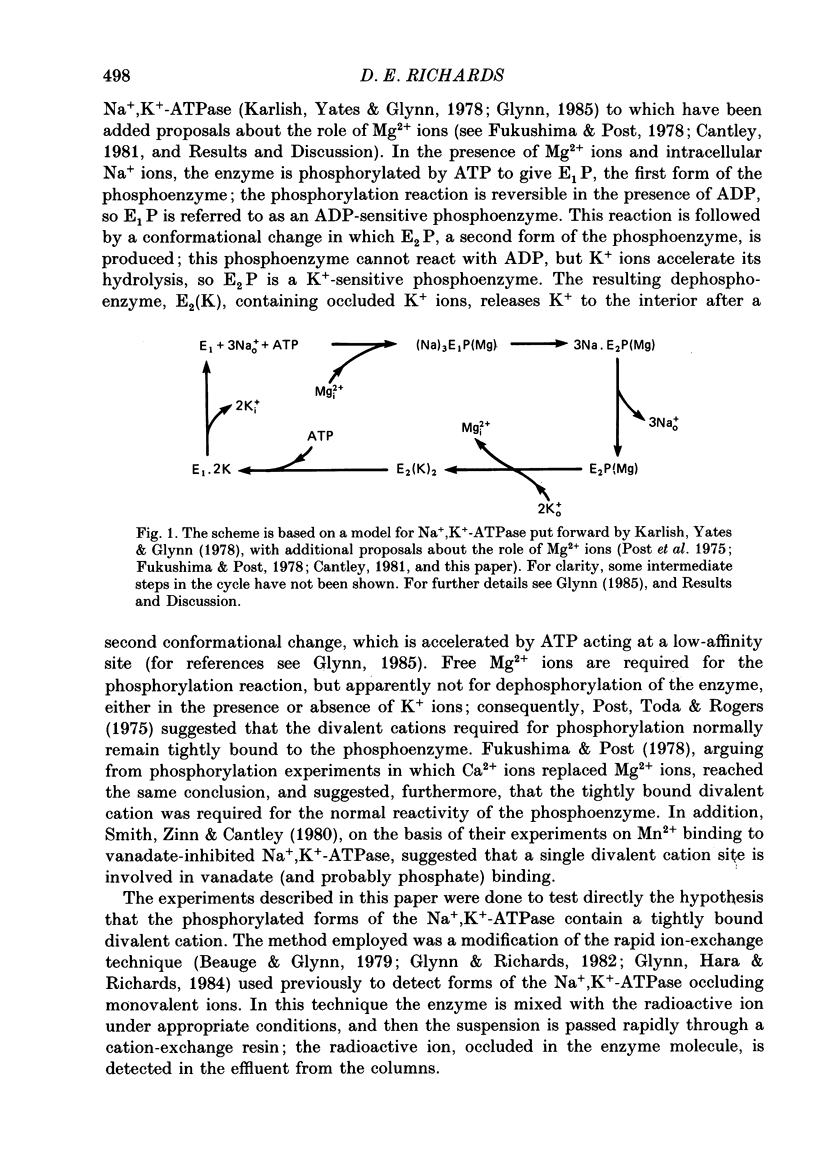
Abstract
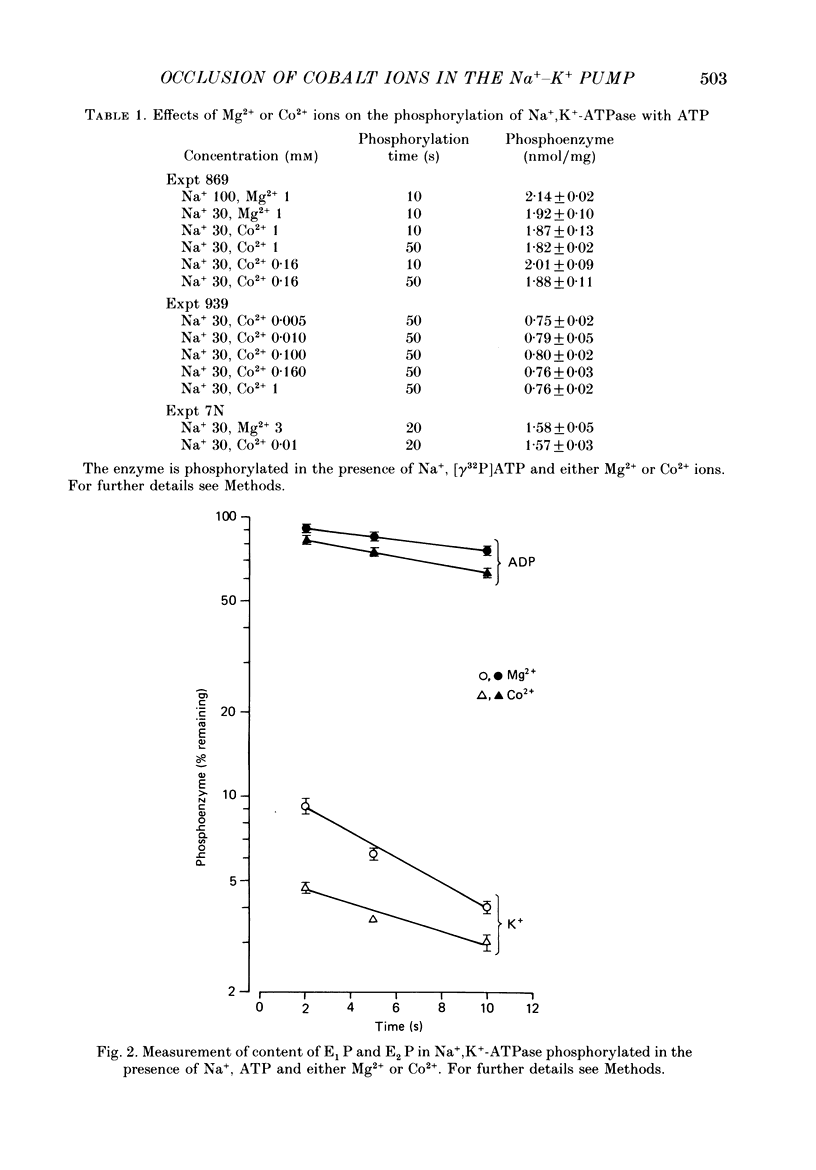
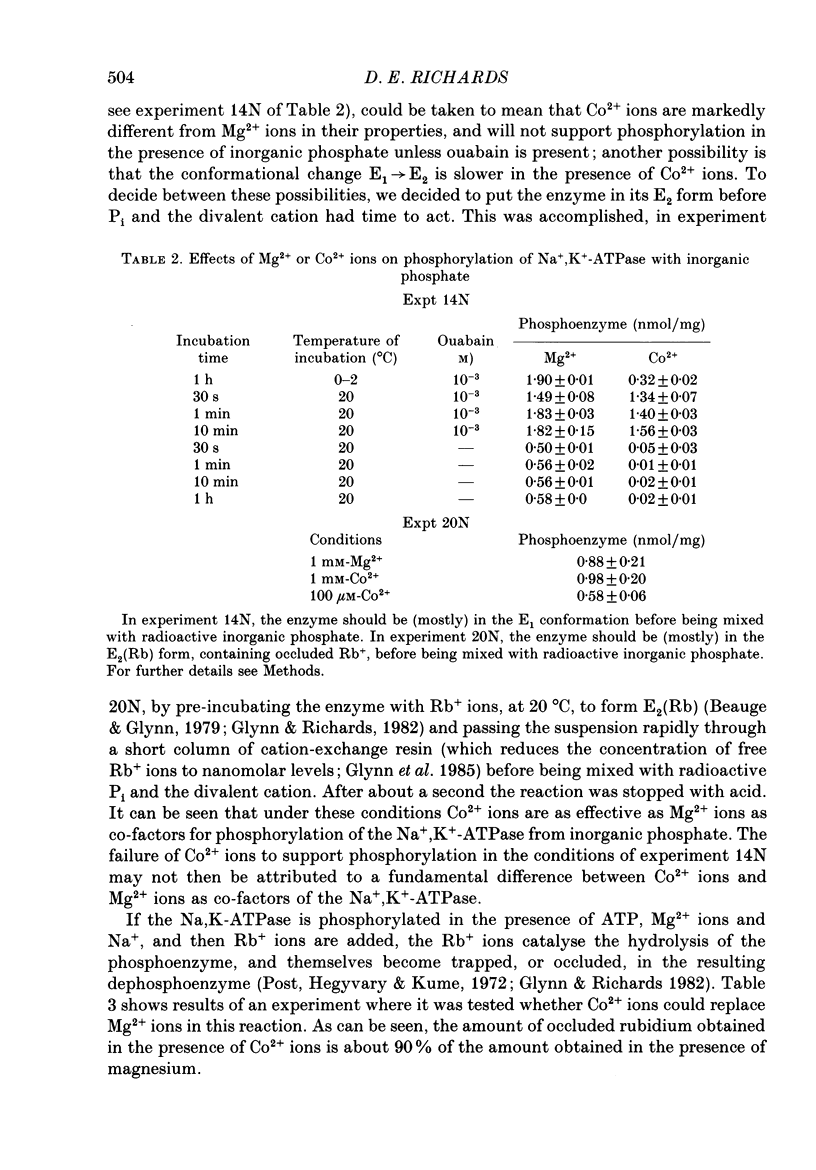
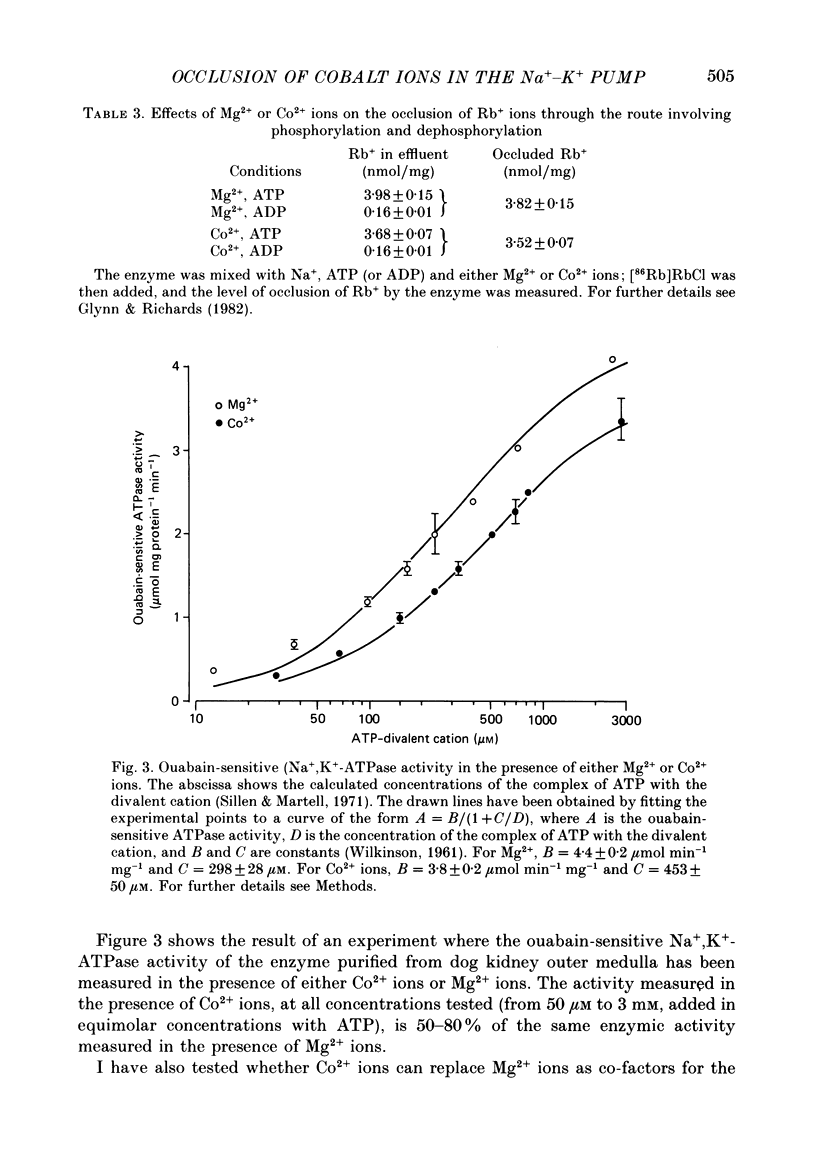
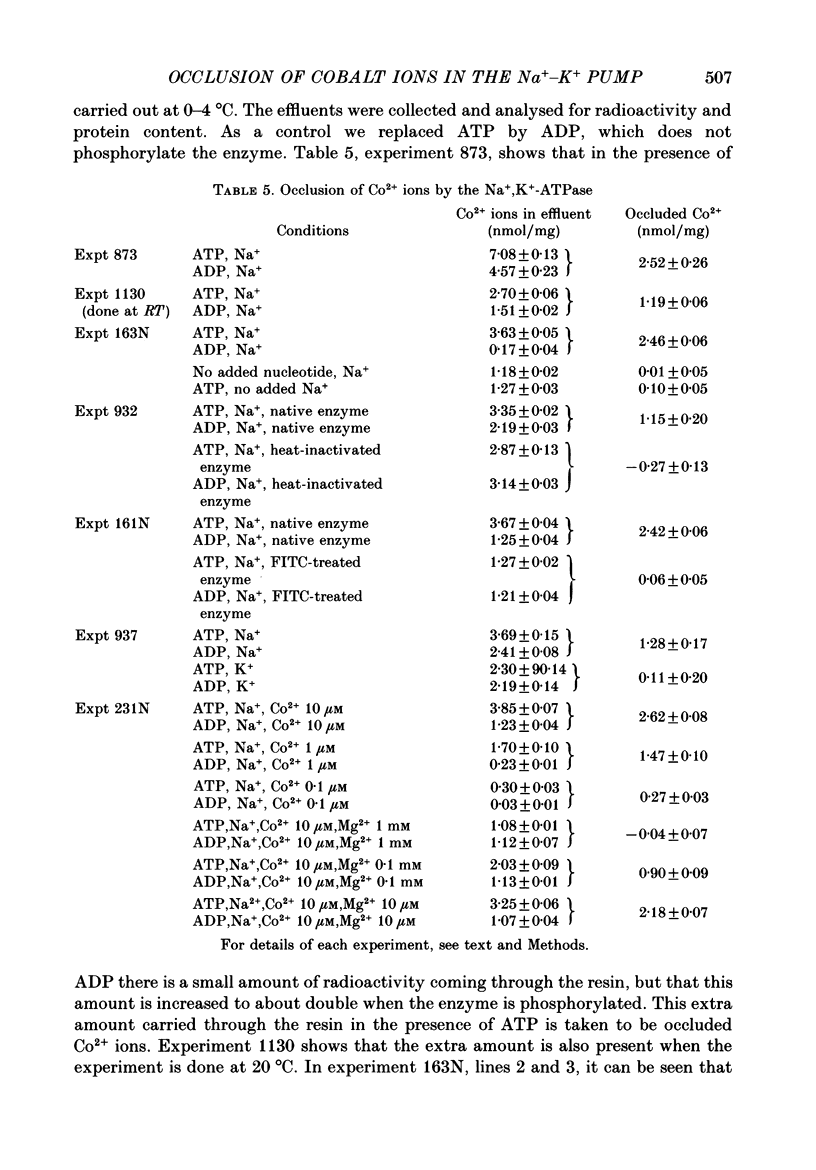
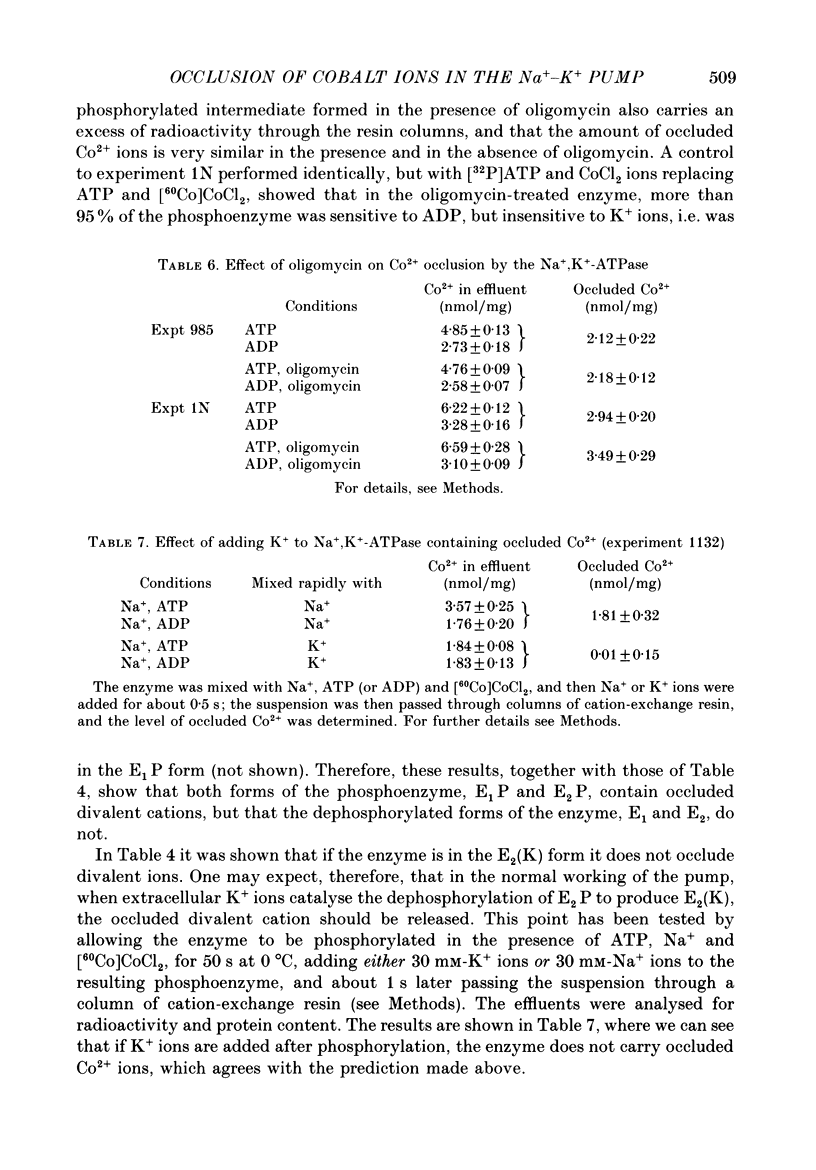
1. Co2+ ions can replace Mg2+ ions as co-factors for the Na+-K+ pump purified from dog kidney outer medulla. The evidence comes from (a) measurement of ouabain-sensitive Na+,K+-ATPase activity, (b) measurement of ATP-dependent 22Na uptake catalysed by the Na+-K+ pump reconstituted into phospholipid vesicles, (c) measurements of phosphorylation of the Na+-K+ pump either in the presence of ATP and sodium ions or in the presence of inorganic phosphate, and (d) measurement of occlusion of rubidium ions through the route involving phosphorylation and dephosphorylation. 2. Purified Na+,K+-ATPase incubated in the presence of ATP, Na+ ions and [60Co]CoCl2, can carry occluded Co2+ ions through a cation-exchange resin. The enzyme fails to occlude the divalent cation (i) if ADP replaces ATP, (ii) if the enzyme is heat-inactivated, (iii) if the enzyme is inactivated by treatment with fluorescein isothiocyanate, (iv) if K+ replaces Na+ in the incubation medium, (v) if Na+ ions are omitted, and (vi) if Mg2+ ions are added in a sufficient concentration. 3. The amount of occluded Co2+ ions is unaffected by pre-treatment of the Na+,K+-ATPase with oligomycin, which stabilizes the phosphoenzyme in the E1P form. 4. The addition of K+ ions to Na+,K+-ATPase that has been phosphorylated in the presence of ATP, Na+ ions and [60Co]CoCl2 releases the occluded Co2+ ions from the enzyme. Under those conditions, K+ ions accelerate the hydrolysis of the phosphoenzyme, and become occluded in the resulting dephosphoenzyme. 5. The stoichiometry of Co2+ ion occlusion is about one occluded Co2+ ion per phosphorylation site. 6. These results support the hypothesis that, in the normal working of the Na+-K+ pump, Mg2+ ions are trapped in the phosphorylated forms of the enzyme, and are released by a K+-dependent dephosphorylation reaction.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaugé L. A., Glynn I. M. Occlusion of K ions in the unphosphorylated sodium pump. Nature. 1979 Aug 9;280(5722):510–512. doi: 10.1038/280510a0. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. The equilibrium between different conformations of the unphosphorylated sodium pump: effects of ATP and of potassium ions, and their relevance to potassium transport. J Physiol. 1980 Feb;299:367–383. doi: 10.1113/jphysiol.1980.sp013130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Nakao M. Changes in affinity of Na+- and K+-transport ATPase for divalent cations during its reaction sequence. J Biol Chem. 1980 Aug 25;255(16):7813–7819. [PubMed] [Google Scholar]
- Fukushima Y., Post R. L. Binding of divalent cation to phosphoenzyme of sodium- and potassium-transport adenosine triphosphatase. J Biol Chem. 1978 Oct 10;253(19):6853–6862. [PubMed] [Google Scholar]
- Glynn I. M., Hara Y., Richards D. E. The occlusion of sodium ions within the mammalian sodium-potassium pump: its role in sodium transport. J Physiol. 1984 Jun;351:531–547. doi: 10.1113/jphysiol.1984.sp015261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Howland J. L., Richards D. E. Evidence for the ordered release of rubidium ions occluded within the Na,K-ATPase of mammalian kidney. J Physiol. 1985 Nov;368:453–469. doi: 10.1113/jphysiol.1985.sp015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Richards D. E. Occlusion of rubidium ions by the sodium-potassium pump: its implications for the mechanism of potassium transport. J Physiol. 1982 Sep;330:17–43. doi: 10.1113/jphysiol.1982.sp014326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Karlish S. J. Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr. 1980 Aug;12(3-4):111–136. doi: 10.1007/BF00744678. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Pick U. Sidedness of the effects of sodium and potassium ions on the conformational state of the sodium-potassium pump. J Physiol. 1981 Mar;312:505–529. doi: 10.1113/jphysiol.1981.sp013641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Conformational transitions between Na+-bound and K+-bound forms of (Na+ + K+)-ATPase, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):252–264. doi: 10.1016/0005-2744(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Kuriki Y., Racker E. Inhibition of (Na+, K+)adenosine triphosphatase and its partial reactions by quercetin. Biochemistry. 1976 Nov 16;15(23):4951–4956. doi: 10.1021/bi00668a001. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Post R. L., Toda G., Rogers F. N. Phosphorylation by inorganic phosphate of sodium plus potassium ion transport adenosine triphosphatase. Four reactive states. J Biol Chem. 1975 Jan 25;250(2):691–701. [PubMed] [Google Scholar]
- RENDI R., UHR M. L. SODIUM, POTASSIUM-REQUIRING ADENOSINETRIPHOSPHATASE ACTIVITY. I. PURIFICATION AND PROPERTIES. Biochim Biophys Acta. 1964 Sep 18;89:520–531. doi: 10.1016/0926-6569(64)90078-1. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Zinn K., Cantley L. C. A study of the vanadate-trapped state of the (Na,K)-ATPase. Evidence against interacting nucleotide site models. J Biol Chem. 1980 Oct 25;255(20):9852–9859. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


