Abstract
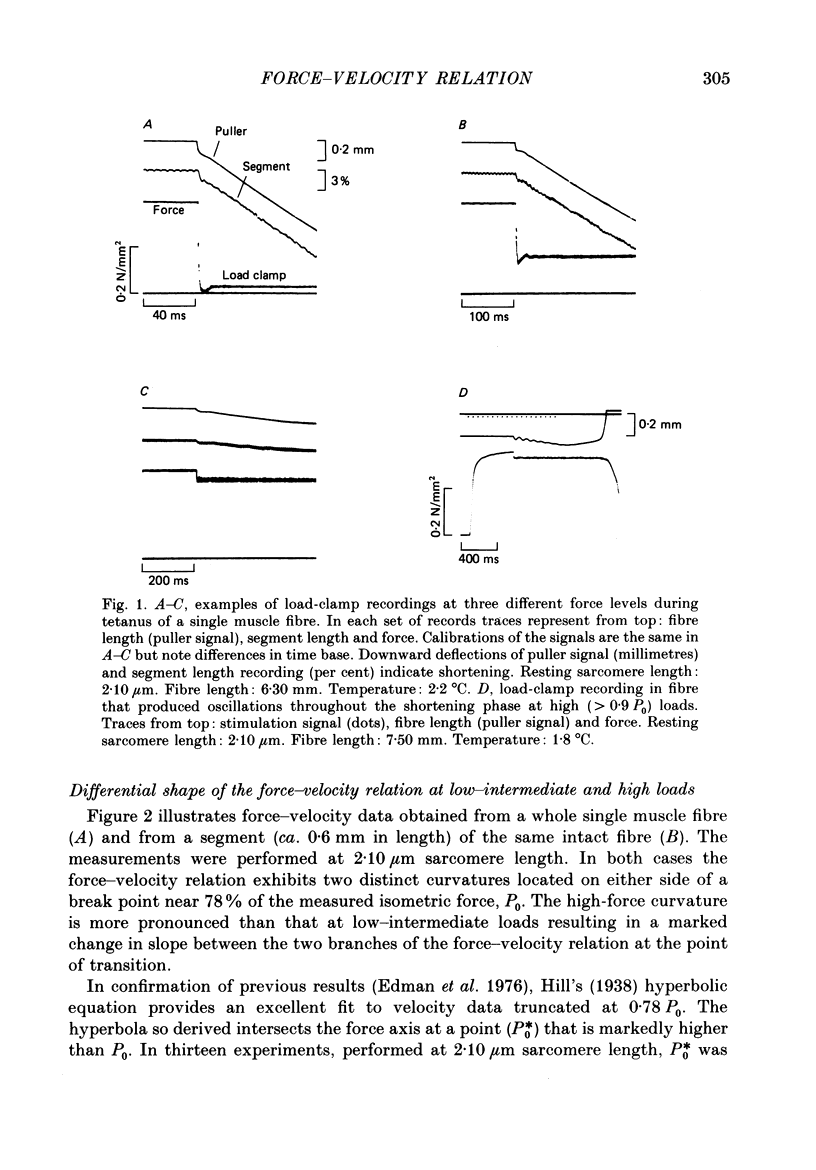
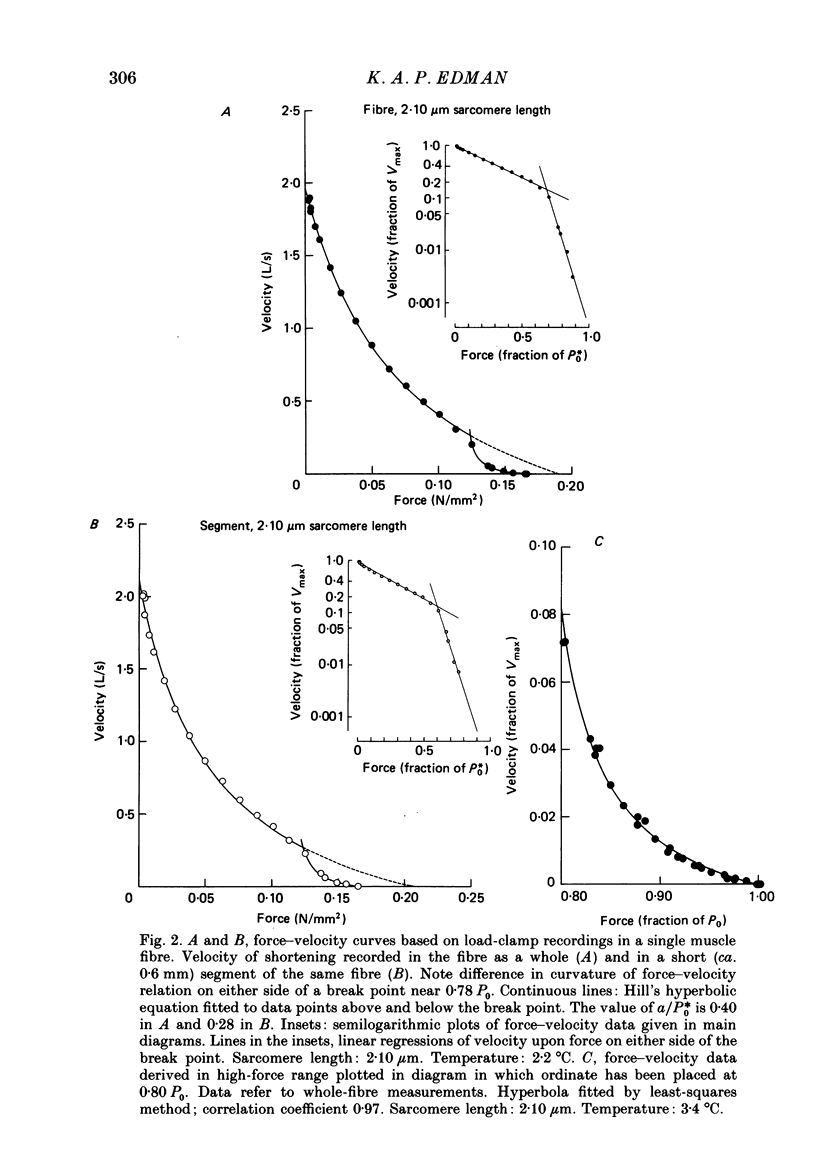
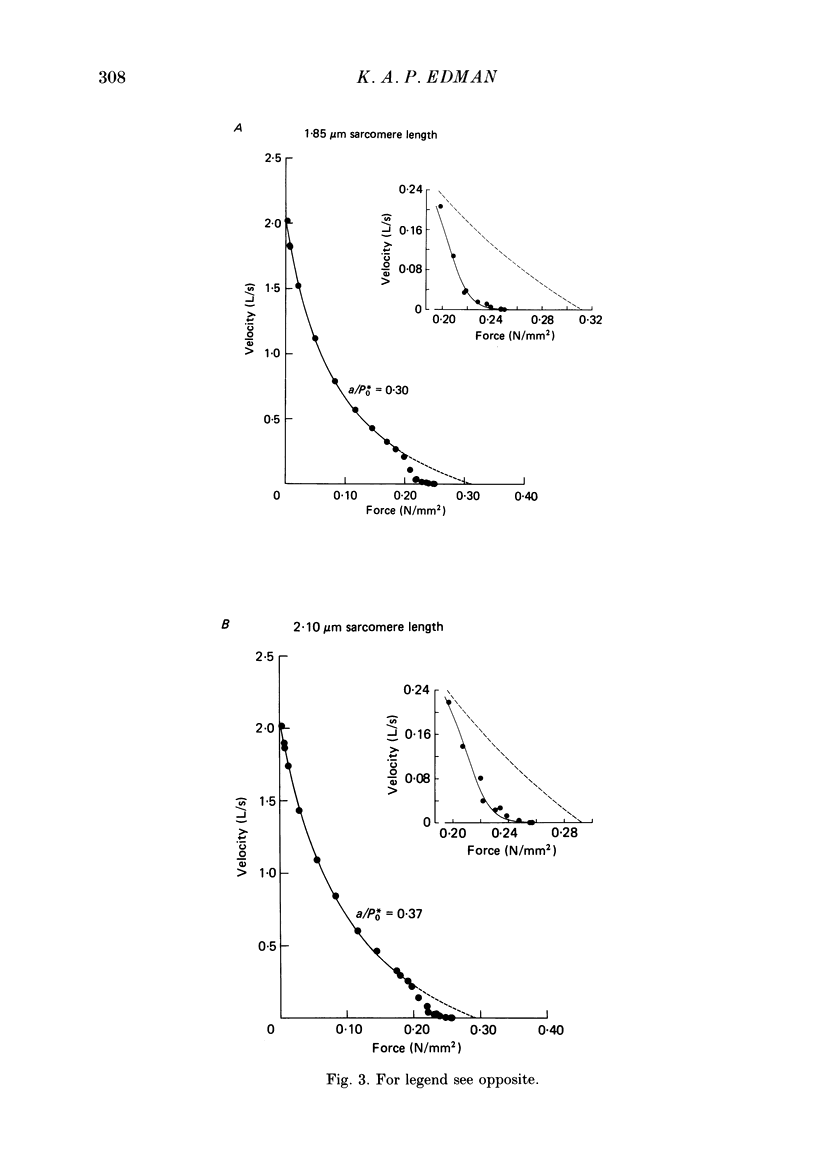
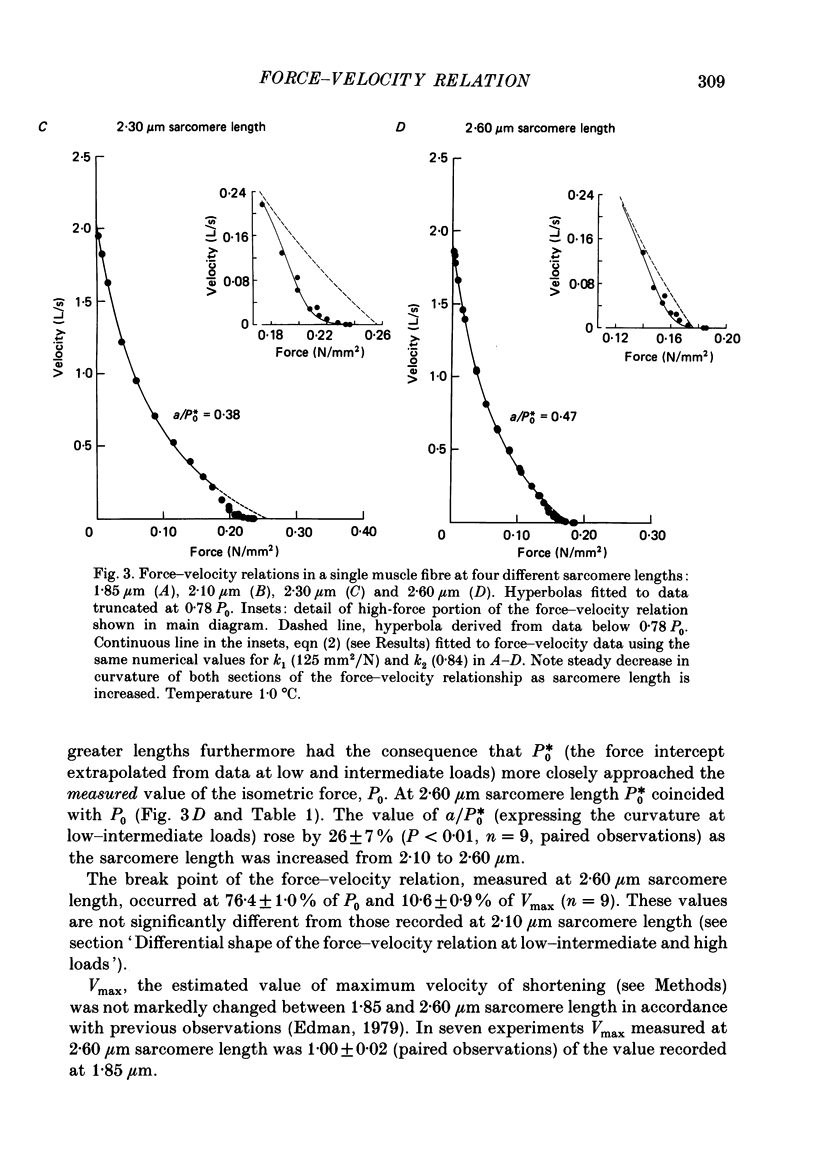
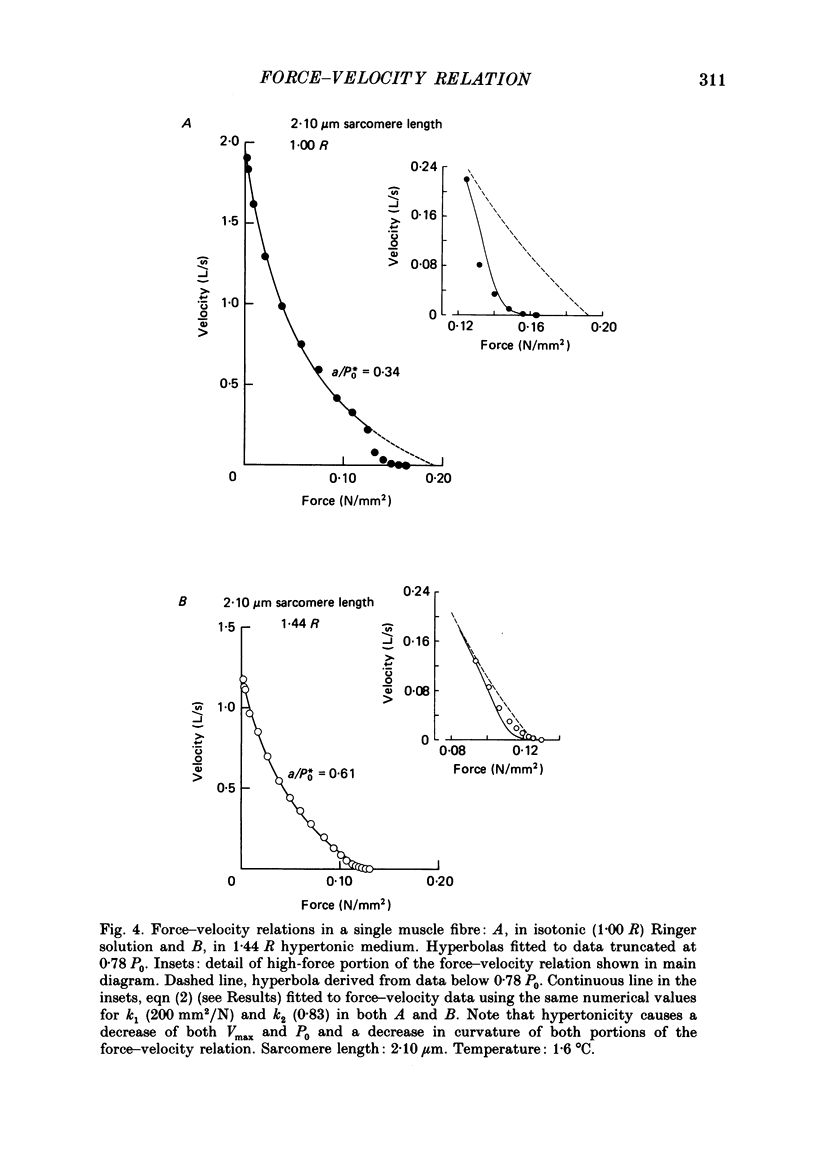
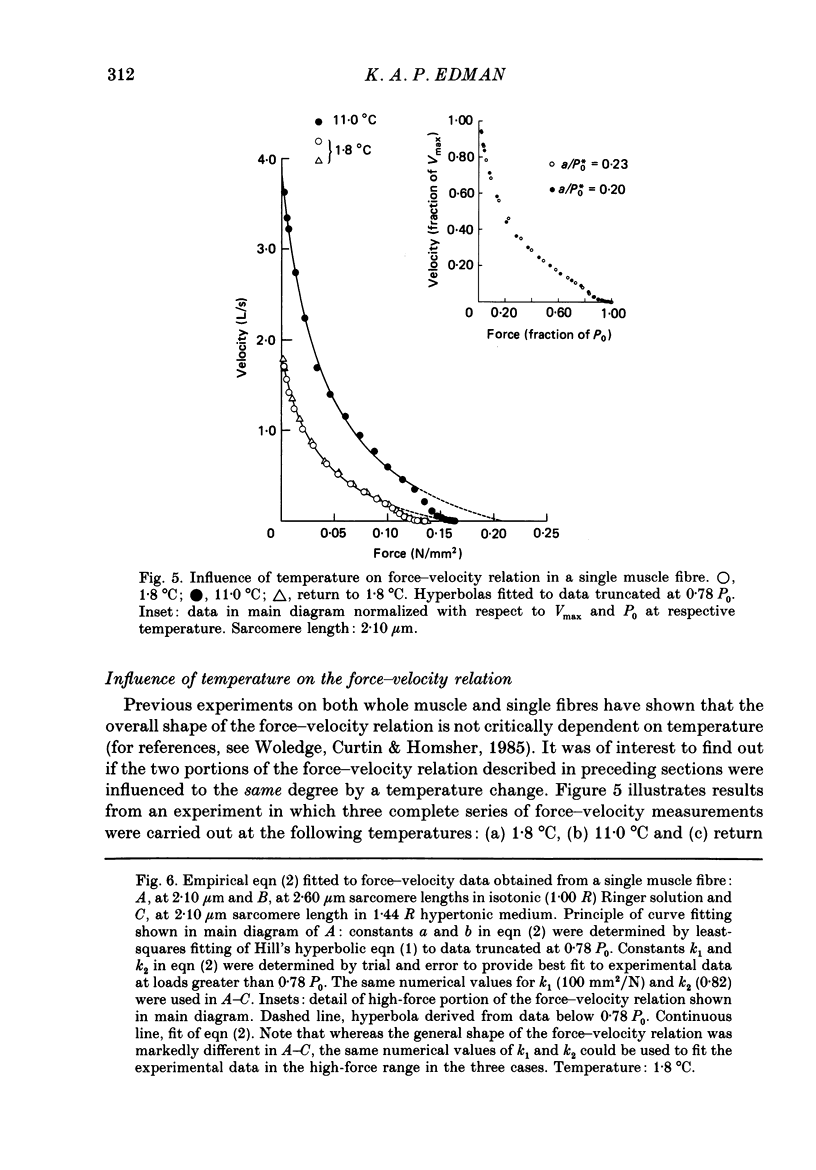
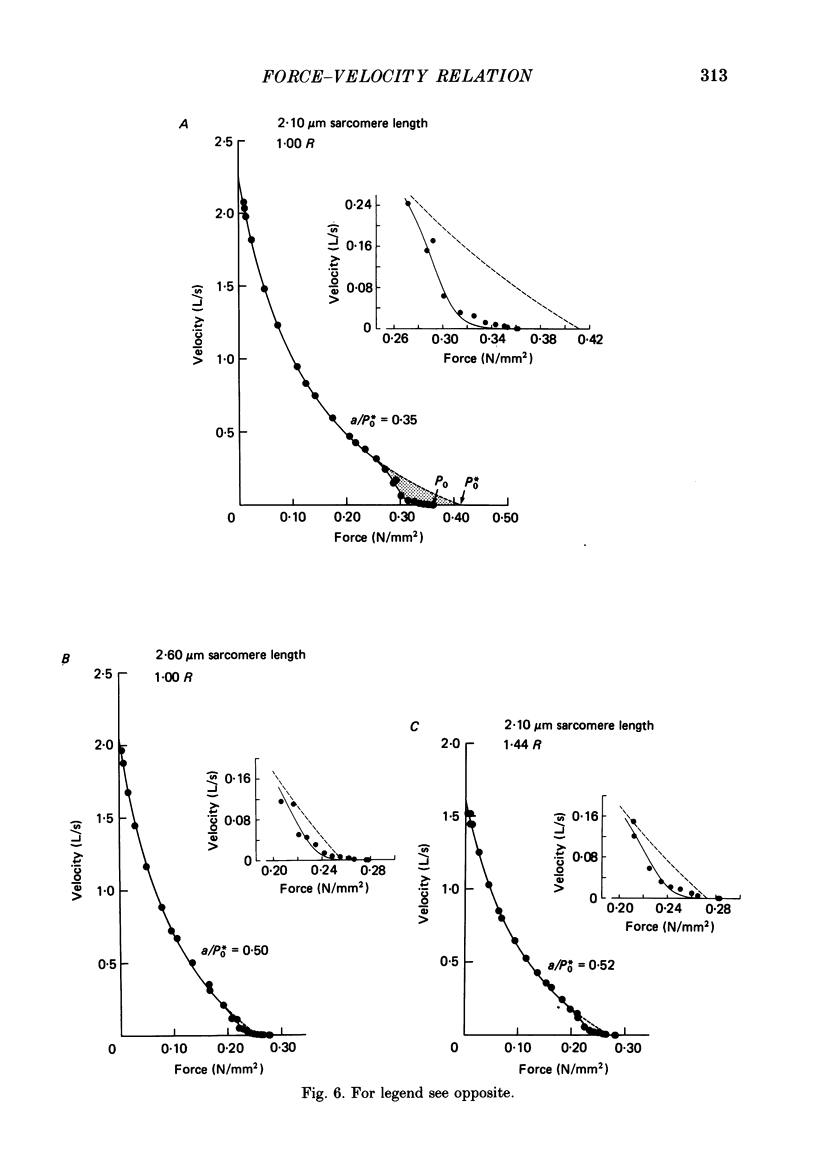
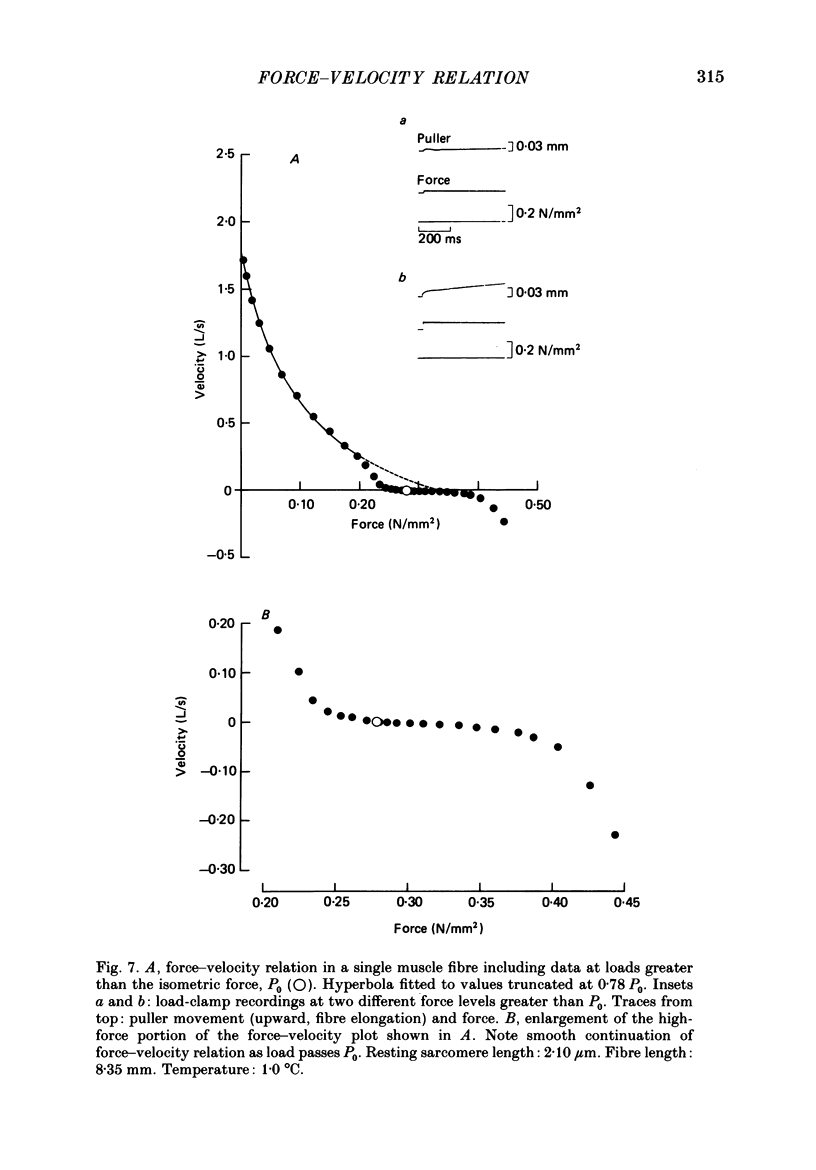
1. The relationship between force and velocity of shortening was studied at 2.10 micron sarcomere length during fused tetani (1-3 degrees C) in single fibres isolated from the anterior tibialis muscle of Rana temporaria. The speed of shortening was recorded from the whole fibre and, in some experiments, simultaneously from a short (ca. 0.6 mm) segment, while the preparation was released to shorten isotonically at selected force levels ('load-clamp' recording). The segment was defined by opaque markers of hair that were placed on the fibre surface. The distance between the markers was recorded by means of a photo-electric detector system. 2. The force-velocity relation had two distinct regions, each one exhibiting an upwards concave shape, that were located within the ranges 0-78 and 78-100% of the measured isometric force (P0), respectively. The two portions of the force-velocity relation could be fitted well by hyperbolic functions or by single-exponential functions. The curvature was more pronounced in the high-force region than at low-intermediate loads. The transition between the two portions of the force-velocity relation (the 'break point' of the force-velocity curve) occurred at 78.4 +/- 0.4% of P0 (mean +/- S.E. of mean, n = 12) corresponding to 10.9 +/- 0.4% of maximum velocity of shortening (Vmax). The general shape of the force-velocity curve, and the appearance of a break point near 78% of P0, was the same when measurements were made from the whole fibre and from a short segment along the same fibre. 3. The 'negative' branch of the force-velocity relation was delineated for loads ranging from P0 to 1.6-1.8 P0 in five experiments. The negative branch formed a smooth continuation of the force-velocity relation recorded between 0.78 P0 and P0. The force-velocity relation was nearly flat between 0.90 P0 and 1.20 P0, the difference in speed of shortening or elongation being 1.8 +/- 0.3% (mean +/- S.E. of mean, n = 5) of Vmax over this range. 4. An increase in sarcomere length from 1.85 to 2.60 micron did not affect Vmax but caused a steady decrease in curvature of the force-velocity relation, both at low-intermediate loads and in the high-force range. Similar changes in shape of the force-velocity relation were produced by osmotic compression of the fibre in a Ringer solution made hypertonic by addition of 98 mM-sucrose.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Critical sarcomere extension required to recruit a decaying component of extra force during stretch in tetanic contractions of frog skeletal muscle fibers. J Gen Physiol. 1981 Oct;78(4):365–382. doi: 10.1085/jgp.78.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., te Kronnie G. Differences in maximum velocity of shortening along single muscle fibres of the frog. J Physiol. 1985 Aug;365:147–163. doi: 10.1113/jphysiol.1985.sp015764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. A cross-bridge model of muscle contraction. Prog Biophys Mol Biol. 1978;33(1):55–82. doi: 10.1016/0079-6107(79)90025-7. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Lowy J., Millman B. M. Low-angle x-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967 Apr 14;25(1):31–45. doi: 10.1016/0022-2836(67)90277-x. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Marsh B. S. Muscular force at different speeds of shortening. J Physiol. 1935 Nov 22;85(3):277–297. doi: 10.1113/jphysiol.1935.sp003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Cross-bridge detachment and sarcomere 'give' during stretch of active frog's muscle. J Physiol. 1978 Mar;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E. Measurement of sarcomere shortening in skinned fibers from frog muscle by white light diffraction. Biophys J. 1987 Jul;52(1):57–68. doi: 10.1016/S0006-3495(87)83188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J. 1987 Jul;52(1):127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., WILKIE D. R. The dynamics of muscular contraction. J Physiol. 1958 Aug 29;143(1):104–113. doi: 10.1113/jphysiol.1958.sp006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H., Tsuchiya T. Isotonic velocity transients in frog muscle fibres following quick changes in load. J Physiol. 1981;319:219–238. doi: 10.1113/jphysiol.1981.sp013903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T. Passive interaction between sliding filaments in the osmotically compressed skinned muscle fibers of the frog. Biophys J. 1988 Mar;53(3):415–423. doi: 10.1016/S0006-3495(88)83118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


