Abstract
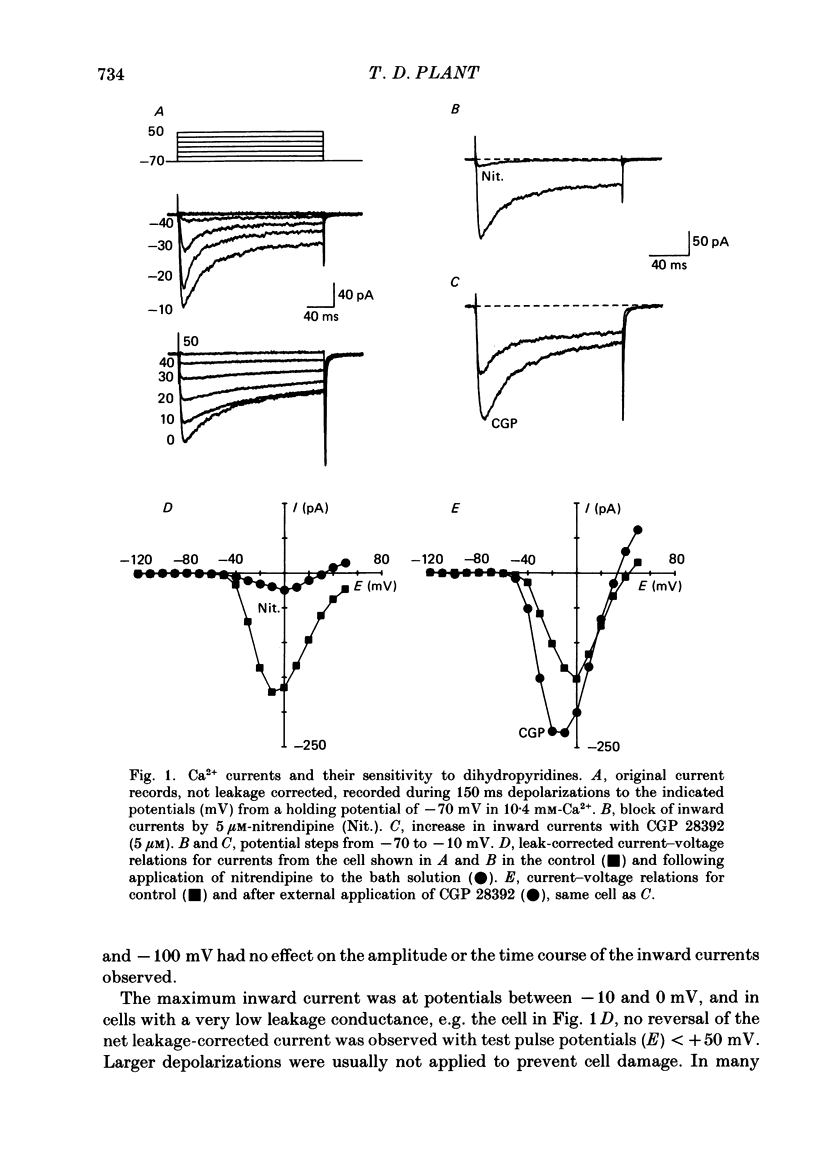
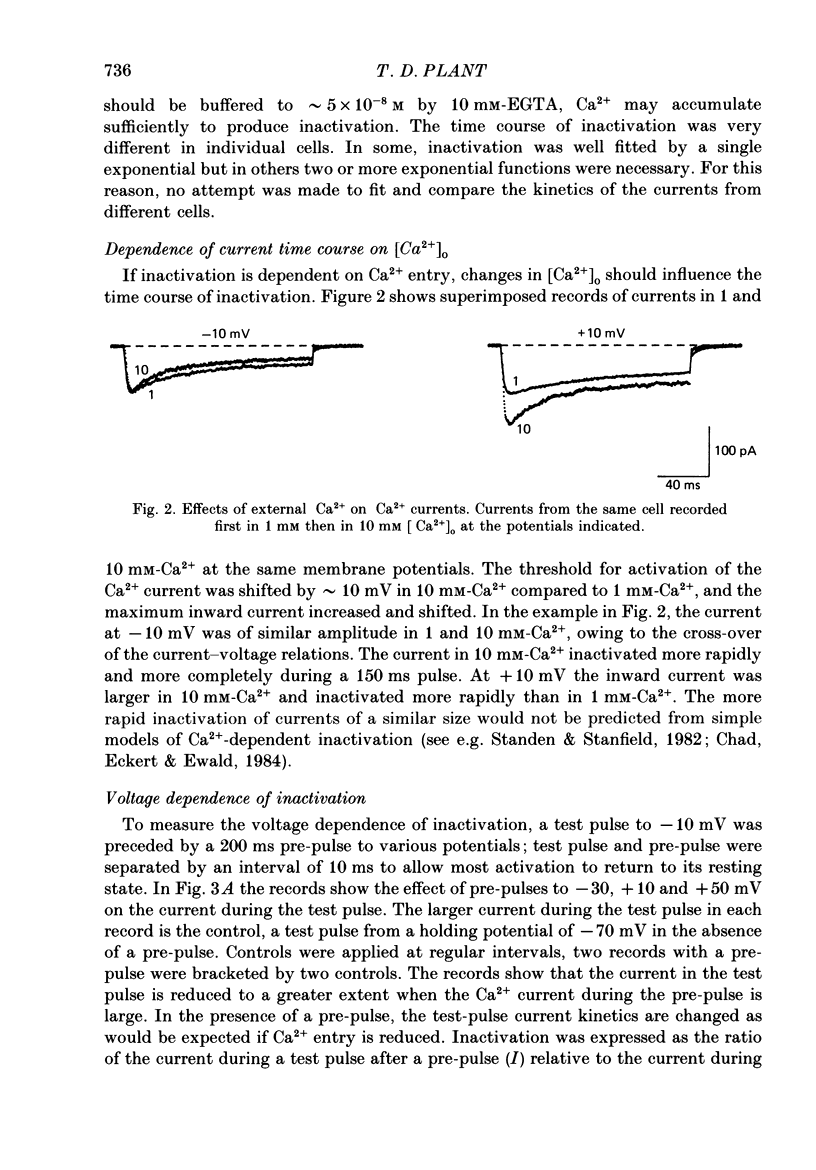
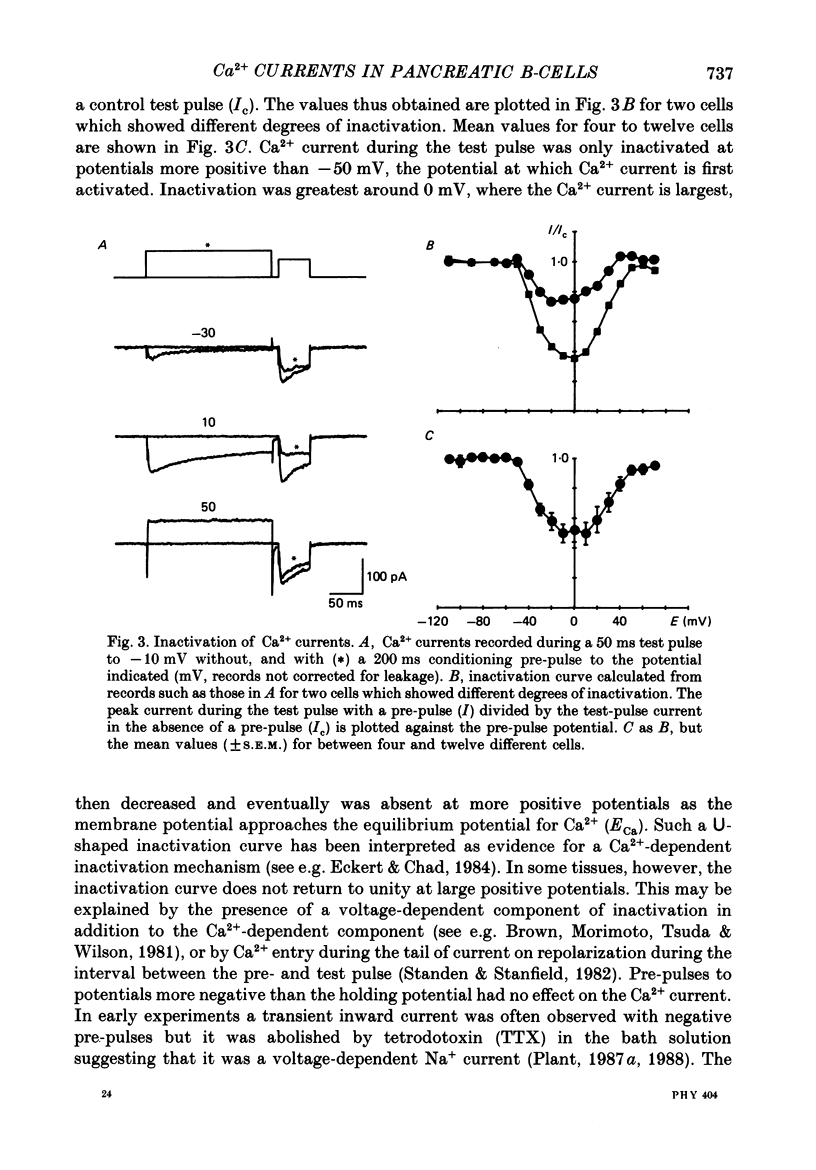
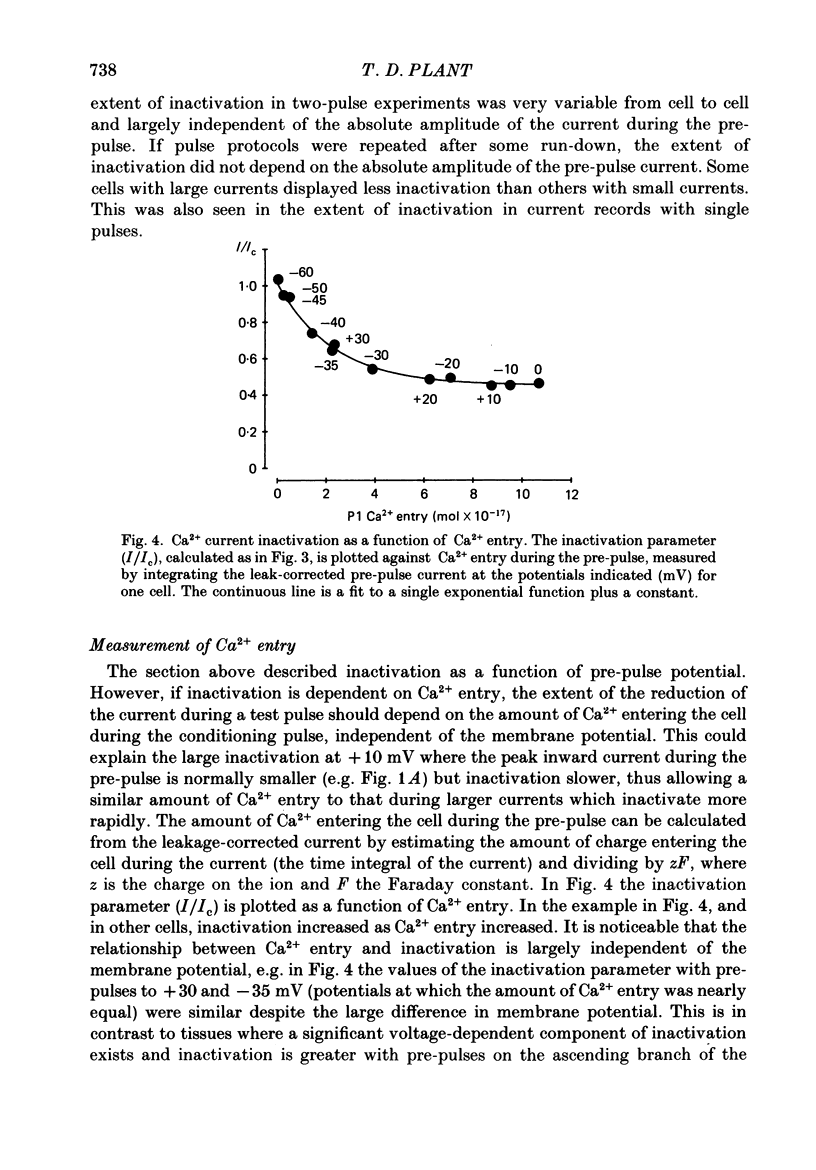
1. Ca2+ currents were recorded using the whole-cell mode of the patch-clamp technique from mouse pancreatic B-cells kept in culture for 1-4 days. B-cells were identified in the cell-attached mode by their response to a change in the glucose concentration from 3 to 15 or 20 mM or by their inward currents. 2. Only one component of Ca2+ current was observed in these cells, which activated at potentials greater than -50 mV and was blocked by nitrendipine (5 microM), and increased in amplitude by CGP 28392 (5 microM). 3. During maintained depolarizations the Ca2+ current inactivated considerably but not completely. Inactivation was most marked at potentials where the Ca2+ currents were large, but in general was slower for currents at potentials greater than 0 mV than at more negative potentials. 4. Two-pulse experiments showed that the inactivation curve for the Ca2+ current was U-shaped, returning to unity at potentials approaching the Ca2+ equilibrium potential. Measurements of Ca2+ entry showed that inactivation was dependent on the amount of Ca2+ entering during the pre-pulse, independent of the pre-pulse potential. 5. Ca2+ currents were not appreciably slowed when BAPTA, a faster buffer of Ca2+, replaced EGTA in the pipette solution. 6. Replacement of Ca2+ in the external solution by Ba2+ increased the amplitude of the inward current and largely abolished inactivation. Large inward currents through Ca2+ channels were observed in the absence of divalent cations in the external solution (+EGTA), which were presumably carried by Na+. These currents did not inactivate during 150 ms depolarizations, but were increased in amplitude by CGP 28392 (5 microM) and blocked by D600 (30 microM). 7. The observations suggest that normal mouse pancreatic B-cells have only one type of Ca2+ channel which is dihydropyridine sensitive and inactivates by a mechanism which is almost purely Ca2+ dependent. Inactivation of the Ca2+ current will probably be important in the control of Ca2+ entry during glucose-induced electrical activity.
Full text
PDF



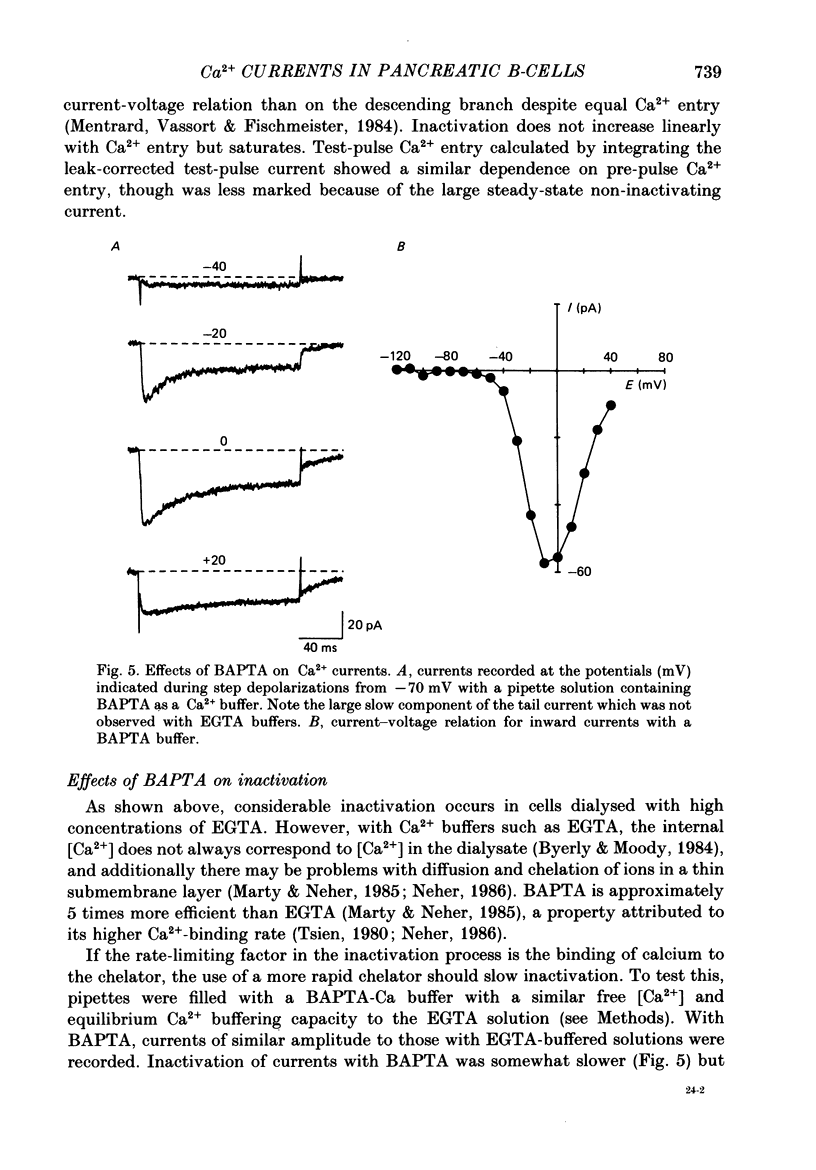
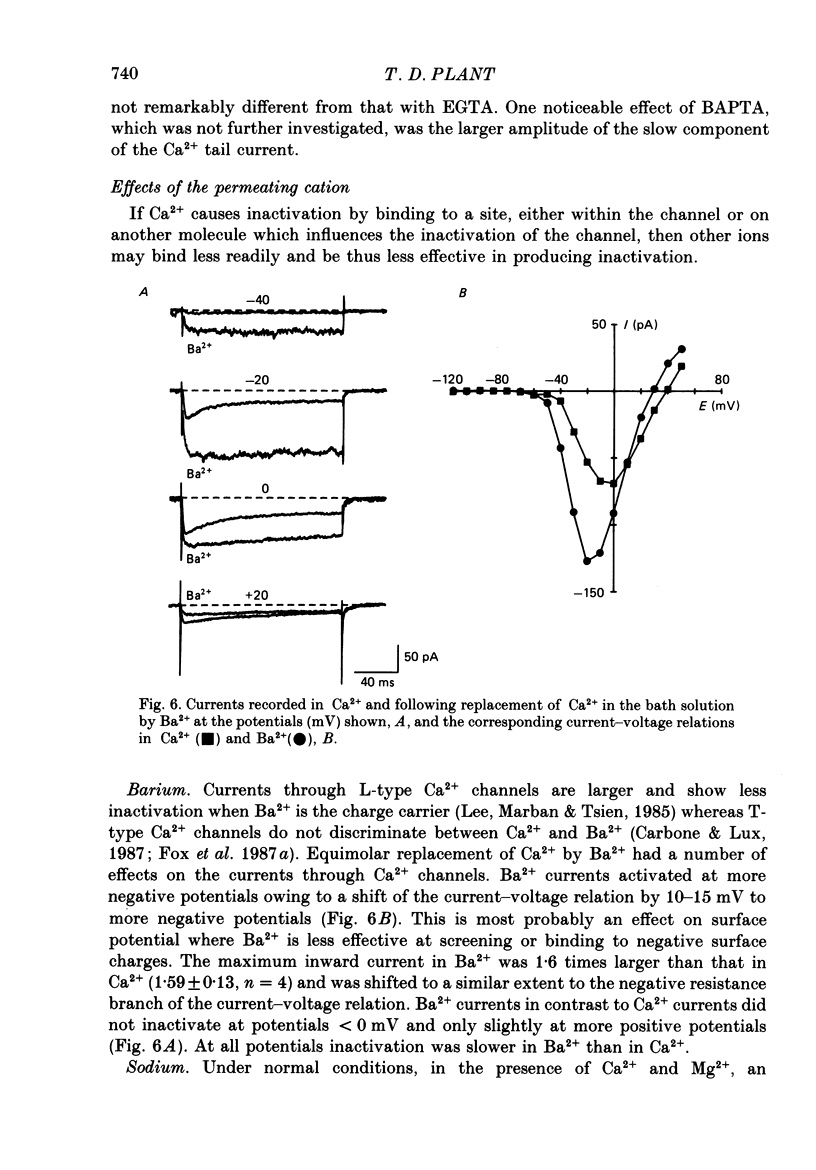
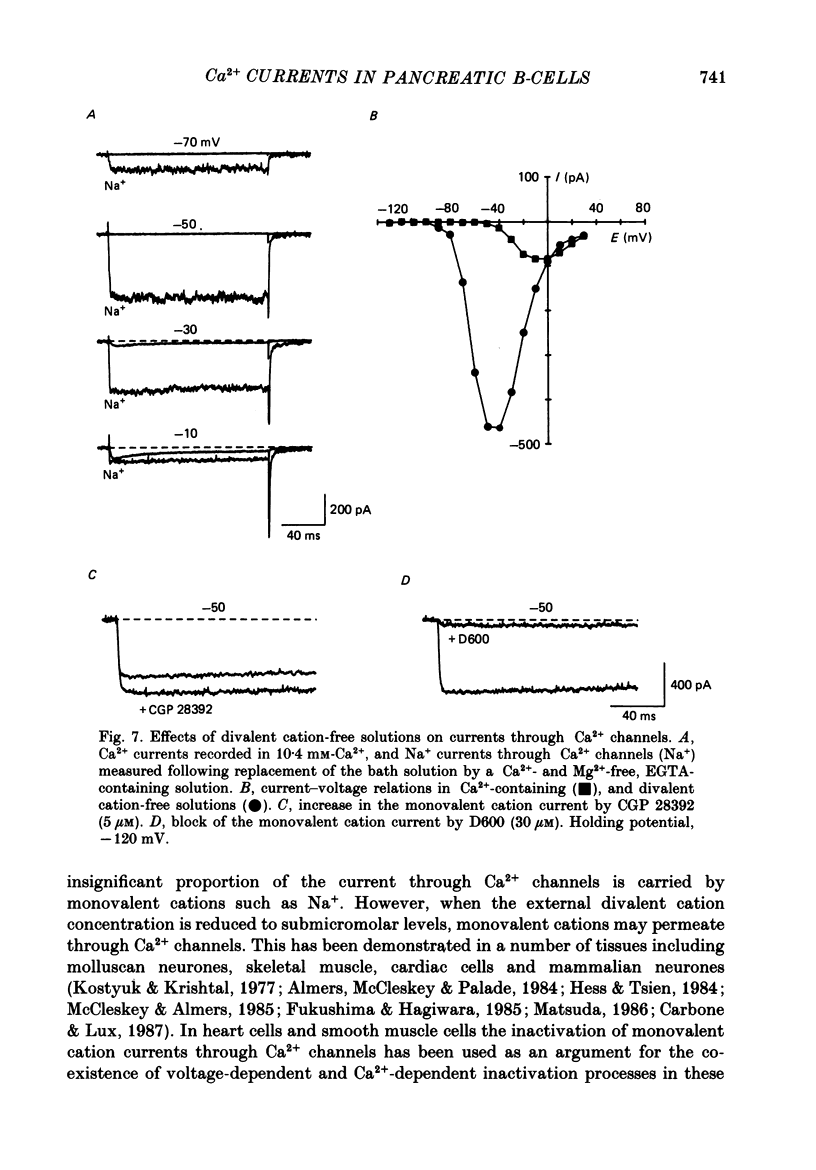






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Atwater I., Beigelman P. M. Dynamic characteristics of electrical activity in pancreatic beta-cells. I. - Effects of calcium and magnesium removal. J Physiol (Paris) 1976 Nov;72(6):769–786. [PubMed] [Google Scholar]
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Intracellular calcium ions and calcium currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jul;352:637–652. doi: 10.1113/jphysiol.1984.sp015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J., Eckert R., Ewald D. Kinetics of calcium-dependent inactivation of calcium current in voltage-clamped neurones of Aplysia californica. J Physiol. 1984 Feb;347:279–300. doi: 10.1113/jphysiol.1984.sp015066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J. Voltage-activated Ca2+ currents in insulin-secreting cells. FEBS Lett. 1985 Sep 23;189(2):281–285. doi: 10.1016/0014-5793(85)81040-1. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J., Smaje L. H., Spencer P. D. Filtration coefficient and osmotic reflection coefficient to albumin in rabbit submandibular gland capillaries. J Physiol. 1988 Apr;398:15–32. doi: 10.1113/jphysiol.1988.sp017026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Schmeer W., Nenquin M., Meissner H. P. Effects of a calcium channel agonist on the electrical, ionic and secretory events in mouse pancreatic B-cells. Biochem Biophys Res Commun. 1985 Sep 16;131(2):980–986. doi: 10.1016/0006-291x(85)91336-1. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Tolbutamide stimulation and inhibition of insulin release: studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia. 1980;18(2):151–160. doi: 10.1007/BF00290493. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hof D. A pulse generating and data recording system based on the microcomputer PDP 11/23. Comput Methods Programs Biomed. 1986 Dec;23(3):309–315. doi: 10.1016/0169-2607(86)90065-9. [DOI] [PubMed] [Google Scholar]
- Jmari K., Mironneau C., Mironneau J. Selectivity of calcium channels in rat uterine smooth muscle: interactions between sodium, calcium and barium ions. J Physiol. 1987 Mar;384:247–261. doi: 10.1113/jphysiol.1987.sp016453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Malaisse-Lagae F. Combined effects of a calcium-agonist and hypoglycemic or hyperglycemic sulfonamides upon insulin release. Res Commun Chem Pathol Pharmacol. 1985 Jul;49(1):71–83. [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflugers Arch. 1986 Nov;407(5):465–475. doi: 10.1007/BF00657502. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Almers W. The Ca channel in skeletal muscle is a large pore. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7149–7153. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Plant T. D. Na+ currents in cultured mouse pancreatic B-cells. Pflugers Arch. 1988 Apr;411(4):429–435. doi: 10.1007/BF00587723. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Abrahamsson H. Cyclic AMP potentiates glucose-induced insulin release from mouse pancreatic islets without increasing cytosolic free Ca2+. Acta Physiol Scand. 1985 Dec;125(4):639–647. doi: 10.1111/j.1748-1716.1985.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Arkhammar P., Berggren P. O. Voltage-activated Na+ currents and their suppression by phorbol ester in clonal insulin-producing RINm5F cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C912–C919. doi: 10.1152/ajpcell.1986.251.6.C912. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin L. S., Cook D. L. Voltage-gated Ca2+ current in pancreatic B-cells. Pflugers Arch. 1985 Aug;404(4):385–387. doi: 10.1007/BF00585354. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Hess P., McCleskey E. W., Rosenberg R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Debuyser A., Joffre M. Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine. Fundam Clin Pharmacol. 1987;1(2):95–113. doi: 10.1111/j.1472-8206.1987.tb00549.x. [DOI] [PubMed] [Google Scholar]



