1.6 × 1.6 × 1.6 mm, number of slices = 80, and FA = 180°. Prior to preprocessing, imaging experts performed quality assessments on all imaging data to exclude images with signal artifacts, distortions, or data loss. The images were converted from DICOM to NIFTI format for subsequent analysis.
sMRI processing
Structural MRI data were processed using voxel-based morphometry (VBM) with the VBM toolbox in FSL (https://fsl.fmrib.ox.ac.uk/fsl). The specific steps included removing non-brain tissue using the Brain Extraction Tool (BET), segmenting brain tissue into gray matter, white matter, and cerebrospinal fluid, and registering gray matter images to the Montreal Neurological Institute (MNI) 152 standard space. After volume correction, images were smoothed using a Gaussian kernel with a full width at half maximum (FWHM) of 3 mm.
DTI processing
DTI data were processed using the FSL toolbox, including corrections for eddy currents and head motion, followed by skull stripping. A tensor model was fitted using the least squares method to obtain voxel-wise fractional anisotropy (FA) values. Tract-Based Spatial Statistics (TBSS) was then applied to the FA data. The FA images were aligned to the FMRIB58_FA template and registered to MNI152 standard space. A mean FA image was created to generate the FA skeleton, onto which individual FA data were projected. The Johns Hopkins University (JHU) ICBM-DTI-81 white matter atlas was used to anatomically locate white matter tracts showing significant differences.
fMRI processing
Functional MRI data were processed using the CONN toolbox (RRID: SCR_009550_22.a) and SPM8 (RRID: SCR_007037_12.7771). Preprocessing steps included removal of initial time points, slice timing correction, realignment (excluding images with translational movements ≥ 3 mm or rotational movements ≥ 3°), normalization, smoothing with a 3 mm Gaussian kernel, detrending, and nuisance covariate regression. The amplitude of low-frequency fluctuation (ALFF) and fractional ALFF (fALFF) were calculated for each group. ALFF was defined as the square root of the power spectrum within the low-frequency range, representing the level of spontaneous neuronal activity. fALFF was calculated as the ratio of the ALFF of each voxel to the total power across the entire frequency range, without band-pass filtering.
Seed-based analysis
Regions showing significant differences and correlations with medication use frequency were selected as regions of interest (ROIs) for calculating resting-state functional connectivity. The time series of these ROIs were correlated with the time series of all brain voxels to obtain Pearson correlation coefficients, which were then transformed into z-values using Fisher’s r-to-z transformation.
Statistical analyses
First, data distribution and variance homogeneity were assessed using Shapiro-Wilk tests and Levene’s tests, respectively. For three-group comparisons (HC, CM + MOH, CM − MOH), normally distributed variables were analyzed using one-way ANOVA, followed by post-hoc tests for pairwise comparisons. Non-normally distributed variables were analyzed using the Kruskal-Wallis H test, followed by post-hoc tests with Bonferroni correction for multiple comparisons. For two-group comparisons (CM + MOH and CM − MOH), normally distributed variables were analyzed using independent t-tests, while non-normally distributed variables were analyzed using Mann-Whitney U tests. Categorical variables were analyzed using chi-square tests or Fisher’s exact tests, as appropriate. Table 1 was restructured to report both overall and pairwise comparisons. The Overall P-value column presents the results of ANOVA or Kruskal-Wallis tests for three-group comparisons, while the Pairwise P-value column reports the results of t-tests or Mann-Whitney U tests for comparisons between CM + MOH and CM − MOH groups, along with the Bonferroni correction results. All statistical analyses were performed using SPSS, with a significance threshold of P < 0.05.
Table 1.
Demographic and clinical characteristics of the participants
| CM − MOH | CM + MOH | HC | P-values | ||
|---|---|---|---|---|---|
| n = 19 | n = 25 | n = 19 | Overall | Group 1 vs. 2 | |
| Sex (Female, n (%)) | 19 (100%) | 25 (100%) | 19 (100%) | 1.000 | NA |
| Age (years) | 39.11 ± 11.25 | 41.76 ± 12.33 | 39.37 ± 11.82 | 0.554※ | NA |
| BMI | 22.93 ± 4.81 | 22.08 ± 3.59 | 21.95 ± 3.25 | 0.695※ | NA |
| Education (years) | 16 (12, 16) | 16 (9, 16) | 16 (9, 16) | 0.710# | NA |
| Headache profile | |||||
| Days of headache per month | 30 (15, 30) | 30 (20, 30) | NA | NA | 0.680^ |
| Days of migraine per month | 19 (9, 21) | 10 (10, 22) | NA | NA | 0.365^ |
| Attack duration (hours) | 20 (5, 24) | 24 (13, 24) | NA | NA | 0.114^ |
| VAS (0–10) | 7 (6, 8) | 8 (7, 9) | NA | NA | 0.067^ |
| Headache laterality | 0.788* | ||||
| Bilateral | 6 (31.58%) | 9 (36.00%) | NA | NA | |
| Alternating | 5 (26.32%) | 4 (16.00%) | NA | NA | |
| Unilateral | 8 (42.11%) | 12 (48.00%) | NA | NA | |
| Associated symptoms | |||||
| Aggravation after exercise | 17 (89.47%) | 24 (96.00%) | NA | NA | 0.570* |
| Nausea | 16 (84.21%) | 22 (88.00%) | NA | NA | 1.000* |
| Vomiting | 11 (57.89%) | 20 (80.00%) | NA | NA | 0.182* |
| Photophobia | 12 (63.16%) | 22 (88.00%) | NA | NA | 0.074* |
| Phonophobia | 17 (89.47%) | 25 (100.00%) | NA | NA | 0.181* |
| Analgesics use profile | |||||
| Frequency (days/month) | 7 (5, 8) | 30 (19, 30) | NA | NA | <0.001 ^ |
| Classes of analgesics | 0.074 * | ||||
| Simple analgesics | 7 (36.84%) | 3 (12.00%) | NA | NA | |
| Triptan | 0 (0.00%) | 1 (4.00%) | NA | NA | |
| Combination analgesic | 12 (63.16%) | 21 (84.00%) | NA | NA | |
| PHQ-9 | 9.26 ± 6.24 | 10.20 ± 7.7 | 2.05 ± 1.87 | <0.001 ※ | 0.865 |
| GAD-7 | 6.79 ± 5.55 | 7.48 ± 5.45 | 1.21 ± 1.87 | <0.001 ※ | 0.602 |
| PSQI | 8.8 ± 4.7 | 8.4 ± 4.2 | 4.21 ± 1.96 | <0.001 ※ | 0.872 |
CM − MOH: Chronic migraine without medication overuse headache; CM + MOH: Chronic migraine with medication overuse headache; HCs: Healthy controls; BMI: Body Mass Index.; VAS: Visual Analogue Scale; NSAID: Non-steroidal anti-inflammatory drug.; PHQ-9: Patient Health Questionnaire-9; GAD-7: General Anxiety Disorder-7; PSQI: Pittsburgh Sleep Quality index. Data following a normal distribution were expressed as mean ± SD, whereas data not meeting the normal distribution criteria were expressed as median and interquartile range (IQR). The P-values (overall) represent the statistical analysis results among the three groups. The P-values (Group 1 vs. 2) represent the -values between the CM − MOH and CM + MOH groups. ※: One-way analysis of variance (ANOVA); #: Kruskal-Wallis H test; ^: Mann-Whitney U test; *: Chi-square test or Fisher’s exact probability test. NA: Not applicable (no pairwise comparison performed if overall P-values was not significant)
For group comparisons of multimodal MRI data, permutation-based non-parametric testing (5000 permutations) was performed.Significant differences in brain components were detected using the Threshold-Free Cluster Enhancement (TFCE) method, with a voxel threshold set at P < 0.001. Multiple comparisons were corrected for by using the Family-Wise Error (FWE) rate, and only clusters containing at least 10 contiguous voxels were considered significant. Generalized Linear Models (GLMs) were applied to analyze the significant clusters, exploring correlations between gray matter volume, ALFF, fALFF values, and white matter integrity with the number of analgesic use days and headache frequency. Age, PHQ-9, GAD-7, and PSQI scores were included as covariates to control for potential confounding variables. The analysis aimed to identify brain region metrics specifically associated with the frequency of acute analgesic use. Statistical significance was determined at P < 0.05.
Results
Table 1 presents the baseline characteristics of the participants, with P-values reflecting subgroup analyses between chronic migraine patient groups (CM − MOH vs. CM + MOH). No significant differences in age or educational years were observed among the CM − MOH, CM + MOH, and HC groups. A significant difference was identified in analgesic use frequency between the CM − MOH and CM + MOH groups (P < 0.001). However, no intergroup differences were observed between CM − MOH and CM + MOH in the following variables: headache attack frequency, migraine attack frequency, attack duration, average headache intensity, headache laterality, associated symptoms, or analgesic classes. Notably, anxiety/depression scores and sleep quality also showed no significant differences between CM − MOH and CM + MOH. In contrast, marked differences were observed when comparing HC with both patient groups: anxiety/depression scores and sleep quality were significantly worse in CM − MOH (P < 0.001) and CM + MOH (P < 0.001) compared to HC. Additionally, no significant differences were observed between the CM − MOH and CM + MOH groups in scores of anxiety and depression, and sleep quality (Table 1).
sMRI
Compared with the CM − MOH group, the CM + MOH group exhibited a significant reduction in gray matter volume in the left parahippocampal gyrus and the right middle occipital gyrus (P < 0.001) (Table 2; Fig. 1). No regions demonstrated a significant increase in gray matter volume. In a generalized linear model adjusted for age, anxiety, depression, and sleep quality scores, these factors were not significantly associated with headache-related indicators or the frequency of medication use.
Table 2.
The brain regions showing differences between the CM − MOH and CM + MOH groups in multimodal magnetic resonance imaging
| Region | MNI Coordinates(mm)x, y, z |
Cluster Size | P-values | t-values | ||
|---|---|---|---|---|---|---|
| Gray matter volume | ||||||
| Parahippocampal gyrus (L) | -14 | -4 | -24 | 36 | <0.001 | 3.64 |
| Middle occipital gyrus (R) | + 30 | -90 | 0 | 15 | <0.001 | 3.70 |
| Fractional anisotropy | ||||||
| Cingulum (L) | + 34 | -17 | -03 | 10 | <0.001 | 3.70 |
| ALFF | ||||||
| Supplementary motor area (L) | -02 | + 20 | + 60 | 27 | <0.001 | 5.08 |
| Superior frontal gyrus, dorsolateral (L) | -12 | + 54 | + 38 | 22 | <0.001 | 4.92 |
| fALFF | ||||||
| Superior frontal gyrus, dorsolateral (R) | + 16 | + 58 | + 04 | 27 | <0.001 | 5.99 |
| Middle frontal gyrus (R) | + 22 | + 28 | + 38 | 22 | <0.001 | 5.46 |
| Supplementary motor area (R) | + 02 | + 16 | + 68 | 19 | <0.001 | 4.40 |
| Precuneus (L) | + 06 | -44 | + 52 | 17 | <0.001 | 4.57 |
| Supramarginal gyrus (L) | -50 | -46 | + 28 | 17 | <0.001 | 4.80 |
| Superior frontal gyrus, orbital part (L) | -24 | + 42 | -12 | 17 | <0.001 | 5.26 |
| Anterior cingulate (L) | -12 | + 50 | -02 | 15 | <0.001 | 5.28 |
| Superior temporal gyrus (L) | + 54 | -34 | + 08 | 14 | <0.001 | 4.26 |
| Middle temporal gyrus (L) | -38 | + 18 | -36 | 14 | <0.001 | 4.43 |
| Precuneus (L) | -08 | -52 | + 52 | 13 | <0.001 | 4.66 |
| Superior frontal gyrus, dorsolateral (L) | -22 | + 04 | + 72 | 12 | <0.001 | 4.51 |
| Superior occipital gyrus (L) | -12 | -84 | + 48 | 12 | <0.001 | 3.61 |
| Superior frontal gyrus, medial (R) | + 10 | + 50 | + 36 | 12 | <0.001 | 4.50 |
| Middle temporal gyrus (R) | + 58 | -44 | + 06 | 12 | <0.001 | 3.58 |
| Cerebelum Crus2 (L) | -20 | -86 | -40 | 12 | <0.001 | 4.10 |
| Inferior frontal gyrus, opercular part (R) | + 38 | + 10 | + 28 | 11 | <0.001 | 4.92 |
| Supramarginal gyrus (R) | + 62 | -42 | + 44 | 10 | <0.001 | 4.31 |
| Lenticular nucleus, putamen (R) | + 28 | -10 | + 02 | 10 | <0.001 | 5.45 |
| Inferior frontal gyrus, orbital part (L) | -54 | + 30 | -10 | 10 | <0.001 | 3.44 |
The table lists all brain regions identified as statistically significant (P < 0.05, corrected) across multimodal imaging. The left hemisphere is denoted as (L) and the right hemisphere as (R)
Fig. 1.
Compared to the CM − MOH group, the CM + MOH group exhibited a significant reduction in gray matter volume in the left parahippocampal gyrus (A) and the right middle occipital gyrus (B). It also had significantly lower ALFF values in the left dorsolateral superior frontal gyrus (C) and the left supplementary motor area (D)
DTI
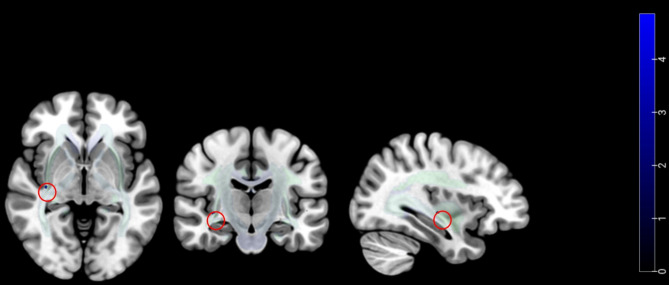
The CM + MOH group showed a significant decrease in fractional anisotropy (FA) within the left cingulum bundle compared to the CM − MOH group (P < 0.001) (Table 2; Fig. 2). No regions exhibited a significant increase in FA. In the generalized linear model controlling for age, anxiety, depression, and sleep quality scores, these covariates showed no significant associations with headache-related metrics or medication use frequency.
Fig. 2.
Compared to the CM − MOH group, the CM + MOH group showed a significant decrease in fractional anisotropy (FA) within the left cingulum bundle
fMRI
Compared to the CM − MOH group, the CM + MOH group exhibited significantly lower ALFF values in the left dorsolateral superior frontal gyrus (DLPFC) and the left supplementary motor area (SMA) (P < 0.001) (Table 2; Fig. 1). Notably, no regions exhibited a significant increase in ALFF. Within the generalized linear model adjusted for age, anxiety, depression, and sleep quality scores, these factors were not significantly correlated with headache-related indicators or the frequency of analgesic use.
Using a voxel-wise threshold of P < 0.001 and a cluster-wise threshold of FWE < 0.05, the CM + MOH group exhibited significantly reduced fALFF values in multiple brain regions compared to the CM − MOH group. These clusters were predominantly located in the frontal lobe, with additional involvement of the parietal, temporal, occipital lobes, cerebellum, and putamen (Table 2; Fig. 3). Further analysis revealed a significant negative correlation between fALFF values in the right putamen and the frequency of acute analgesic use, indicating that higher analgesic consumption is associated with lower spontaneous neural activity in this region (coefficient = − 297.96, 95%CI = − 497.5–−98.3, P value = 0.0058, with adjustment for age, GAD7, PHQ9, and PSQI scores) (Supplementary Fig. 1). To determine whether the alteration in right putamen fALFF is a distinguishing feature between CM + MOH and CM − MOH groups, both patient groups were individually compared to healthy controls. No significant differences in fALFF within the putamen were observed between either patient group and the healthy controls. These findings suggest that the reduced fALFF in the right putamen is specifically related to medication overuse.
Fig. 3.
Compared with the CM − MOH group, the CM + MOH group exhibited significantly reduced fALFF values in multiple brain regions, including the frontal, parietal, temporal, and occipital lobes, the cerebellum, and the putamen. Detailed brain regions are listed in Table 2
The right putamen was designated as the region of interest (ROI) for functional connectivity analyses across the entire brain. In the CM + MOH group, significant alterations in functional connectivity with right putamen were observed in two clusters located in right precuneus cortex and right superior frontal gyrus (Fig. 4 and Supplementary Table 1). Furthermore, the connectivity values between the right putamen and other brain regions in Automated Anatomical Labeling 3 (AAL3) were also analyzed. Compared to the CM − MOH group, the CM + MOH group demonstrated enhanced functional connectivity between the right putamen and the frontal lobe (including the bilateral middle frontal gyrus and the right dorsolateral superior frontal gyrus), bilateral lingual gyrus, bilateral middle cingulate gyrus, and the right precuneus. Moreover, the strength of these functional connections positively correlated with the frequency of acute analgesic use (P < 0.01) (Fig. 5 and Supplementary Table 2), indicating that more frequent analgesic consumption is associated with stronger functional connectivity between the right putamen and these cortical regions. All identified differences met the significance threshold of P < 0.01.
Fig. 4.
Compared with the CM − MOH group, the CM + MOH group exhibited significantly enhanced functional connectivity between the right putamen and 21 brain regions
Fig. 5.
Correlation between changes in brain region function and the frequency of acute analgesic use. The frequency of acute analgesic use shows a positive correlation with the FC between the right putamen and (A) Lingual L, (B) Lingual R, (C) Cingulum Mid L, (D) Precuneus R, (E) Frontal Mid R, (F) Cingulum Mid R, (G) Frontal Sup R, and (H) Frontal Mid L. Orange represents CM − MOH, and green represents CM + MOH
Discussion
This study utilized ultrahigh-field multimodal MRI to investigate structural and functional brain differences between CM + MOH and CM − MOH patients. The CM + MOH group exhibited significant reductions in gray matter volume (e.g., parahippocampal and occipital regions) and fractional anisotropy in the cingulum bundle. Notably, the right putamen demonstrated reduced fALFF values and enhanced functional connectivity with other brain regions, both of which correlated with acute analgesic use frequency. These results support the pivotal involvement of the putamen and its associated neural networks in the pathophysiology of medication overuse headache, thereby highlighting their potential as targets for therapeutic intervention.
This study identified significant reductions in gray matter volume in the Parahippocampal Gyrus (PHG) and Middle Occipital Gyrus (MOG) in CM + MOH patients compared to CM − MOH patients. The PHG, a pivotal region for emotional processing and contextual memory regulation, exhibited reduced volume, indicating potential impairments in these functions. Structural remodeling in the PHG was further supported by increased intrinsic correlations reported in previous studies on MOH [16]. Similarly, the MOG, essential for visual processing, attentional regulation, and visual memory formation [17, 18], also showed significant gray matter reduction in CM + MOH patients, consistent with prior findings in MOH populations [19, 20]. These changes imply diminished capacities for visual processing and attentional control, emphasizing the visual network’s role in the neural adaptations associated with chronic pain and medication dependence. Collectively, the decreased gray matter volumes in both the PHG and the MOG in MOH patients likely reflect a functional impairment in memory, emotion, and visual networks. These findings provide new insights into the neurobiological mechanisms underlying medication dependence and chronic pain, and suggest that the PHG and MOG could serve as potential targets for future therapeutic interventions.
Diffusion tensor imaging (DTI) enables the visualization of white matter microstructure by quantifying the diffusion properties of water molecules. Fractional anisotropy (FA) values, as sensitive indicators of white matter integrity, can reflect the extent of myelin sheath disruption, axonal damage, or fiber disarray [21]. This study found that, compared to CM − MOH patients, CM + MOH patients exhibited a significant reduction in FA values of the left cingulum bundle. The cingulum bundle serves as a major white matter tract connecting the frontal, parietal, and temporal lobes, playing a pivotal role in emotion regulation, memory consolidation, and cognitive control [21]. Previous studies have demonstrated that MOH patients exhibit reduced FA values in white matter fibers associated with pain perception compared to healthy controls [22]. Our findings further highlight that excessive medication use in CM + MOH patients may exacerbate white matter damage, specifically in the left cingulum bundle. Notably, compulsive medication-taking behavior, a key characteristic of MOH, demonstrates behavioral and neurobiological parallels with obsessive compulsive disorder (OCD). MOH patients tend to score higher on the Yale-Brown Obsessive Compulsive Scale and Leeds Dependence Questionnaire compared to those with episodic or chronic migraine without medication overuse, indicating a stronger predisposition to compulsive tendencies [23, 24]. In addition, widespread reductions in FA values, particularly in the cingulum bundle, have been observed in OCD patients [25, 26]. Based on the findings of this study, the reduction in FA values of the left cingulum bundle in CM + MOH patients may reflect the pathological mechanisms underlying compulsive medication-taking behavior. These findings provide novel insights into the neurobiological mechanisms of CM + MOH and suggest that the cingulum bundle could serve as a potential target for future interventions.
This study revealed a significant reduction in fALFF values within the right putamen in CM + MOH patients compared to CM − MOH patients. ALFF and fALFF are crucial indicators of spontaneous neural activity, with fALFF being particularly sensitive to detecting changes in regional spontaneous activity by mitigating the influence of physiological noise [27]. The observed decrease in fALFF values in the right putamen of CM + MOH patients, along with its negative correlation with the frequency of acute analgesic use, suggests that spontaneous activity in the putamen is markedly suppressed under conditions of chronic headache and medication overuse. The putamen, a key structure within the basal ganglia, forms the dorsal striatum along with the caudate nucleus and participates in circuits with the prefrontal cortex and limbic system to collaboratively regulate functions such as motivation, decision-making, and motor control [28]. Furthermore, the putamen plays a central role in the reward system, contributing to reward-driven behaviors through dopaminergic projections [29]. Research indicates that the medication overuse behaviors observed in MOH patients share behavioral, genetic, and neuronal pathway mechanisms with addiction [30]. Approximately two-thirds of MOH patients meet the substance dependence criteria outlined in the DSM-IV [31, 32]. Additionally, these patients often exhibit psychological traits such as pain avoidance, emotional distress, and reward-seeking tendencies [33], which may reinforce medication use behaviors due to factors such as uncertainty regarding acute medication responses and concerns about headache exacerbation [34]. The adaptive changes in the putamen’s reward mechanism may be associated with this phenomenon, while the reduction in fALFF may reflect the inefficiency of reward function under chronic pain conditions. Furthermore, impaired risk-based decision-making is a prominent feature of MOH patients. Studies using the Iowa Gambling Task have shown that MOH patients are more likely to make disadvantageous decisions under conditions of uncertainty, with their performance negatively correlating with the frequency of medication use [35]. This suggests that dysfunction in the putamen may impair impulse control and decision-making abilities, preventing patients from effectively analyzing complex situations and leading to impulsive medication intake. Moreover, animal studies have demonstrated that chronic drug exposure can disrupt glutamate metabolism in the putamen, further supporting the role of the putamen in drug addiction [36]. In the current study, we observed that fALFF values in the putamen of CM + MOH patients were negatively correlated with the frequency of acute analgesic use, indicating that more frequent acute analgesic consumption is associated with a further suppression of putamen function. Therefore, the alterations in the putamen observed in CM + MOH patients likely result from the combined effects of chronic headache and medication use, highlighting the complex interplay between pain management and neural adaptations in MOH.
In addition to the reduced local brain activity, this study found that patients with CM + MOH exhibited significantly increased functional connectivity between the putamen and multiple brain regions, including the middle frontal gyrus, dorsolateral superior frontal gyrus, middle cingulate gyrus, lingual gyrus, and precuneus. These enhanced connections were positively correlated with the frequency of acute analgesic use, suggesting that the putamen may play a key role in medication overuse through its interactions with other brain regions.
The increased functional connectivity between the putamen and both the middle frontal gyrus and dorsolateral superior frontal gyrus may reflect a compensatory mechanism related to cognitive control. These regions are key components of the Central Executive Network (CEN), which plays a crucial role in complex cognitive processes such as planning, decision-making, and problem-solving [37]. In CM + MOH patients, a higher frequency of medication use is associated with enhanced CEN functional connectivity, possibly reflecting an attempt to regulate pain and medication use through cognitive control. Additionally, these patients may experience a higher cognitive load as they strive to balance reward-driven impulses and cognitive regulation.
The heightened functional connectivity between the putamen and the middle cingulate gyrus suggests altered activity within the Salience Network in CM + MOH patients. Both the putamen and the middle cingulate gyrus are key nodes of the Salience Network, which is primarily involved in identifying the salient stimuli from both internal and external sources, and in coordinating the allocation of attentional resources [38]. Previous research has shown that acute analgesic medications can modulate the glutamate cycle, thereby affecting excitatory neural activity in the middle cingulate gyrus, potentially contributing to the development of MOH [39]. In the current study, the internal functional connectivity of the Salience Network was significantly enhanced and positively correlated with the frequency of analgesic use, suggesting that CM + MOH patients may be excessively focused on pain-related information, thereby reinforcing medication overuse behaviors. Machine learning studies have demonstrated that the functional connectivity between the dorsal rostral putamen (DRP) and the whole brain can effectively distinguish MOH patients from non-MOH individuals. Furthermore, the connectivity between this region and the middle cingulate cortex has been identified as a key discriminative feature for MOH classification [40]. These findings are partially consistent with the current study, both emphasizing the importance of the putamen and its specific functional connections, particularly with the middle cingulate gyrus, in MOH patients.
The enhanced functional connectivity between the putamen and the lingual gyrus may reflect a coupling mechanism linking analgesic overuse with visual processing. The lingual gyrus is a key region for visual processing, particularly in object shape recognition and color perception. It is also involved in the pathway from primary visual feature reception to higher-level information integration [41]. Additionally, the lingual gyrus may interact with reward-related networks, integrating visual cues with motivational information. This increased connectivity suggests that patients may experience heightened sensitivity to analgesic cues, thereby reinforcing medication overuse behaviors through visual stimuli. Similarly, the enhanced functional connectivity between the putamen and the precuneus may suggest altered regulation of self-referential processing under chronic pain and acute medication overuse. The precuneus, located on the medial surface of the parietal lobe, is a key node of the Default Mode Network, and is involved in processes such as self-perception and introspection [42]. Despite the observed reduction in local activity within both the putamen and the precuneus, the increased functional connectivity could indicate an adaptive compensatory mechanism aimed at sustaining cognitive and emotional regulation.
Limitations
The primary limitations of this study include the relatively small sample size and the exclusive inclusion of female patients, which may limit the generalizability of the findings. While CM and MOH are more prevalent in females, future studies should include both genders to enhance external validity. Estrogen, not measured here, may influence migraine susceptibility and brain alterations, suggesting the need for hormonal profiling in future studies. This study did not further stratify participants based on the type of medication used. Given that different medications may have distinct effects on patients [43], future studies should examine the role of specific drug classes by categorizing participants according to their medication types. Additionally, reliance on self-reported data introduces recall bias, and future research could incorporate electronic diaries for more accurate tracking. Due to the difficulty in precisely defining the interictal phase in chronic migraine patients, we ensured that participants were not experiencing a migraine attack at the time of MRI scanning and had not had an attack in the 24 h preceding the scan. However, this approach may have inadvertently included patients who had recently experienced a attack, potentially influencing the results. Lastly, the cross-sectional design limits causal inference, and longitudinal studies with repeated neuroimaging assessments are necessary to explore temporal relationships and causality.
Conclusion
This study utilized 7 Tesla multimodal MRI to investigate alterations in brain structure, white matter integrity, and neural activity in CM patients with and without MOH. The results demonstrated that CM + MOH patients exhibited reduced gray matter volume in the parahippocampal and middle occipital gyri, along with decreased FA in the left cingulum bundle. Additionally, the fALFF in the right putamen was decreased and negatively correlated with analgesic use frequency, whereas its functional connectivity with multiple brain regions was increased and positively correlated with analgesic use frequency. These findings provide novel neuroimaging evidence for the pathophysiological mechanisms underlying MOH and suggest that dysfunction of the putamen may contribute to the formation of habitual analgesic overuse and dependency, resembling neural mechanisms observed in substance use disorders. This underscores the putamen’s potential role as a biomarker for MOH diagnosis and treatment, while also offering new insights into the addictive characteristics associated with MOH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank all participants in the study.
Abbreviations
- CM
Chronic migraine
- MOH
Medication overuse headache
- MRI
Magnetic resonance imaging
- DTI
Diffusion tensor imaging
- fMRI
Functional magnetic resonance imaging
- CM − MOH
Chronic migraine without medication overuse headache
- CM + MOH
Chronic migraine with medication overuse headache
- FA
Fractional anisotropy or Flip angle
- PHQ-9
Patient Health Questionnaire-9
- GAD-7
Generalized Anxiety Disorder-7
- PSQI
Pittsburgh Sleep Quality Index
- sMRI
Structural magnetic resonance imaging
- TR
Repetition time
- TE
Echo time
- FOV
Field of view
- VBM
Voxel-based morphometry
- MNI
Montreal Neurological Institute
- FWHM
Full Width at Half Maximum
- TBSS
Tract-Based Spatial Statistics
- ALFF
Amplitude of Low-Frequency Fluctuation
- fALFF
Fractional Amplitude of Low-Frequency Fluctuation
- ROIs
Regions of interest
- TFCE
Threshold-Free Cluster Enhancement
- FWE
Family-Wise Error
- GLM
Generalized Linear Model
- NA
Not Applicable
- BMI
Body Mass Index
- HCs
Healthy controls
- NSAID
Non-steroidal anti-inflammatory drug
- VAS
Visual Analogue Scale
- DLPFC
Dorsolateral superior frontal gyrus
- PHG
Parahippocampal Gyrus
- MOG
Middle Occipital Gyrus
- SMA
Supplementary Motor Area
- DRP
Dorsal Rostral Putamen
- OCD
Obsessive compulsive disorder
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition
- ICHD
International Classification of Headache Disorders
- JHU
Johns Hopkins University
- L
Left
- R
Right
- Cingulum_Mid
Middle Cingulate Cortex
- Frontal_Mid
Middle Frontal Gyrus
- Frontal_Sup
Superior Frontal Gyrus
- Lingual
Lingual Gyrus
Author contributions
Study concept and design: YS, LTM, XLou, and ZD. Acquisition of data: YS, SW, CHD, XYW, XBB, SQW, DQZ, SYX, SHZ, YYL, XXL, RBW, XLiu, SYY. Data analysis and writing the manuscript: YS and LTM. Review and editing, funding acquisition, supervision: XLou and ZD. All authors contributed intellectual content to the revised manuscript and read and approved the final manuscript. YS and LTM contributed equally to this article.
Funding
This study was supported by the National Key R&D Program of China (2023YFC2508702), the National Natural Science Foundation of China (grants 82171208 to Z.D.), and Protect of Chinese Research Hospital Association (Y2023FH-TTYGJZA12).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Chinese PLA General Hospital in accordance with the ethical principles of the Declaration of Helsinki. (S2023-459-01).
Consent for publication
All authors consent for the publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yin Sun and Longteng Ma contributed equally to the work as first authors.
Contributor Information
Xin Lou, Email: louxin@301hospital.com.cn.
Zhao Dong, Email: dong_zhaozhao@126.com.
References
- 1.Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L et al (2010) Global prevalence of chronic migraine: a systematic review. Cephalalgia 30(5):599–609 [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS) (2018) The international classification of headache disorders, 3rd edition. Cephalalgia 38(1):1–211 [DOI] [PubMed]
- 3.Vandenbussche N, Laterza D, Lisicki M, Lloyd J, Lupi C, Tischler H et al (2018) Medication-overuse headache: a widely recognized entity amidst ongoing debate. J Headache Pain.;19(1) [DOI] [PMC free article] [PubMed]
- 4.Westergaard ML, Hansen EH, Glümer C, Olesen J, Jensen RH (2013) Definitions of medication-overuse headache in population-based studies and their implications on prevalence estimates: A systematic review. Cephalalgia 34(6):409–425 [DOI] [PubMed] [Google Scholar]
- 5.Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J et al (2012) The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache 52(4):582–591 [DOI] [PubMed] [Google Scholar]
- 6.Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF et al (2017) Global, regional, and National burden of neurological disorders during 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol 16(11):877–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planchuelo-Gomez A, Garcia-Azorin D, Guerrero AL, Aja-Fernandez S, Rodriguez M, de Luis-Garcia R (2020) Structural connectivity alterations in chronic and episodic migraine: A diffusion magnetic resonance imaging connectomics study. Cephalalgia 40(4):367–383 [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Zhou M, Wu X, Zhao F, Luo X, Li K et al (2023) Increased iron deposition in nucleus accumbens associated with disease progression and chronicity in migraine. BMC Med 21(1):136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez C, Lopez A, Ramos-Cabrer P, Vieites-Prado A, Perez-Mato M, Villalba C et al (2019) Iron deposition in periaqueductal Gray matter as a potential biomarker for chronic migraine. Neurology 92(10):e1076–e85 [DOI] [PubMed] [Google Scholar]
- 10.Schwedt TJ, Chong CD (2017) Medication overuse headache: pathophysiological insights from structural and functional brain MRI research. Headache 57(7):1173–1178 [DOI] [PubMed] [Google Scholar]
- 11.Wu CH, Chang FC, Wang YF, Lirng JF, Wu HM, Pan LLH et al (2024) Impaired glymphatic and meningeal lymphatic functions in patients with chronic migraine. Ann Neurol 95(3):583–595 [DOI] [PubMed] [Google Scholar]
- 12.Christensen RH, Ashina H, Al-Khazali HM, Zhang Y, Tolnai D, Poulsen AH et al (2024) Differences in cortical morphology in people with and without migraine: A registry for migraine (REFORM) MRI study. Neurology 102(9):e209305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäki-Marttunen V, Kies DA, Pijpers JA, Louter MA, van der Wee NJ, Rombouts SARB et al (2023) Functional connectivity of the visual cortex in chronic migraine before and after medication withdrawal therapy. NeuroImage: Clin.;40 [DOI] [PMC free article] [PubMed]
- 14.Niddam DM, Wu S-W, Lai K-L, Yang Y-Y, Wang Y-F, Wang S-J (2023) An altered reward system characterizes chronic migraine with medication overuse headache. Cephalalgia.;43(4) [DOI] [PubMed]
- 15.Fagan AJ, Bitz AK, Bjorkman-Burtscher IM, Collins CM, Kimbrell V, Raaijmakers AJE et al (2021) 7T MR safety. J Magn Reson Imaging 53(2):333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Chen X, Chen Z, Liu M, He H, Ma L et al (2017) Alteration of Gray matter texture features over the whole brain in medication-overuse headache using a 3-dimentional texture analysis. J Headache Pain.;18(1) [DOI] [PMC free article] [PubMed]
- 17.Amedi A, Stern WM, Camprodon JA, Bermpohl F, Merabet L, Rotman S et al (2007) Shape conveyed by visual-to-auditory sensory substitution activates the lateral occipital complex. Nat Neurosci 10(6):687–689 [DOI] [PubMed] [Google Scholar]
- 18.Yu Q, Panichello MF, Cai Y, Postle BR, Buschman TJ (2020) Delay-period activity in frontal, parietal, and occipital cortex tracks noise and biases in visual working memory. PLoS Biol 18(9):e3000854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer M, Di Scala G, Edde M, Dilharreguy B, Radat F, Allard M et al (2017) Brain structural investigation and hippocampal tractography in medication overuse headache: a native space analysis. Behav Brain Funct.;13(1) [DOI] [PMC free article] [PubMed]
- 20.Lai T-H, Chou K-H, Fuh J-L, Lee P-L, Kung Y-C, Lin C-P et al (2016) Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia 36(14):1324–1333 [DOI] [PubMed] [Google Scholar]
- 21.Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018) The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev 92:104–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michels L, Christidi F, Steiger VR, Sándor PS, Gantenbein AR, Landmann G et al (2016) Pain modulation is affected differently in medication-overuse headache and chronic myofascial pain– A multimodal MRI study. Cephalalgia 37(8):764–779 [DOI] [PubMed] [Google Scholar]
- 23.Cupini LM, De Murtas M, Costa C, Mancini M, Eusebi P, Sarchielli P et al (2009) Obsessive-Compulsive disorder and migraine with Medication‐Overuse headache. Headache: J Head Face Pain 49(7):1005–1013 [DOI] [PubMed] [Google Scholar]
- 24.Sarchielli P, Corbelli I, Messina P, Cupini LM, Bernardi G, Bono G et al (2015) Psychopathological comorbidities in medication-overuse headache: a multicentre clinical study. Eur J Neurol 23(1):85–91 [DOI] [PubMed] [Google Scholar]
- 25.Koch K, Reess TJ, Rus OG, Zimmer C, Zaudig M (2014) Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. J Psychiatr Res 54:26–35 [DOI] [PubMed] [Google Scholar]
- 26.Radua J, Grau M, van den Heuvel OA, Thiebaut de Schotten M, Stein DJ, Canales-Rodríguez EJ et al (2014) Multimodal Voxel-Based Meta-Analysis of white matter abnormalities in Obsessive–Compulsive disorder. Neuropsychopharmacology 39(7):1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ et al (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172(1):137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balleine BW, Delgado MR, Hikosaka O (2007) The role of the dorsal striatum in reward and decision-making. J Neurosci 27(31):8161–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice ME, Patel JC (2015) Somatodendritic dopamine release: recent mechanistic insights. Philos Trans R Soc Lond B Biol Sci.;370(1672) [DOI] [PMC free article] [PubMed]
- 30.Takahashi TT, Ornello R, Quatrosi G, Torrente A, Albanese M, Vigneri S et al (2021) Medication overuse and drug addiction: a narrative review from addiction perspective. J Headache Pain.;22(1) [DOI] [PMC free article] [PubMed]
- 31.Bottiroli S, Galli F, Ballante E, Pazzi S, Sances G, Guaschino E et al (2021) Validity of the severity of dependence scale for detecting dependence behaviours in chronic migraine with medication overuse. Cephalalgia 42(3):209–217 [DOI] [PubMed] [Google Scholar]
- 32.Fuh J-L, Wang S-J, Lu S-R, Juang K-D (2005) Does medication overuse headache represent a behavior of dependence? Pain 119(1–3):49–55 [DOI] [PubMed] [Google Scholar]
- 33.Radat F, Lanteri-Minet M (2010) What is the role of Dependence‐Related behavior in Medication‐Overuse headache?? Headache: J Head Face Pain 50(10):1597–1611 [DOI] [PubMed] [Google Scholar]
- 34.Lau CI, Liu M-N, Chen W-H, Walsh V, Wang S-J (2020) Clinical and biobehavioral perspectives: is medication overuse headache a behavior of dependence? Update on emerging treatments for migraine. Progress in brain research p. 371–402 [DOI] [PubMed]
- 35.Lau CI, Chen WH, Wang HC, Walsh V (2023) Decision-making impairment under ambiguity but not under risk May underlie medication overuse in patients with chronic migraine. Headache: J Head Face Pain 63(6):822–833 [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Jensen JE, Gillis TE, Zuo CS, Prescot AP, Brimson M et al (2011) Chronic cocaine exposure induces putamen glutamate and glutamine metabolite abnormalities in squirrel monkeys. Psychopharmacology 217(3):367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL (2013) Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25(1):74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of Insula function. Brain Struct Funct 214(5–6):655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niddam DM, Lai K-L, Tsai S-Y, Lin Y-R, Chen W-T, Fuh J-L et al (2020) Brain metabolites in chronic migraine patients with medication overuse headache. Cephalalgia 40(8):851–862 [DOI] [PubMed] [Google Scholar]
- 40.Torta DM, Costa T, Luda E, Barisone MG, Palmisano P, Duca S et al (2016) Nucleus accumbens functional connectivity discriminates medication-overuse headache. NeuroImage: Clin 11:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, He S, Weng X (2018) Localization and functional characterization of an occipital visual word form sensitive area. Sci Rep 8(1):6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guterstam A, Bio BJ, Wilterson AI, Graziano M (2021) Temporo-parietal cortex involved in modeling one’s own and others’ attention. Elife.;10 [DOI] [PMC free article] [PubMed]
- 43.Thorlund K, Sun-Edelstein C, Druyts E, Kanters S, Ebrahim S, Bhambri R et al (2016) Risk of medication overuse headache across classes of treatments for acute migraine. J Headache Pain.;17(1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.