Abstract
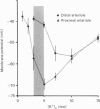
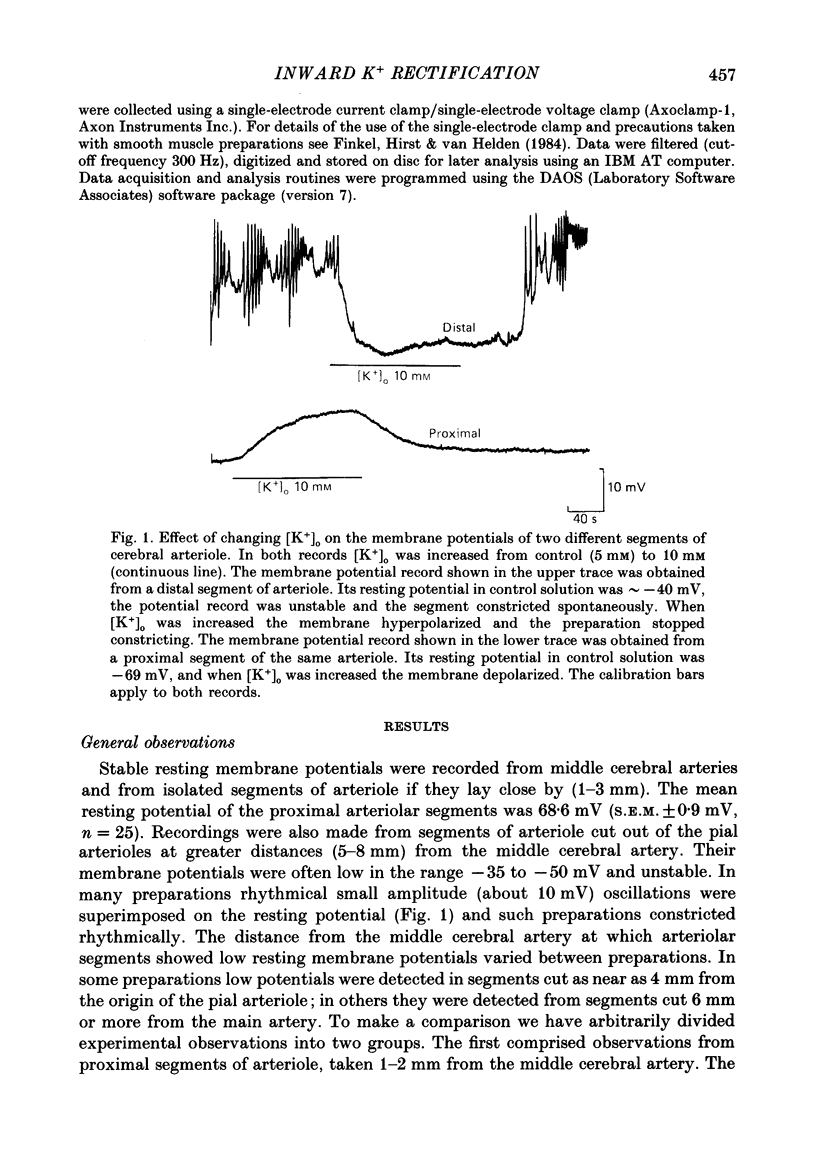
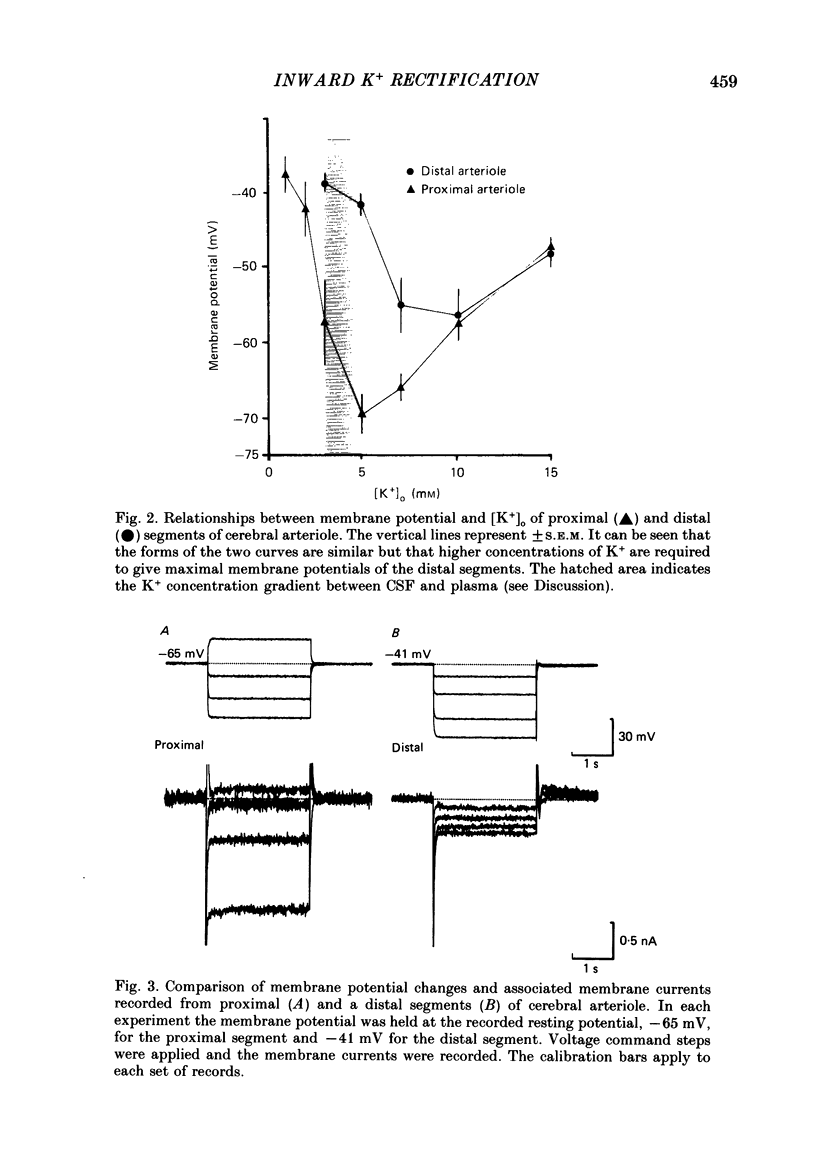
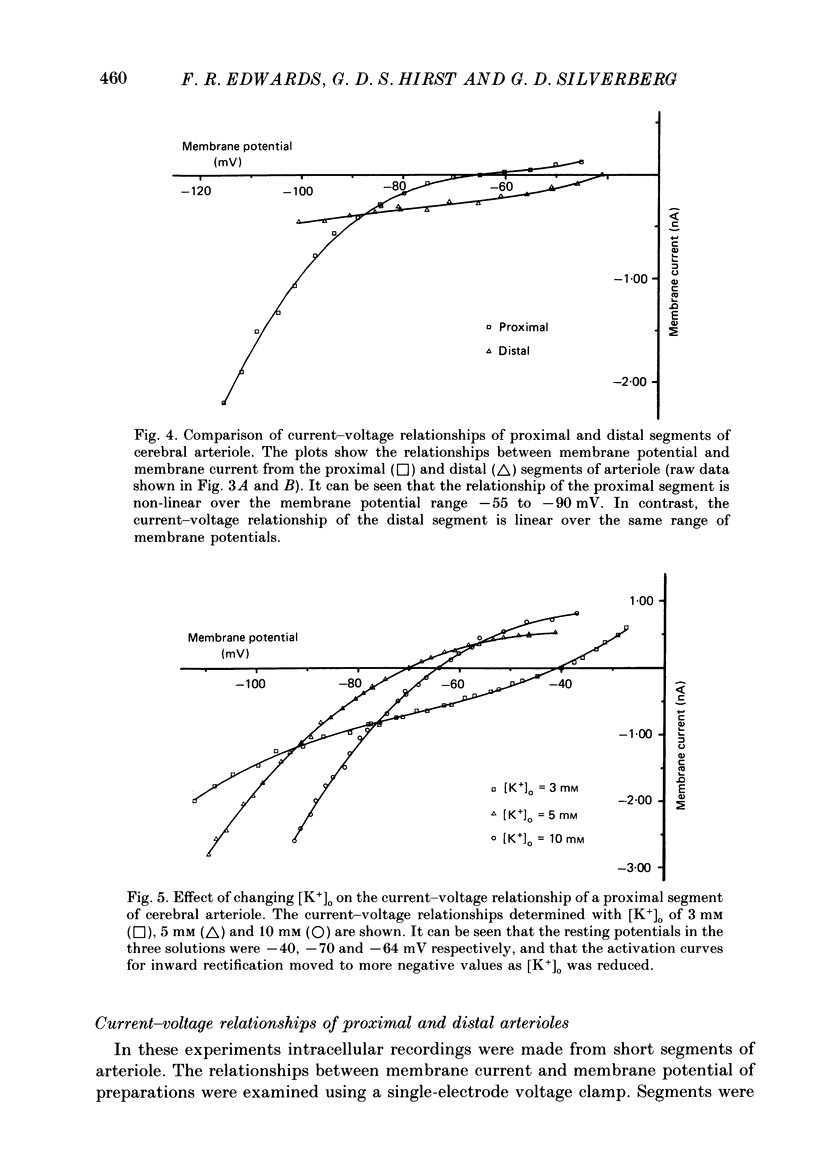
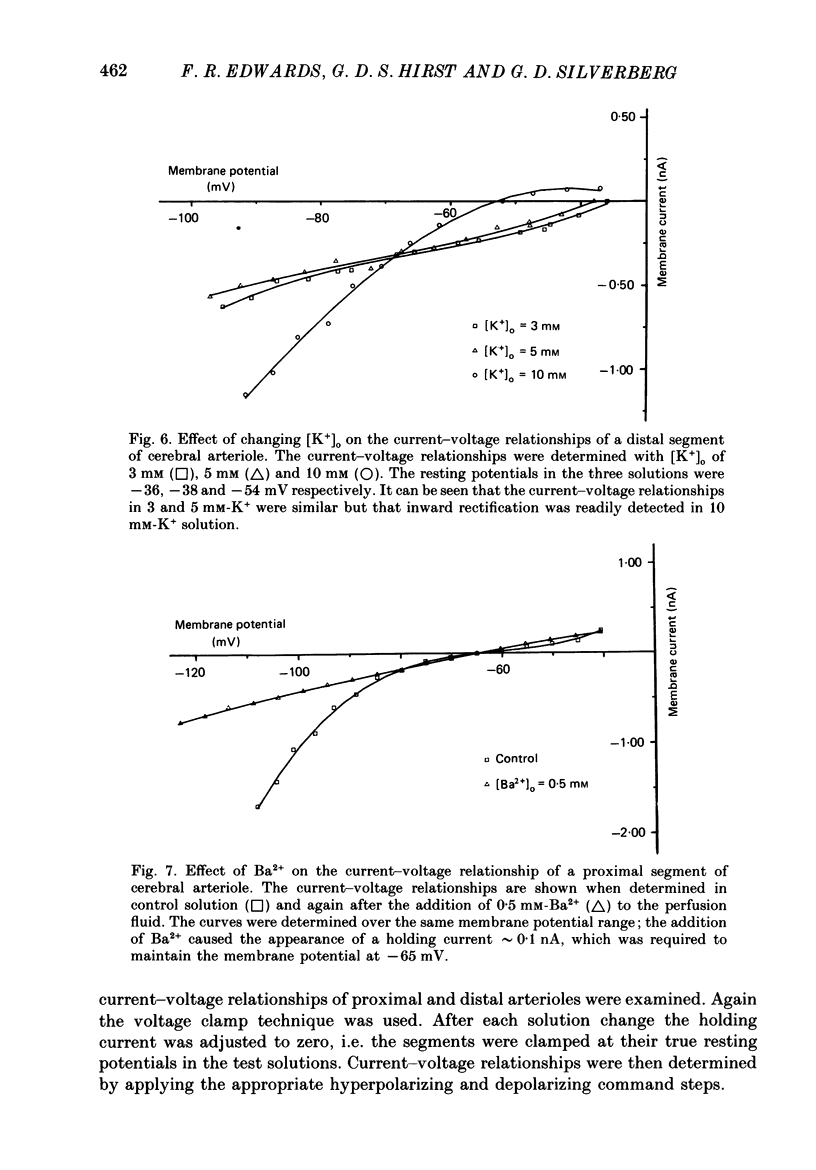
1. The resting membrane potentials of proximal and distal segments of the arterioles which arise from the rat middle cerebral artery were determined. Proximal segments had stable membrane potentials with a mean value of -69 mV. The membrane potentials of distal segments were less negative and often unstable. 2. When the extracellular concentration of potassium ions [( K+]o) was increased proximal segments of arteriole were depolarized whereas distal ones were hyperpolarized. When [K+]o was decreased both proximal and distal segments were depolarized, the changes being more marked in proximal arterioles. 3. The membranes of proximal segments of arteriole displayed inward rectification at potentials near rest; inward rectification in distal segments of arteriole, when detected, was less pronounced. 4. The activation curve for inward rectification in proximal segments of arteriole was changed by changing the extracellular concentration of K+. A reduction in [K+]o caused the activation curve to move to such negative potentials that the inward rectifier no longer contributed to the resting conductance. 5. Increasing [K+]o changed the activation curve for inward rectification in distal segments of arteriole so that more K+ current flowed at potentials near resting. At the same time the membrane potential hyperpolarized. 6. The results are discussed in relation to autoregulatory changes which occur following changes in the K+ concentration of cerebrospinal fluid.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtorp O., Sorensen S. C. The ontogenetic development of concentration differences for protein and ions between plasma and cerebrospinal fluid in rabbits and rats. J Physiol. 1974 Dec;243(2):387–400. doi: 10.1113/jphysiol.1974.sp010759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988 Oct;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Hirst G. D., Van Helden D. F. Some properties of excitatory junction currents recorded from submucosal arterioles of guinea-pig ileum. J Physiol. 1984 Jun;351:87–98. doi: 10.1113/jphysiol.1984.sp015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U., Lux H. D., Gutnick M. J. Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977 Mar 30;27(3-4):237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., Silverberg G. D., van Helden D. F. Sympathetic innervation and excitability of arterioles originating from the rat middle cerebral artery. J Physiol. 1986 Feb;371:305–316. doi: 10.1113/jphysiol.1986.sp015976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silverberg G. D., van Helden D. F. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986 Feb;371:289–304. doi: 10.1113/jphysiol.1986.sp015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., van Helden D. F. Ionic basis of the resting potential of submucosal arterioles in the ileum of the guinea-pig. J Physiol. 1982 Dec;333:53–67. doi: 10.1113/jphysiol.1982.sp014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Keep R. F. The control of potassium concentration in the cerebrospinal fluid and brain interstitial fluid of developing rats. J Physiol. 1987 Feb;383:441–453. doi: 10.1113/jphysiol.1987.sp016419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W., Wahl M., Bosse O., Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res. 1972 Aug;31(2):240–247. doi: 10.1161/01.res.31.2.240. [DOI] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercombe R., Lacombe P., Aubineau P., Mamo H., Pinard E., Reynier-Rebuffel A. M., Seylaz J. Is there an active mechanism limiting the influence of the sympathetic system on the cerebral vascular bed? Evidence for vasomotor escape from sympathetic stimulation in the rabbit. Brain Res. 1979 Mar 23;164:81–102. doi: 10.1016/0006-8993(79)90008-8. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M., Kuschinsky W. The dilatatory action of adenosine on pial arteries of cats and its inhibition by theophylline. Pflugers Arch. 1976 Mar 11;362(1):55–59. doi: 10.1007/BF00588681. [DOI] [PubMed] [Google Scholar]