Abstract
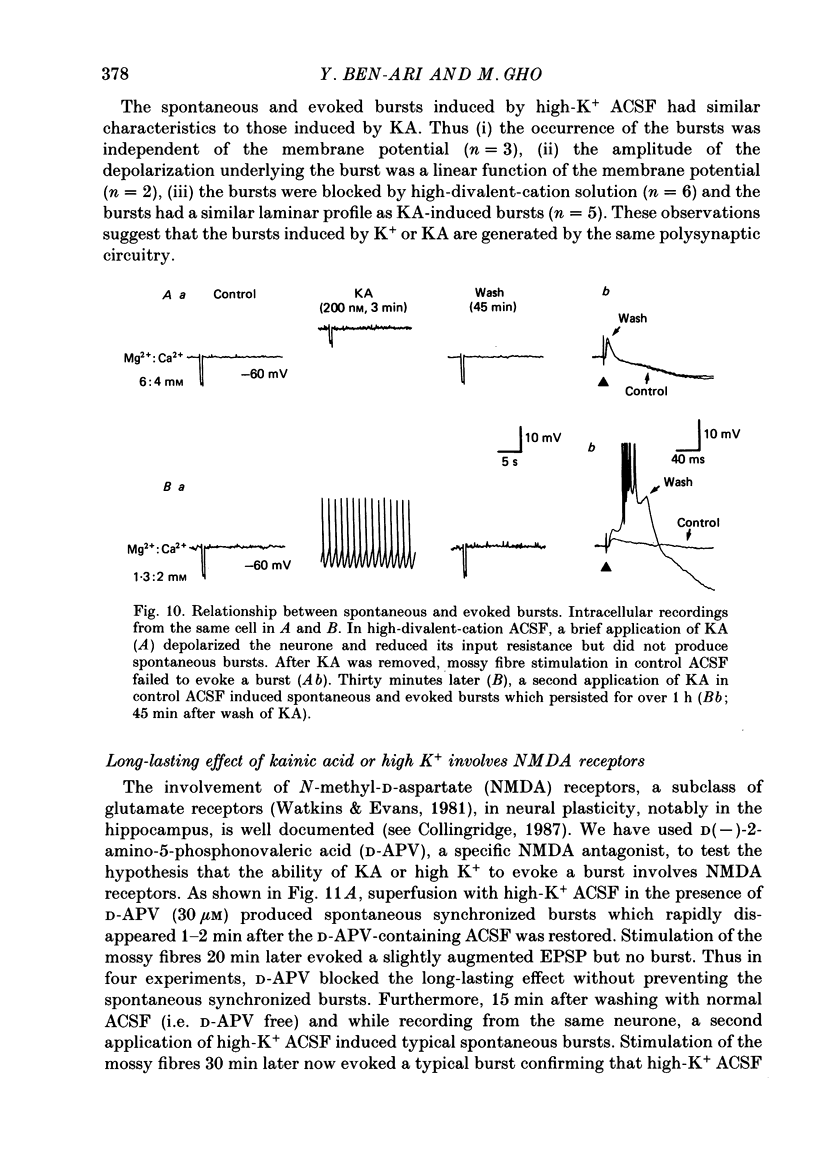
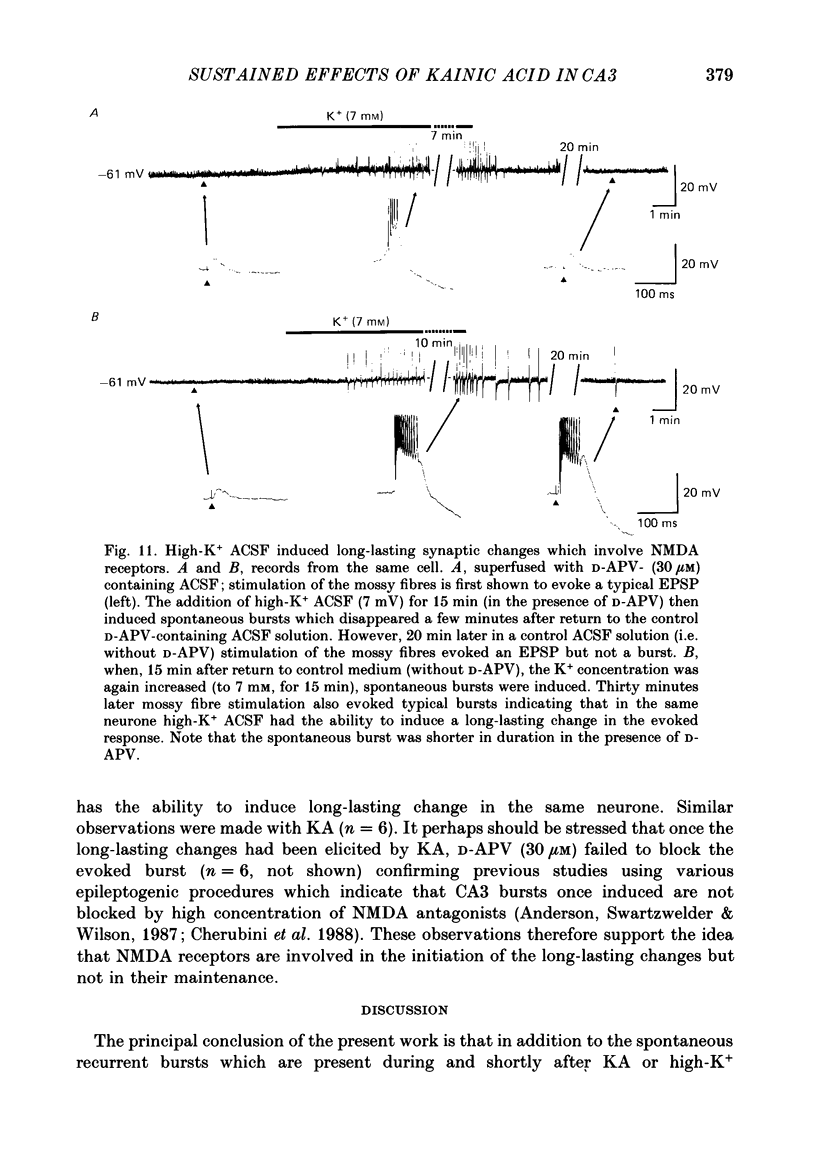
1. The action of a short bath application of kainic acid (KA, 200-250 nM, 3-5 min) on the CA3 region of rat hippocampal slices has been studied with intracellular and extracellular recording techniques. 2. KA evoked bursts which persisted for 10-15 min. In addition, after KA, electrical stimulation of various inputs to CA3 which elicited an EPSP-IPSP sequence in control conditions evoked an EPSP followed by a burst. This evoked response persisted for several hours after removal of KA suggesting the occurrence of a long-lasting modification of the synaptic properties of CA3 neurones. 3. Intracellular recordings showed the spontaneous and evoked bursts to consist of five to ten action potentials riding on a depolarizing shift 10-25 mV in amplitude and 40-100 ms in duration. Both spontaneous and evoked bursts were followed by a long-lasting hyperpolarization 15-25 mV in amplitude and 1-1.5 s in duration. 4. We propose that both spontaneous and evoked synchronized bursts are generated by a polysynaptic network since: (a) intracellularly recorded bursts were synchronized with the bursts in extracellular field recording; (b) bursts disappeared when synaptic transmission or Na+ action potential were blocked by cobalt (1 mM) or TTX (1 microM) respectively; (c) bursts were suppressed by elevated divalent cation concentration; (d) burst occurrence was independent of the membrane potential of the cell; (e) the depolarization shift that underlies the bursts was a linear function of the membrane potential and reversed in polarity at 0 mV. In addition, the evoked bursts were all-or-none events with a variable latency. 5. Laminar profile analysis of the spontaneous and evoked bursts suggests that they were generated by synapses located on the distal apical segments of the dendrites of CA3 pyramidal cells. 7. The persistence of the evoked bursts was neither due to a persistent change in cell excitability nor to a long-lasting reduction in GABAergic synaptic inhibition. 8. Bath application of a high concentration of potassium (7 mM) also induced spontaneous and evoked bursts; the latter also persisted several hours after return to control medium. 9. The N-methyl-D-aspartate (NMDA) antagonist, D-APV (D(-)-2-amino-5-phosphonovaleric acid) (30 microM), did not block the spontaneous discharges induced by KA or high potassium, but prevented the long-lasting effects on the synaptic responses.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


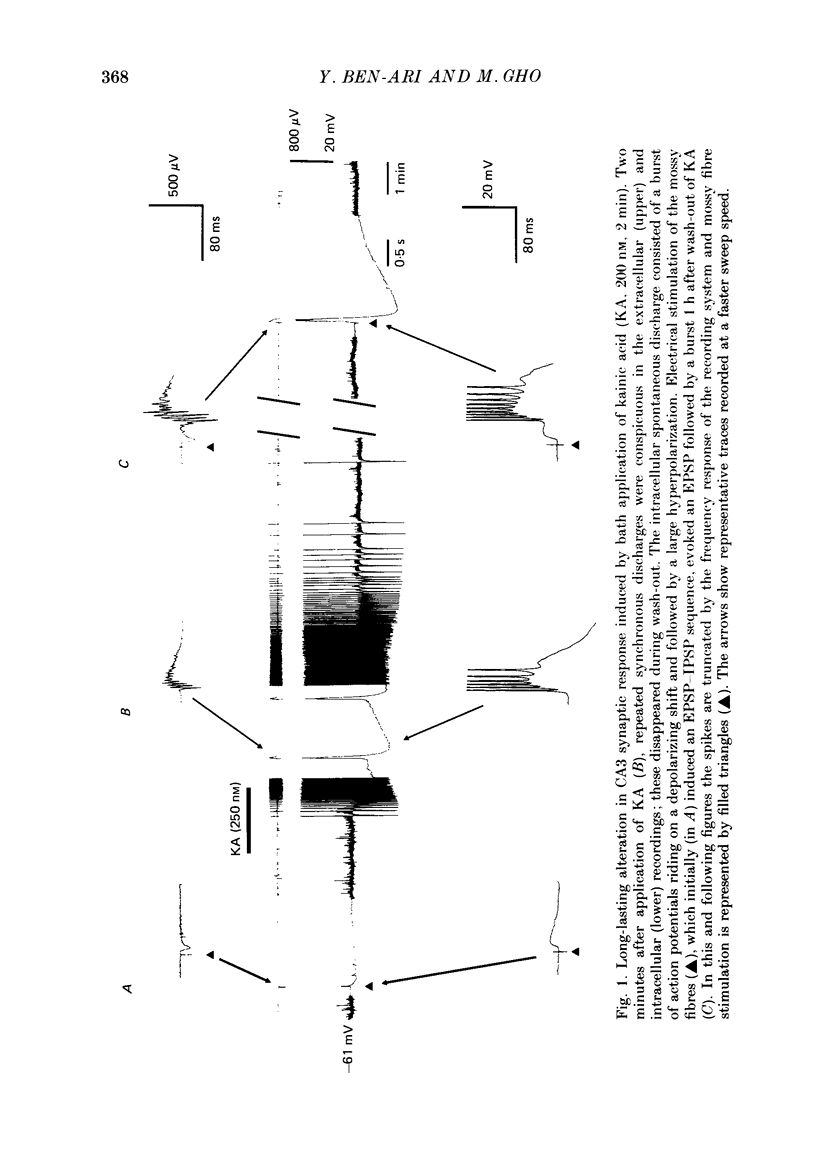

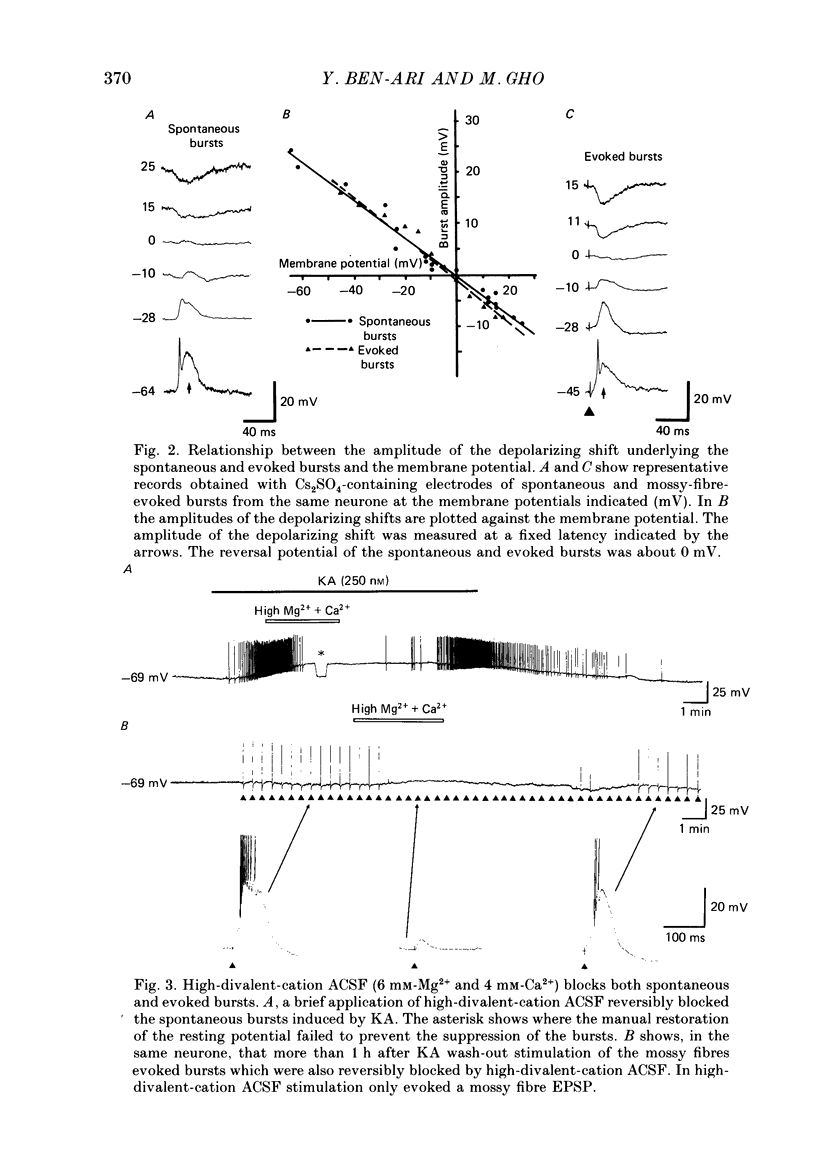
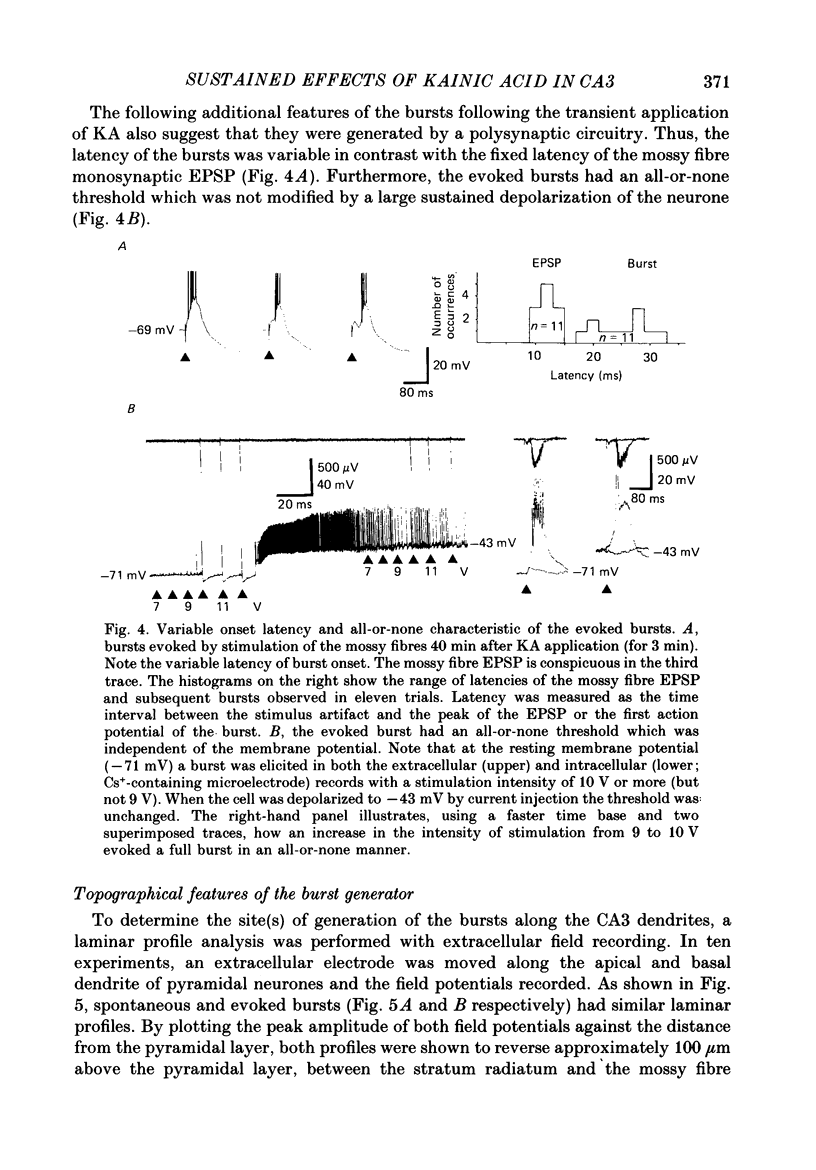
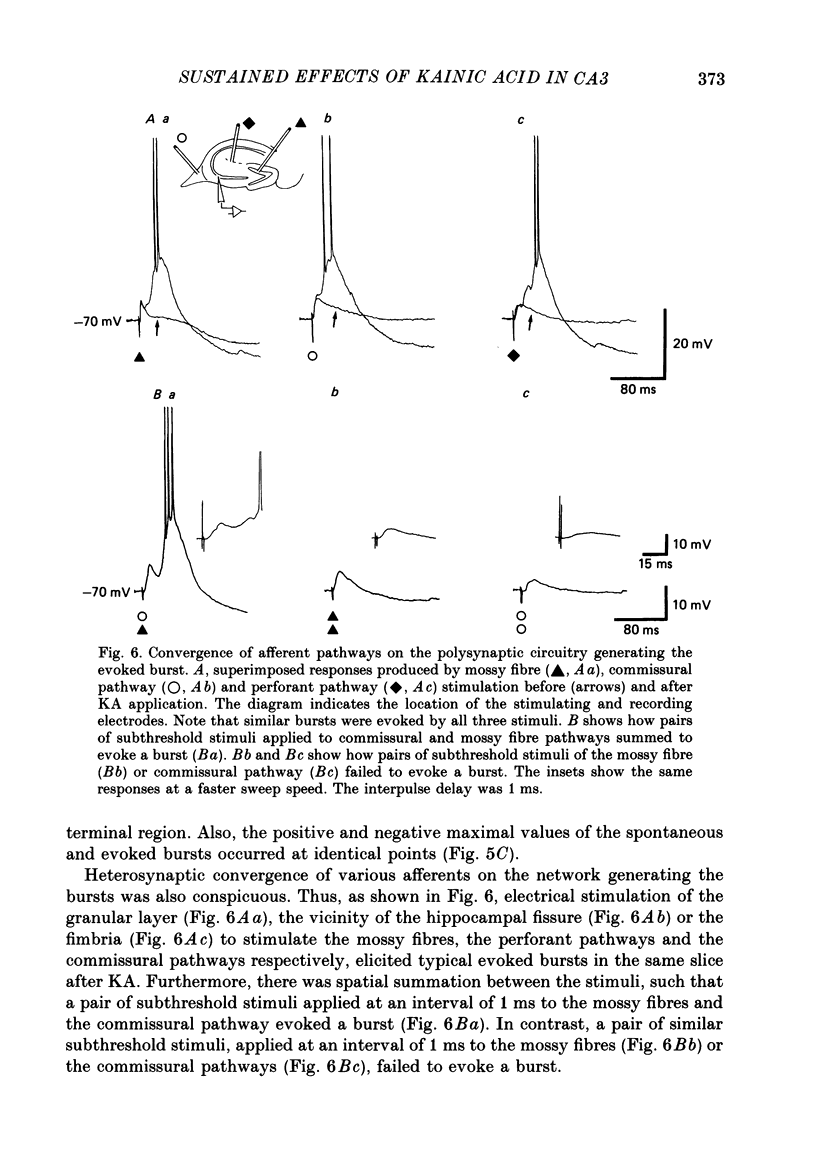
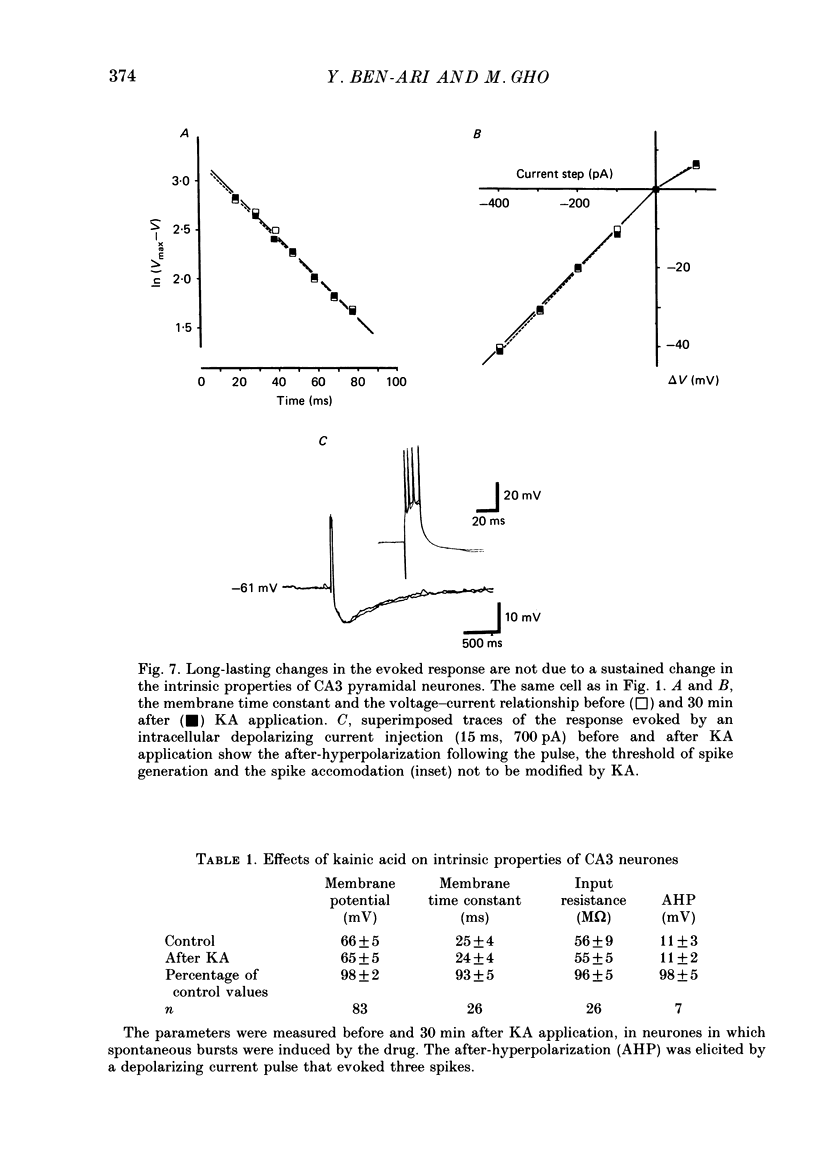

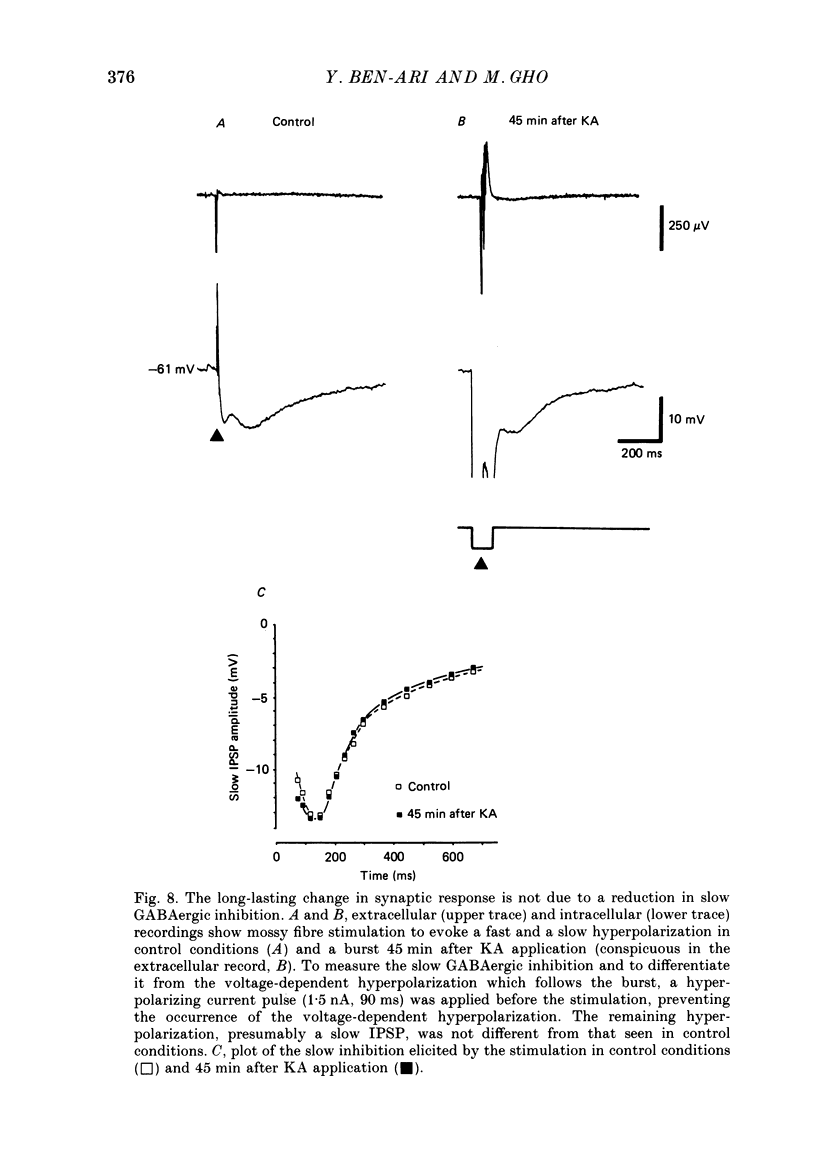
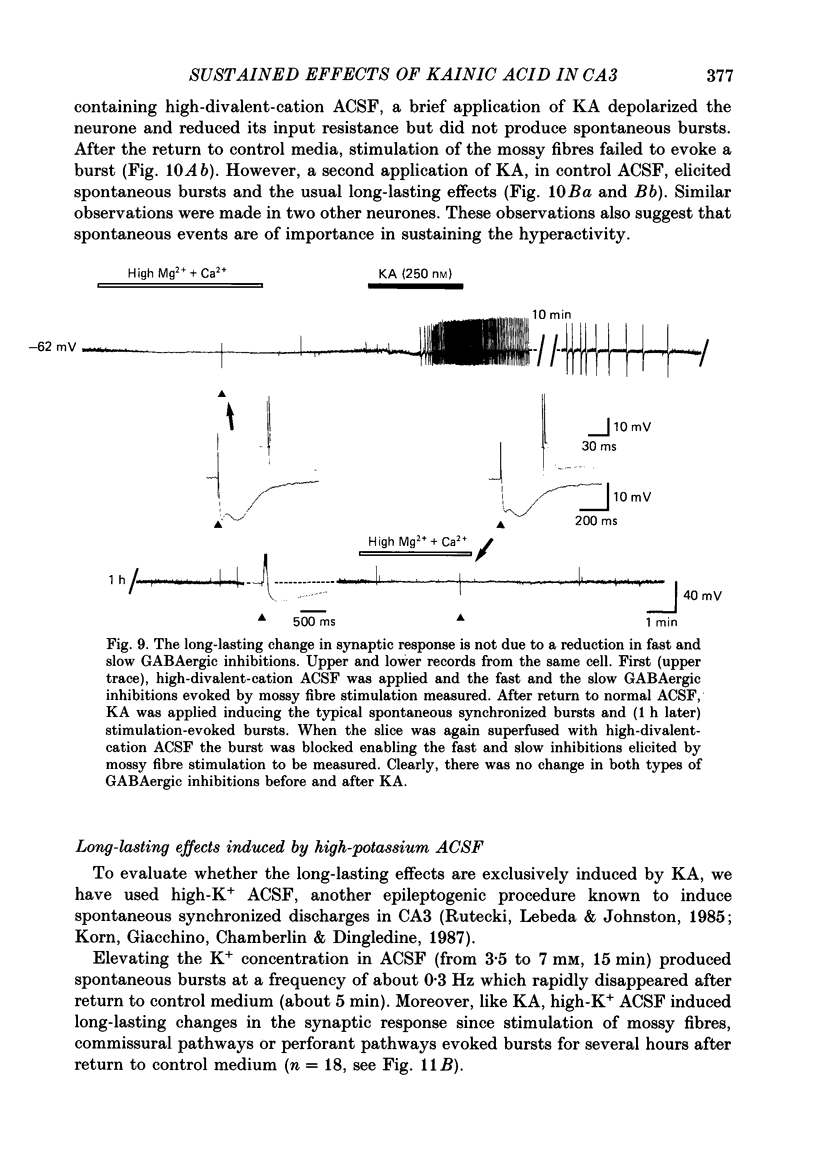





Selected References
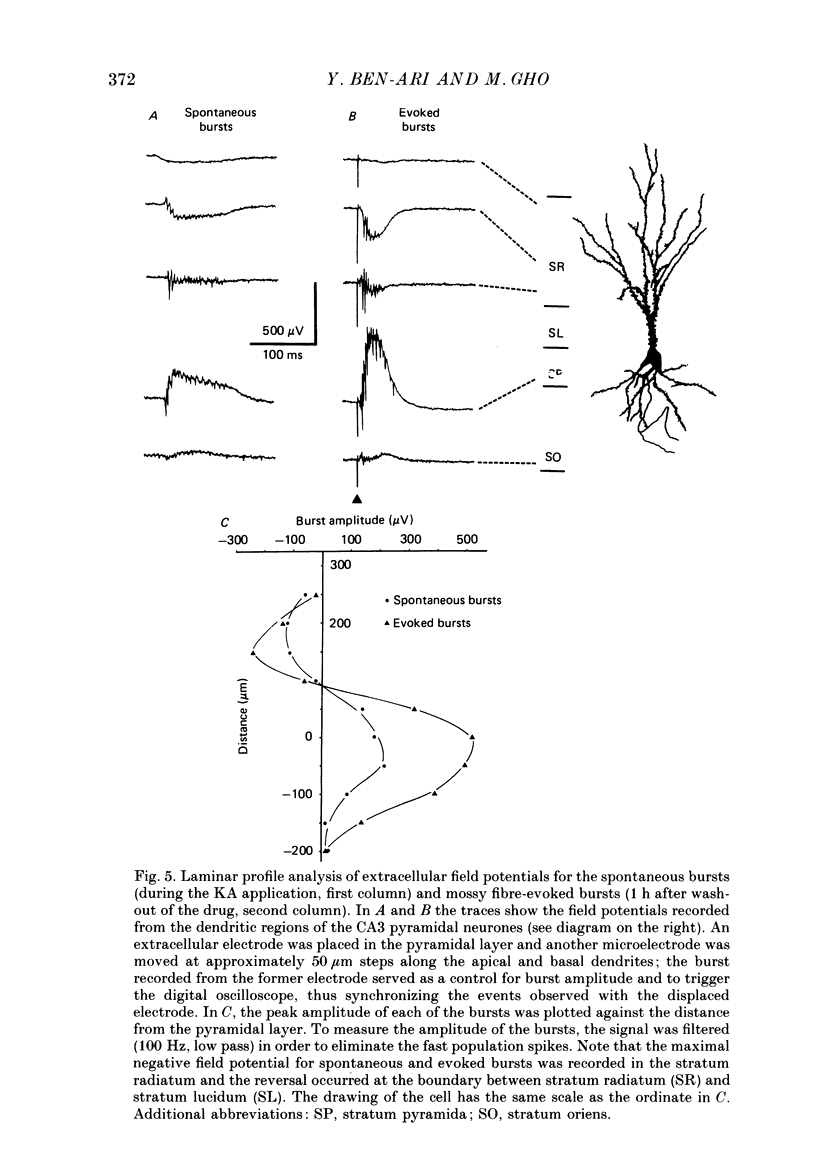
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Gustafsson B., Wigström H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol. 1987 Dec;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1984 Nov;52(5):892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. W., Swartzwelder H. S., Wilson W. A. The NMDA receptor antagonist 2-amino-5-phosphonovalerate blocks stimulus train-induced epileptogenesis but not epileptiform bursting in the rat hippocampal slice. J Neurophysiol. 1987 Jan;57(1):1–21. doi: 10.1152/jn.1987.57.1.1. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985 Feb;14(2):375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Bingmann D., Speckmann E. J. Actions of pentylenetetrazol (PTZ) on CA3 neurons in hippocampal slices of guinea pigs. Exp Brain Res. 1986;64(1):94–104. doi: 10.1007/BF00238204. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Johnston D. Voltage-clamp analysis of mossy fiber synaptic input to hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):487–507. doi: 10.1152/jn.1983.50.2.487. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Neuman R., Rovira C., Ben Ari Y. Epileptogenic properties of the mast cell degranulating peptide in CA3 hippocampal neurones. Brain Res. 1988 Mar 29;445(1):91–100. doi: 10.1016/0006-8993(88)91077-3. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Rovira C., Gho M., Ben-Ari Y. Effects of kainate on CA1 hippocampal neurons recorded in vitro. Adv Exp Med Biol. 1986;203:475–484. doi: 10.1007/978-1-4684-7971-3_36. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., Loo R., McLennan H. Effects of kainic and other amino acids on synaptic excitation in rat hippocampal slices: 1. Extracellular analysis. Exp Brain Res. 1983;52(2):170–178. doi: 10.1007/BF00236625. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. Synaptic plasticity. The role of NMDA receptors in learning and memory. Nature. 1987 Dec 17;330(6149):604–605. doi: 10.1038/330604a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Alger B. E. Electrophysiological mechanisms of kainic acid-induced epileptiform activity in the rat hippocampal slice. J Neurosci. 1984 May;4(5):1312–1323. doi: 10.1523/JNEUROSCI.04-05-01312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN J. D. THE HIPPOCAMPUS. Physiol Rev. 1964 Oct;44:561–608. doi: 10.1152/physrev.1964.44.4.561. [DOI] [PubMed] [Google Scholar]
- Gho M., King A. E., Ben-Ari Y., Cherubini E. Kainate reduces two voltage-dependent potassium conductances in rat hippocampal neurons in vitro. Brain Res. 1986 Oct 22;385(2):411–414. doi: 10.1016/0006-8993(86)91093-0. [DOI] [PubMed] [Google Scholar]
- Griffith W. H., Brown T. H., Johnston D. Voltage-clamp analysis of synaptic inhibition during long-term potentiation in hippocampus. J Neurophysiol. 1986 Apr;55(4):767–775. doi: 10.1152/jn.1986.55.4.767. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Johnston D. Endogenous nature of spontaneous bursting in hippocampal pyramidal neurons. Cell Mol Neurobiol. 1981 Dec;1(4):325–334. doi: 10.1007/BF00716267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J., Thalmann R. H. Conductance changes underlying a late synaptic hyperpolarization in hippocampal CA3 neurons. J Neurophysiol. 1987 Jul;58(1):160–179. doi: 10.1152/jn.1987.58.1.160. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Johnston D. Passive cable properties of hippocampal CA3 pyramidal neurons. Cell Mol Neurobiol. 1981 Mar;1(1):41–55. doi: 10.1007/BF00736038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Giacchino J. L., Chamberlin N. L., Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987 Jan;57(1):325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO H., MARSAN C. A. CORTICAL CELLULAR PHENOMENA IN EXPERIMENTAL EPILEPSY: INTERICTAL MANIFESTATIONS. Exp Neurol. 1964 Apr;9:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980 Feb 17;184(1):220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987 Oct 22;329(6141):724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983 Nov 24;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985 Jan;65(1):37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney J. W., Rhee V., Ho O. L. Kainic acid: a powerful neurotoxic analogue of glutamate. Brain Res. 1974 Sep 13;77(3):507–512. doi: 10.1016/0006-8993(74)90640-4. [DOI] [PubMed] [Google Scholar]
- Represa A., Tremblay E., Schoevart D., Ben-Ari Y. Development of high affinity kainate binding sites in human and rat hippocampi. Brain Res. 1986 Oct 1;384(1):170–174. doi: 10.1016/0006-8993(86)91234-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. H., Deadwyler S. A. Kainic acid produces depolarization of CA3 pyramidal cells in the vitro hippocampal slice. Brain Res. 1981 Sep 21;221(1):117–127. doi: 10.1016/0006-8993(81)91067-2. [DOI] [PubMed] [Google Scholar]
- Rutecki P. A., Lebeda F. J., Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985 Nov;54(5):1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975 May 16;89(1):107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Bragdon A. C., Wilson W. A. Induction of epileptiform activity in hippocampal slices by trains of electrical stimuli. Brain Res. 1985 Oct 7;344(2):296–302. doi: 10.1016/0006-8993(85)90807-8. [DOI] [PubMed] [Google Scholar]
- Swann J. W., Brady R. J., Friedman R. J., Smith E. J. The dendritic origins of penicillin-induced epileptogenesis in CA3 hippocampal pyramidal cells. J Neurophysiol. 1986 Dec;56(6):1718–1738. doi: 10.1152/jn.1986.56.6.1718. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982 May 14;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Wamsley J. K. Autoradiographic localization of high-affinity [3H]kainic acid binding sites in the rat forebrain. Eur J Pharmacol. 1983 Jan 21;86(3-4):361–371. doi: 10.1016/0014-2999(83)90185-1. [DOI] [PubMed] [Google Scholar]
- Walther H., Lambert J. D., Jones R. S., Heinemann U., Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986 Aug 29;69(2):156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Lothman E. W. Cellular and synaptic basis of kainic acid-induced hippocampal epileptiform activity. Brain Res. 1983 Aug 22;273(1):97–109. doi: 10.1016/0006-8993(83)91098-3. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Traub R. D. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2-CA3 region. J Neurophysiol. 1983 Feb;49(2):442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]


