Abstract
1. We describe here a technique which allows the long-term in vitro survival of the perfused isolated brain stem-cerebellum of adult guinea-pig. The viability of this preparation was assessed by comparing the electrophysiological properties of individual neurones and of neuronal pools to those obtained in vivo or in brain slices. The areas investigated included the cerebellar cortex, the inferior olive and the pontine nuclei. 2. Cerebellar field potential and intra- and extracellular single-cell recordings could be obtained for as long as 15 h after the preparation was initially isolated. The waveforms of field potentials recorded at various depths in the cerebellar cortex following surface folial stimulation were similar to those recorded in vivo. Extracellular recordings from single Purkinje cells following white matter stimulation demonstrated antidromic as well as mossy- and climbing fibre-mediated excitation. Stimulation of the cerebellar surface elicited orthodromic parallel fibre excitation of Purkinje cells and basket-stellate and Golgi cell inhibition. 3. Intrasomatic and intradendritic recordings from Purkinje cells reproduced all the phenomenology described earlier under in vivo conditions and in vitro slice preparations. In addition, spontaneous excitatory synaptic potentials generating simple spikes (mossy fibre-parallel fibre-mediated activity) and complex spikes (climbing fibre-mediated activity) were consistently observed. 4. Extracellular field potentials and extra- and intracellular recordings from inferior olive neurones were similar to those previously shown for the mammalian inferior olive. 5. Intracellular recordings were also obtained from pontine nuclei neurones, a major source of mossy fibre afferents to the cerebellum. Stimulation of the contralateral superior cerebellar peduncle produced antidromic invasion of these neurones whereas stimulation of the ipsilateral inferior cerebral peduncle resulted in their orthodromic activation. 6. The preparation responded to pharmacological challenge in a manner which demonstrated a sequential activation of sets of synaptic links in a given pathway. Thus, harmaline generated oscillations of inferior olivary neurones which were similar to those observed in vivo and which produced climbing fibre EPSPs in Purkinje cells at the same frequency as the inferior olivary oscillations. Climbing fibre activation of the Purkinje cells generated powerful inhibitory potentials in the cerebellar nuclear neurones at the same frequency.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

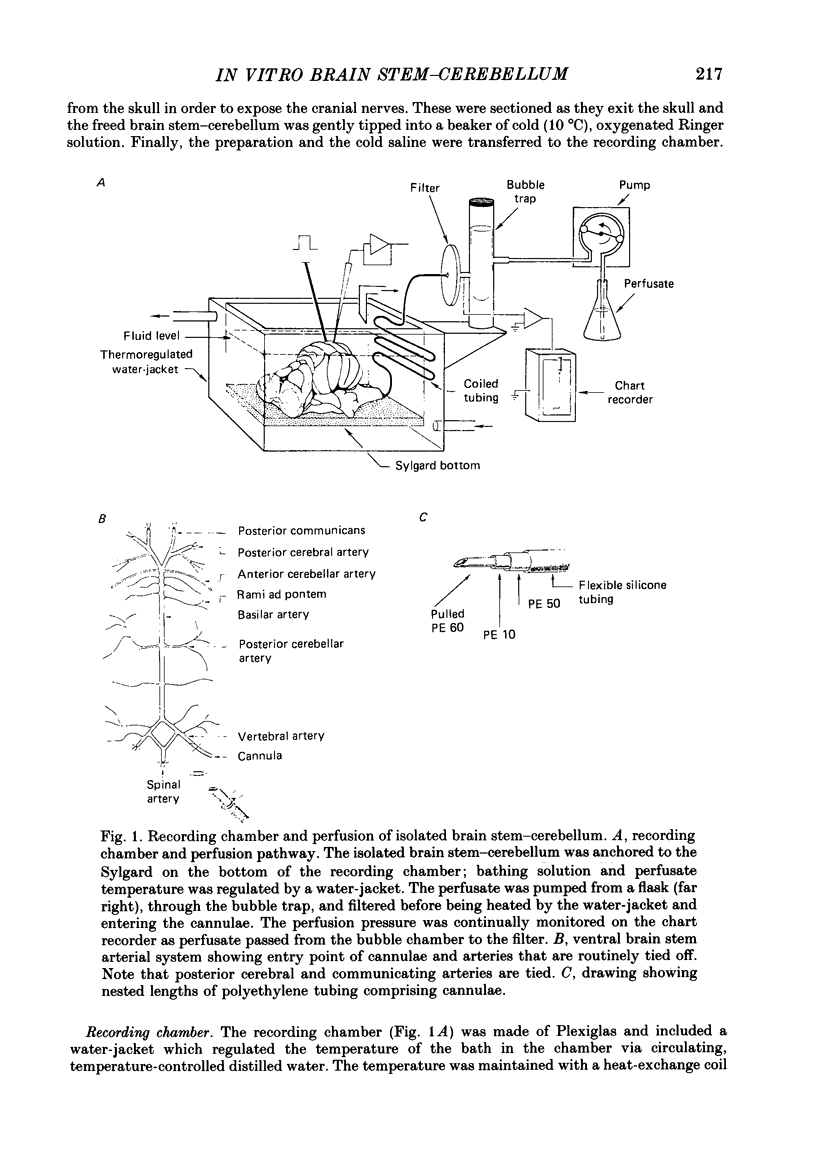



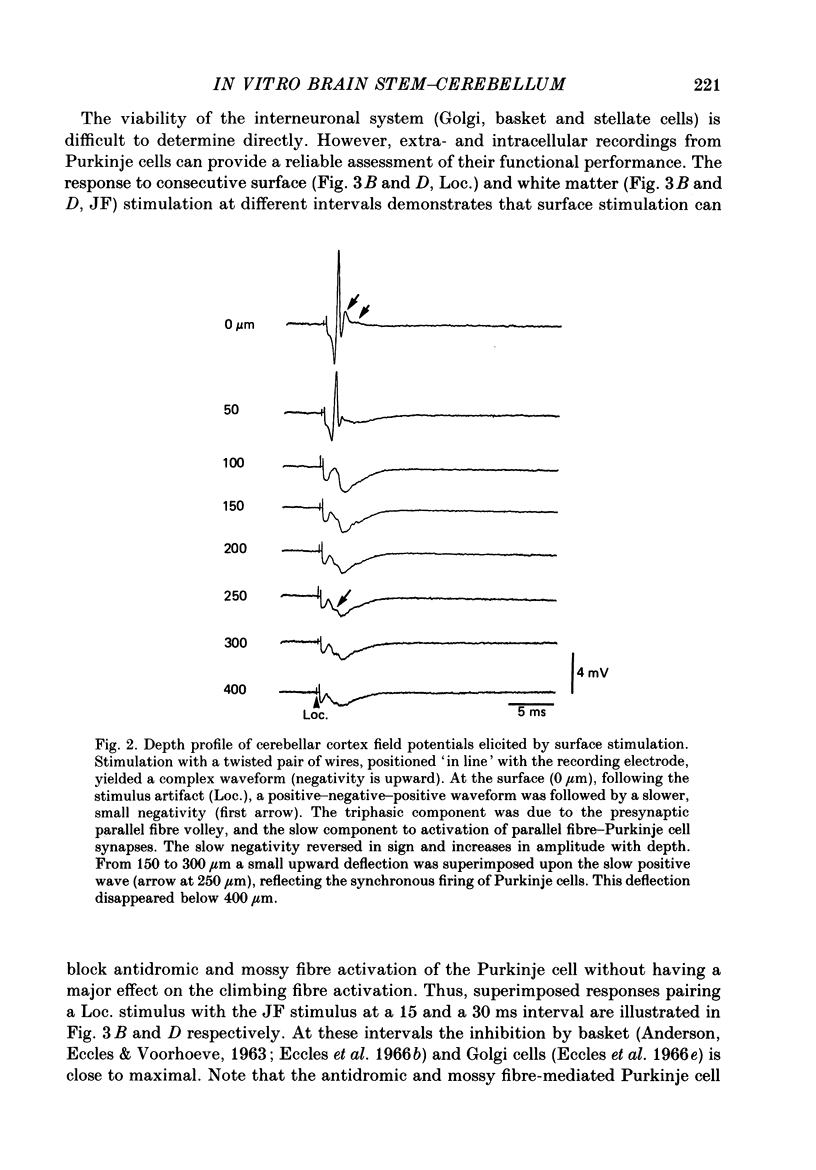
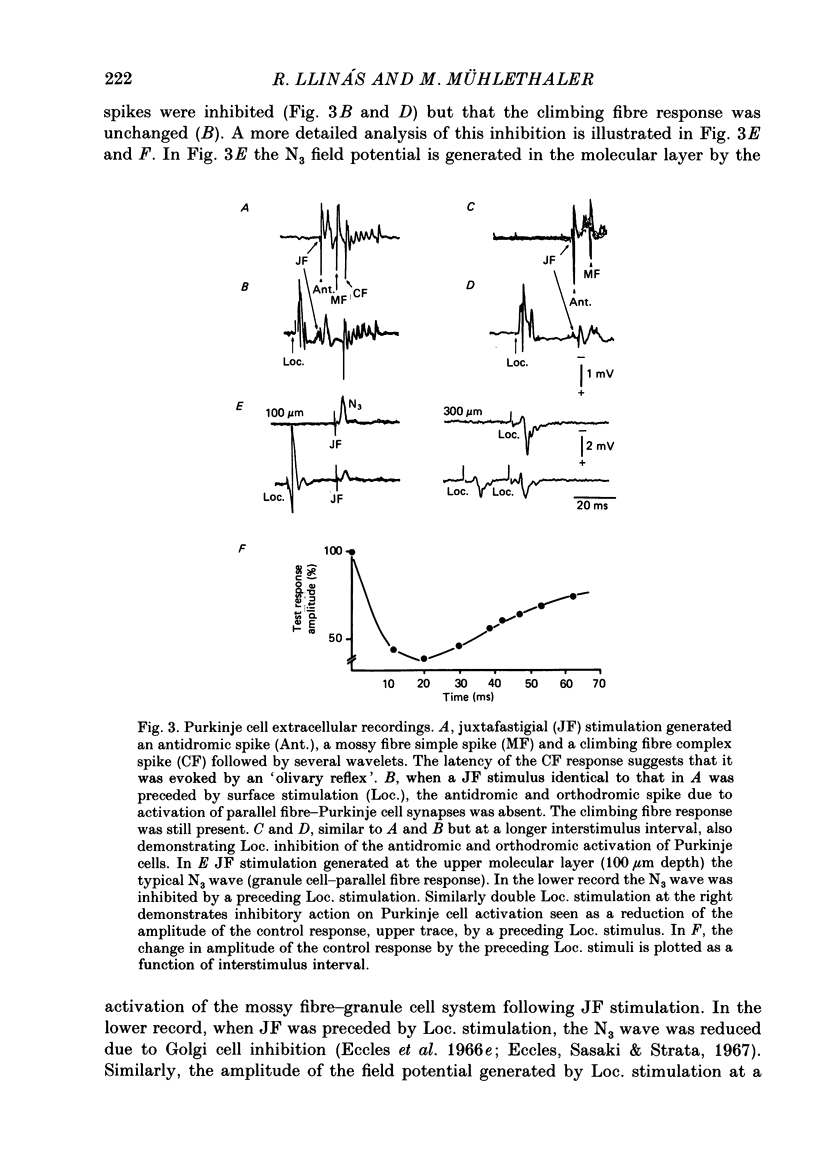
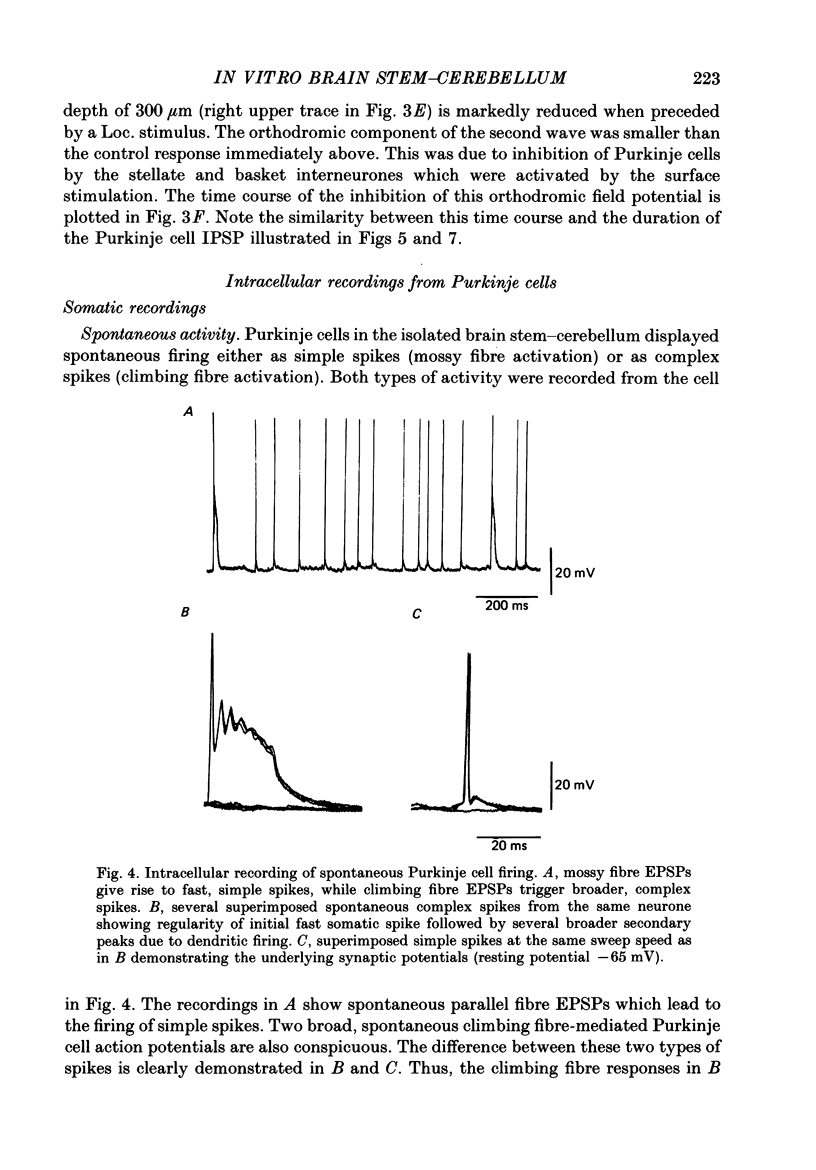



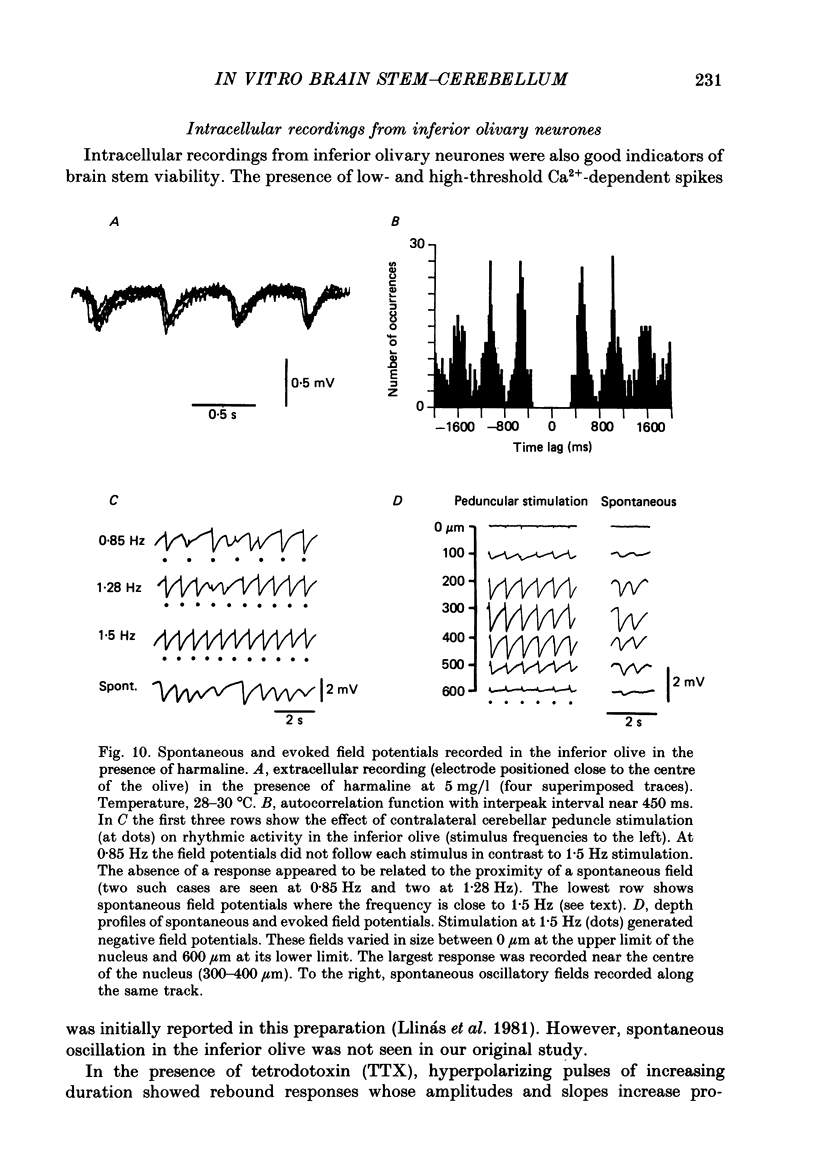
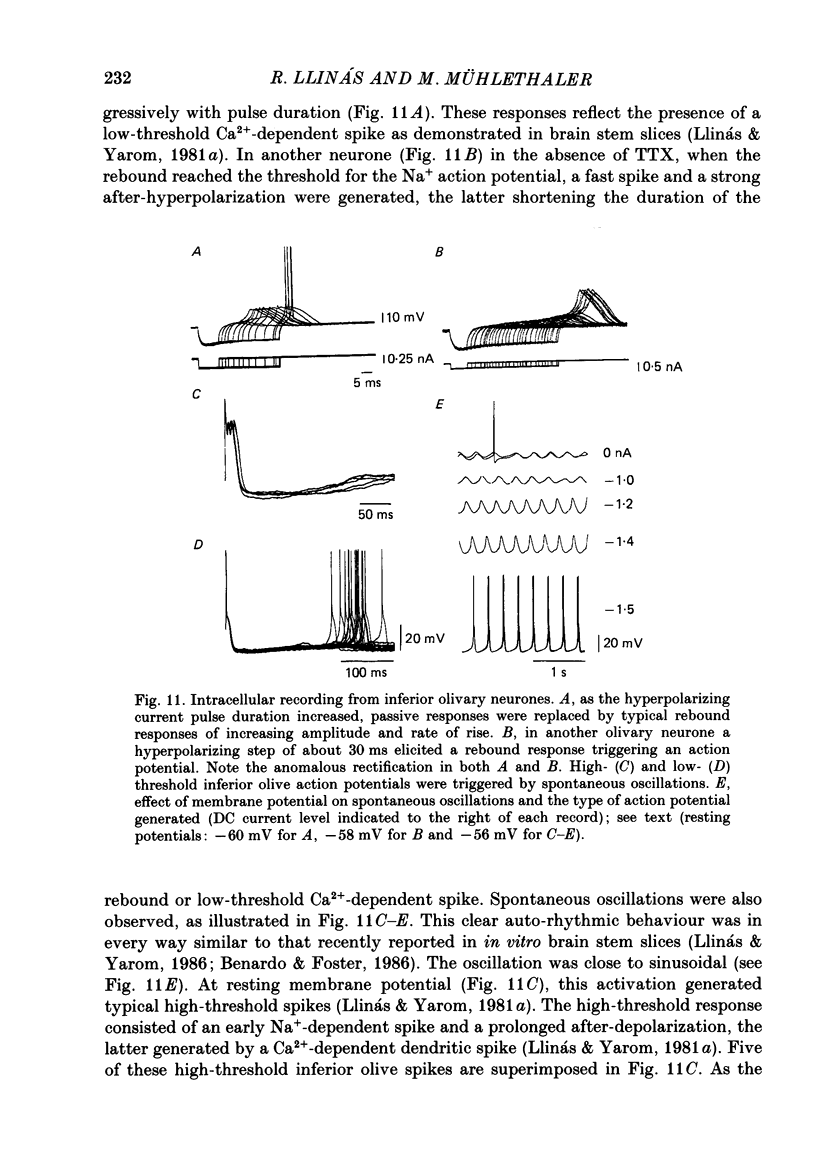

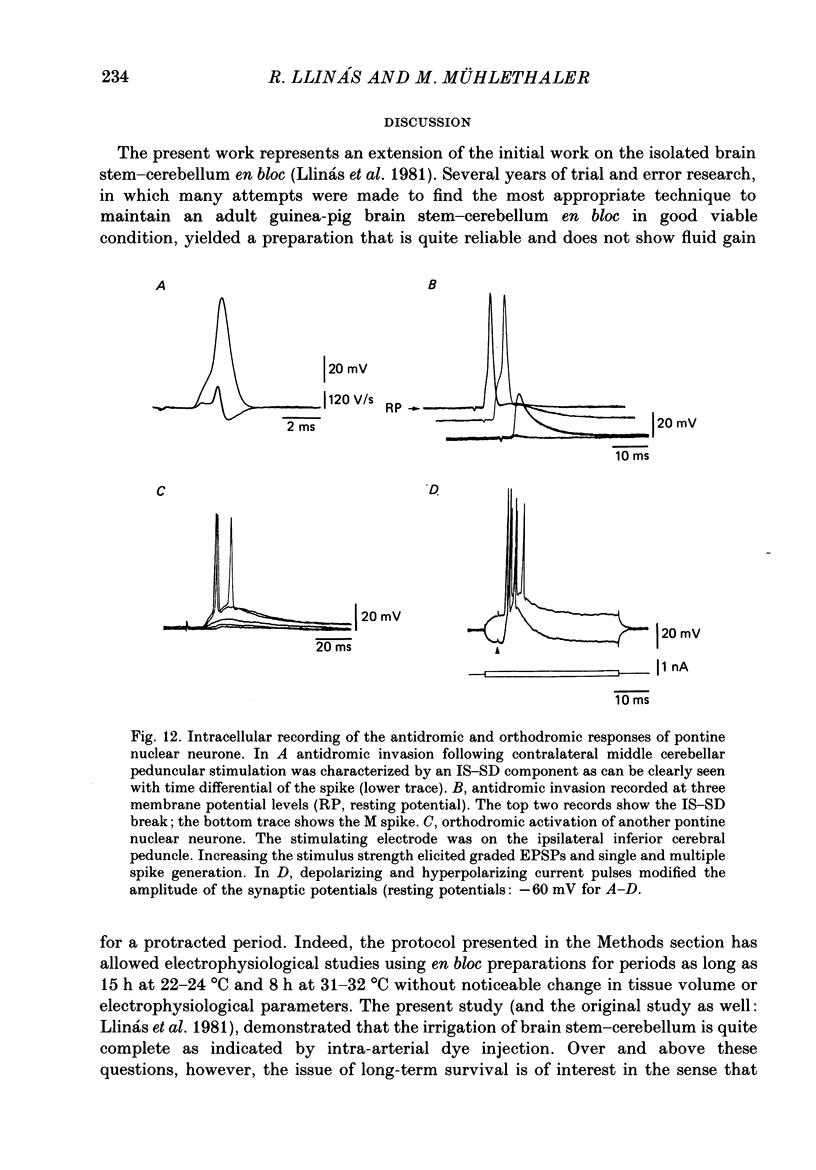






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J., VOORHOEVE P. E. INHIBITORY SYNAPSES ON SOMAS OF PURKINJE CELLS IN THE CEREBELLUM. Nature. 1963 Aug 17;199:655–656. doi: 10.1038/199655a0. [DOI] [PubMed] [Google Scholar]
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980 Oct;307:273–299. doi: 10.1113/jphysiol.1980.sp013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B. D., Harvey R. J. Responses in the inferior olive to stimulation of the cerebellar and cerebral cortices in the cat. J Physiol. 1966 Dec;187(3):553–574. doi: 10.1113/jphysiol.1966.sp008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Foster R. E. Oscillatory behavior in inferior olive neurons: mechanism, modulation, cell aggregates. Brain Res Bull. 1986 Dec;17(6):773–784. doi: 10.1016/0361-9230(86)90089-4. [DOI] [PubMed] [Google Scholar]
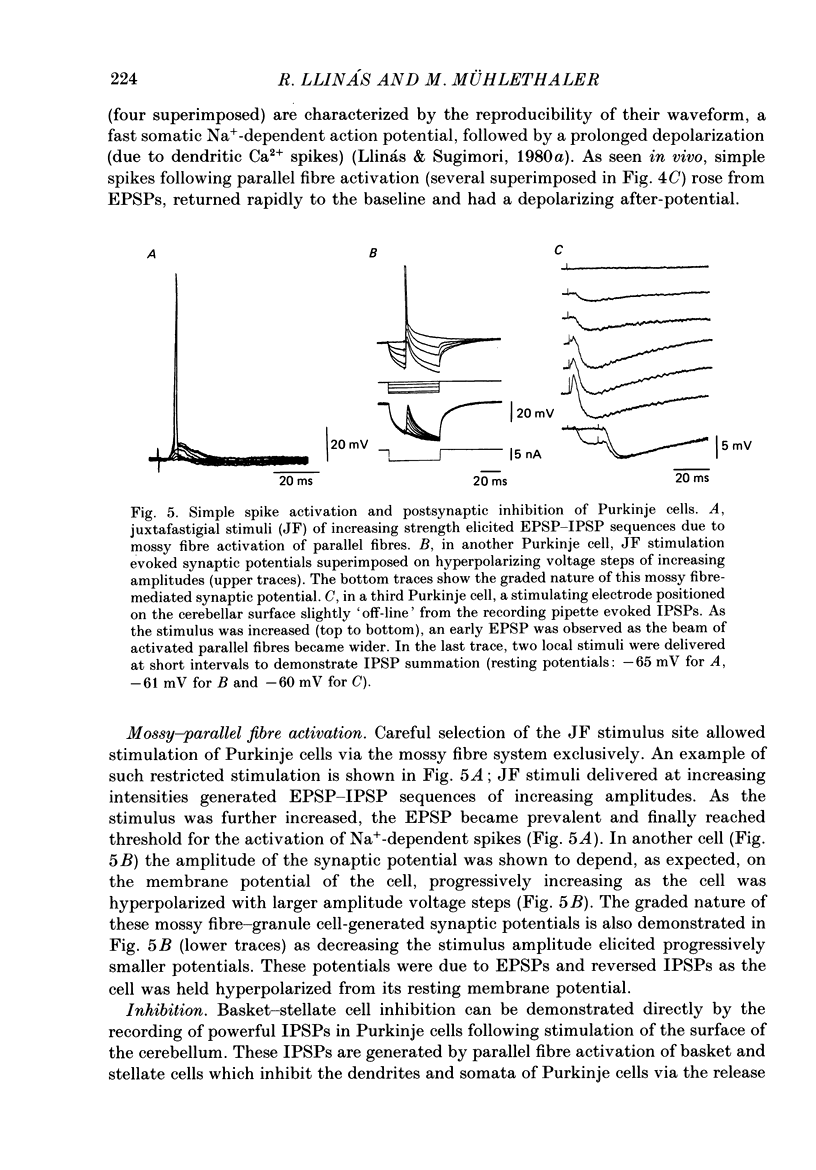
- Bisti S., Iosif G., Marchesi G. F., Strata P. Pharmacological properties of inhibitions in the cerebellar cortex. Exp Brain Res. 1971;14(1):24–37. doi: 10.1007/BF00234908. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. A perfused in vitro preparation of hypothalamus for electrophysiological studies on neurosecretory neurons. J Neurosci Methods. 1983 Mar;7(3):203–214. doi: 10.1016/0165-0270(83)90002-x. [DOI] [PubMed] [Google Scholar]
- Brodal A. Cerebrocerebellar pathways. Anatomical data and some functional implications. Acta Neurol Scand Suppl. 1972;51:153–195. [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N. Distribution of climbing fibres on cerebellar Purkinje cells in X-irradiated rats. An electrophysiological study. J Physiol. 1979 May;290(2):97–112. doi: 10.1113/jphysiol.1979.sp012762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Intracellularly recorded responses of the cerebellar Purkinje cells. Exp Brain Res. 1966;1(2):161–183. doi: 10.1007/BF00236869. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1(1):82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sasaki K., Strata P. Interpretation of the potential fields generated in the cerebellar cortex by a mossy fibre volley. Exp Brain Res. 1967;3(1):58–80. doi: 10.1007/BF00234470. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Oscarsson O. Prolonged depolarization elicited in Purkinje cell dendrites by climbing fibre impulses in the cat. J Physiol. 1981 Sep;318:207–221. doi: 10.1113/jphysiol.1981.sp013859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Baker R., Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974 May;37(3):560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Mühlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988 Oct;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Volkind R. A. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973 Aug 31;18(1):69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986 Jul;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y., Sugimori M. Isolated mammalian brain in vitro: new technique for analysis of electrical activity of neuronal circuit function. Fed Proc. 1981 Jun;40(8):2240–2245. [PubMed] [Google Scholar]
- Pegg D. E., Farrant J. Vascular resistance and edema in the isolated rabbit kidney perfused with a cell-free solution. Cryobiology. 1969 Nov-Dec;6(3):200–210. doi: 10.1016/s0011-2240(69)80350-0. [DOI] [PubMed] [Google Scholar]
- Ross G., White F. N., Brown A. W., Kolin A. Regional blood flow in the rat. J Appl Physiol. 1966 Jul;21(4):1273–1275. doi: 10.1152/jappl.1966.21.4.1273. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K., Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983 Nov 14;278(1-2):387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]
- Yarom Y., Llinás R. Long-term modifiability of anomalous and delayed rectification in guinea pig inferior olivary neurons. J Neurosci. 1987 Apr;7(4):1166–1177. doi: 10.1523/JNEUROSCI.07-04-01166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg W., Morgan C. D., Anderson D. E. Blood viscosity, erythrocyte sedimentation rate, packed cell volume, osmolality, and plasma viscosity of the Wistar rat. Lab Anim Sci. 1971 Oct;21(5):740–742. [PubMed] [Google Scholar]
- de Montigny C., Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973 Apr 13;53(1):81–95. doi: 10.1016/0006-8993(73)90768-3. [DOI] [PubMed] [Google Scholar]


