Abstract
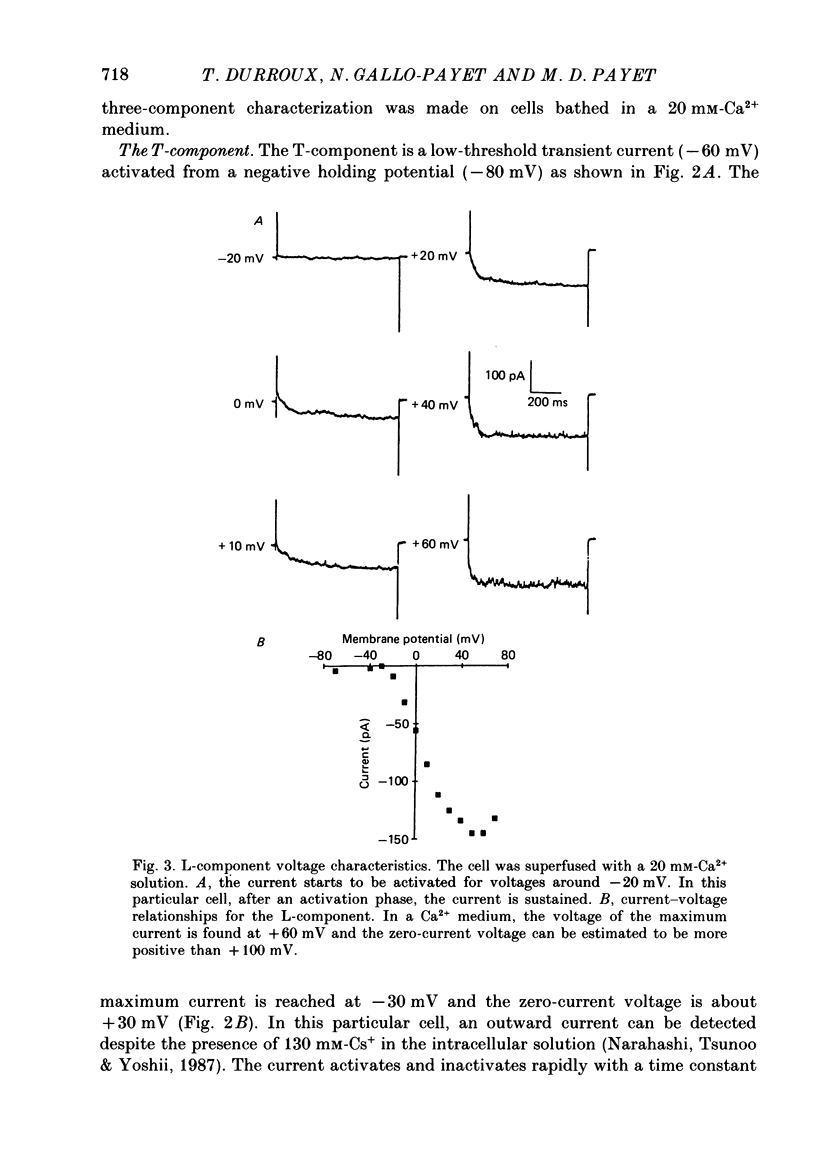
1. Ca2+ channels were studied in cultured glomerulosa cells from the rat adrenal gland. The whole-cell configuration of the patch-clamp technique was used. Cs+-filled pipettes were used in order to block K+ channels. 2. Three Ca2+ components were found, namely, T, L and N, according to the nomenclature proposed by Nowycky, Fox & Tsien (1985). The T-component was a fast transient component activated in the range -60 to -40 mV; the L-component did not inactivate for a sustained depolarization and activated at voltages around -30 mV; the third component, the N-component, was transient and was activated at voltages close to -20 mV. 3. A statistical analysis made on seventy-one experiments showed that the L-component was the most frequent (65% of the experiments), followed by the T- and finally the N- components (59 and 29% of the experiments, respectively). 4. The substitution of Ba2+ ions for Ca2+ ions greatly enhanced the L-component's amplitude (iBa/iCa = 4) while the N-component was unaffected and the T-component was reduced (iBa/iCa = 0.4). 5. A comparison of the voltage-dependent steady-state inactivation of the three components showed that the T-component was inactivated at -60 mV while the inactivation of the L- and N-components was complete at -25 and 0 mV, respectively. 6. A run-down effect was detected in some cells. The time stability of the L-component was lower than that of the T-component. The N-component seemed to be insensitive for at least 1 h. The results for the L- and T-components were obtained in cells which presented no run-down of the current or only a weak one. 7. Cd2+ ions (5 x 10(-5)M) completely blocked the long-lasting component (L-component) and slightly decreased the T-component. 8. Bay K 8644, a dihydropyridine agonist, enhanced the L-component at a concentration of 2.5 microM but decreased it for a higher concentration (5 microM). The T-component was decreased in a reversible way by 1 microM-Bay K 8644. Nifedipine, a well-known antagonist, blocked completely the L-component. This effect was reversed by the addition of Bay K 8644 to the perfusion medium. The T-component was also blocked by nifedipine, a result which is in keeping with the fact that Bay K 8644 has a weak effect on this current.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Catt K. J. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986 Jan;118(1):112–118. doi: 10.1210/endo-118-1-112. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechem M., Schramm M. Calcium-agonists. J Mol Cell Cardiol. 1987 May;19 (Suppl 2):63–75. doi: 10.1016/s0022-2828(87)80005-6. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Braley L. M., Menachery A. I., Brown E. M., Williams G. H. Comparative effect of angiotensin II, potassium, adrenocorticotropin, and cyclic adenosine 3',5'-monophosphate on cytosolic calcium in rat adrenal cells. Endocrinology. 1986 Sep;119(3):1010–1019. doi: 10.1210/endo-119-3-1010. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. Dual effects of dihydropyridines on whole cell and unitary calcium currents in single ventricular cells of guinea-pig. J Physiol. 1986 Oct;379:495–514. doi: 10.1113/jphysiol.1986.sp016266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G. V., Marchetti C., Brown A. M. Involvement of sodium channels and two types of calcium channels in the regulation of adrenocorticotropin release. Endocrinology. 1987 May;120(5):2059–2069. doi: 10.1210/endo-120-5-2059. [DOI] [PubMed] [Google Scholar]
- Cota G. Calcium channel currents in pars intermedia cells of the rat pituitary gland. Kinetic properties and washout during intracellular dialysis. J Gen Physiol. 1986 Jul;88(1):83–105. doi: 10.1085/jgp.88.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denechaud M., Israel J. M., Mishal Z., Vincent J. D. Influence of cell cycle phases on the electrical activity and hormone release in a transformed line of anterior pituitary cells. Life Sci. 1987 Jun 15;40(24):2377–2384. doi: 10.1016/0024-3205(87)90512-1. [DOI] [PubMed] [Google Scholar]
- Dube G. P., Baik Y. H., Vaghy P. L., Schwartz A. Nitrendipine potentiates Bay k 8644-induced contraction of isolated porcine coronary artery: evidence for functionally distinct dihydropyridine receptor subtypes. Biochem Biophys Res Commun. 1985 May 16;128(3):1295–1302. doi: 10.1016/0006-291x(85)91081-2. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Aizawa T., Hinkle P. M. Interaction of dihydropyridine Ca2+ agonist Bay K 8644 with normal and transformed pituitary cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C95–102. doi: 10.1152/ajpcell.1986.250.1.C95. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Sheu S. S., Hinkle P. M. Dihydropyridine modulators of voltage-sensitive Ca2+ channels specifically regulate prolactin production by GH4C1 pituitary tumor cells. J Biol Chem. 1987 Mar 5;262(7):3154–3159. [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume J. R. Comparative interactions of organic Ca++ channel antagonists with myocardial Ca++ and K+ channels. J Pharmacol Exp Ther. 1985 Jul;234(1):134–140. [PubMed] [Google Scholar]
- Janis R. A., Rampe D., Sarmiento J. G., Triggle D. J. Specific binding of a calcium channel activator, [3H]BAY k 8644, to membranes from cardiac muscle and brain. Biochem Biophys Res Commun. 1984 May 31;121(1):317–323. doi: 10.1016/0006-291x(84)90725-3. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Ogata E. Direct demonstration of adrenocorticotropin-induced changes in cytoplasmic free calcium with aequorin in adrenal glomerulosa cell. J Biol Chem. 1986 Jul 25;261(21):9832–9838. [PubMed] [Google Scholar]
- Lymangrover J. R. Adrenocorticotrophic hormone and cyclic adenosine monophosphate effect on mouse adrenal cortical cell membrane potential. Experientia. 1980 May 15;36(5):613–614. doi: 10.1007/BF01965834. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Childs G. V., Brown A. M. Membrane currents of identified isolated rat corticotropes and gonadotropes. Am J Physiol. 1987 Mar;252(3 Pt 1):E340–E346. doi: 10.1152/ajpendo.1987.252.3.E340. [DOI] [PubMed] [Google Scholar]
- Matsunaga H., Maruyama Y., Kojima I., Hoshi T. Transient Ca2+-channel current characterized by a low-threshold voltage in zona glomerulosa cells of rat adrenal cortex. Pflugers Arch. 1987 Apr;408(4):351–355. doi: 10.1007/BF00581128. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Saffran M. Ionic dependence of adrenal steroidogenesis and ACTH-induced changes in the membrane potential of adrenocortical cells. J Physiol. 1973 Oct;234(1):43–64. doi: 10.1113/jphysiol.1973.sp010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natke E., Jr, Kabela E. Electrical responses in cat adrenal cortex: possible relation to aldosterone secretion. Am J Physiol. 1979 Aug;237(2):E158–E162. doi: 10.1152/ajpendo.1979.237.2.E158. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Benabderrazik M., Gallo-Payet N. Excitation-secretion coupling: ionic currents in glomerulosa cells: effects of adrenocorticotropin and K+ channel blockers. Endocrinology. 1987 Sep;121(3):875–882. doi: 10.1210/endo-121-3-875. [DOI] [PubMed] [Google Scholar]
- Payet N., Lehoux J. G. Aldosterone and corticosterone stimulation by ACTH in isolated rat adrenal glomerulosa cells: interaction with vasopressin. J Physiol (Paris) 1982;78(3):317–321. [PubMed] [Google Scholar]
- Quinn S. J., Cornwall M. C., Williams G. H. Electrical properties of isolated rat adrenal glomerulosa and fasciculata cells. Endocrinology. 1987 Mar;120(3):903–914. doi: 10.1210/endo-120-3-903. [DOI] [PubMed] [Google Scholar]
- Quinn S. J., Cornwall M. C., Williams G. H. Electrophysiological responses to angiotensin II of isolated rat adrenal glomerulosa cells. Endocrinology. 1987 Apr;120(4):1581–1589. doi: 10.1210/endo-120-4-1581. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Holz G. G., 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987 Aug;409(4-5):361–366. doi: 10.1007/BF00583789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. A variety of calcium channels. Nature. 1985 Aug 1;316(6027):391–391. doi: 10.1038/316391a0. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Grupp I. L., Grupp G., Williams J. S., Vaghy P. L. Effects of dihydropyridine calcium channel modulators in the heart: pharmacological and radioligand binding correlations. Biochem Biophys Res Commun. 1984 Nov 30;125(1):387–394. doi: 10.1016/s0006-291x(84)80380-0. [DOI] [PubMed] [Google Scholar]
- Tabares L., López-Barneo J. Calcium action potentials in cultured adrenocortical cells. Pflugers Arch. 1986 Aug;407(2):163–165. doi: 10.1007/BF00580669. [DOI] [PubMed] [Google Scholar]
- Tabares L., López-Barneo J., de Miguel C. Calcium- and voltage-activated potassium channels in adrenocortical cell membranes. Biochim Biophys Acta. 1985 Mar 28;814(1):96–102. doi: 10.1016/0005-2736(85)90423-7. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Circ Res. 1985 Jun;56(6):868–875. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]


