Abstract
The emergence of diverse infections worldwide, which is a serious global threat to human existence, necessitates the urgent development of novel therapeutic candidates that can combat these diseases with efficacy. Molecular hybridization has been established as an efficient technique in designing bioactive molecules capable of fighting infections. Isatin, a core nucleus of an array of compounds with diverse biological properties can be modified at different positions leading to the creation of novel drug targets, is an active area of medicinal chemistry. This review containing published articles from 2005 to 2022 highlights isatin hybrids which have been synthesized and reported in the literature alongside a discussion on their biological properties. The enriched structure–activity relationship studies discussed provides insights for the rational design of novel isatin hybrids with tailored biological properties as effective therapeutic candidates inspired by nature.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11030-024-10883-z.
Keywords: Isatin hybrids, Biological properties, Pharmacophore, Molecular hybridization, Structure–activity relationship
Introduction
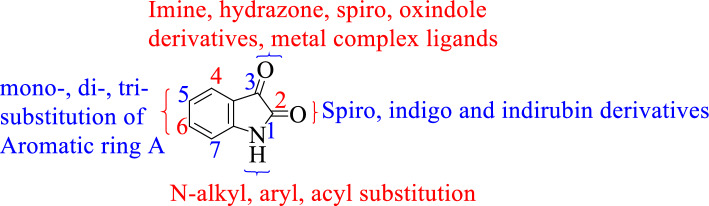
Isatin 1 (indol-2,3-dione: Fig. 1), a secondary metabolite of tryptophan, has been found to be widely distributed in the central nervous system, mammalian tissues, and body fluids of humans [1–3]. This oxidized indole has been used as the core structure in the formulation of several compounds which have been tested and identified as potent inhibitors of Apoptosis [4–9], Anticonvulsants[10, 11], antiviral [5, 12–16], antitubercular [17–19], antifungal [20, 21], antimicrobial [22, 23], antioxidant [24, 25], Antimalarial [26, 27], and Anti-inflammatory agents [28, 29]. Among the known heterocyclic compounds, quinoline and its derivatives have been used for the development of novel drug entities, thus gaining significant attention among 21st-century scientists [30]. Triazole hybrid compounds and aminoquinoline and derivatives have shown promise in the development of next generation antimalarials [31, 32]. Xanthone conjugated amino acids, N-methylpicolinamides, and dihydrazones were recently shown to portray anticancer activities against a broad array of cancer cell lines [33–35], while dihydrazone analogs have shown promise as potential antibacterials [36]. Isatin is also considered a versatile and favorable precursor for pharmacophore development as a privileged scaffold [9] because the moiety can be modified at various positions (N-1, C‐3, C‐4, C‐5, and C‐7 positions) as illustrated in Fig. 1, resulting in different derivatives with diverse biological properties [37, 38]. The modifications at the N‐1, C‐3, and C‐5 positions are much more favorable with the mono-substitution at the C-5 position considered the most favorable. The C-5 position is beneficial to control the electronic effect, lipophilicity, and physicochemical properties.
Fig. 1.
The various possible modification positions on the isatin scaffold [12]
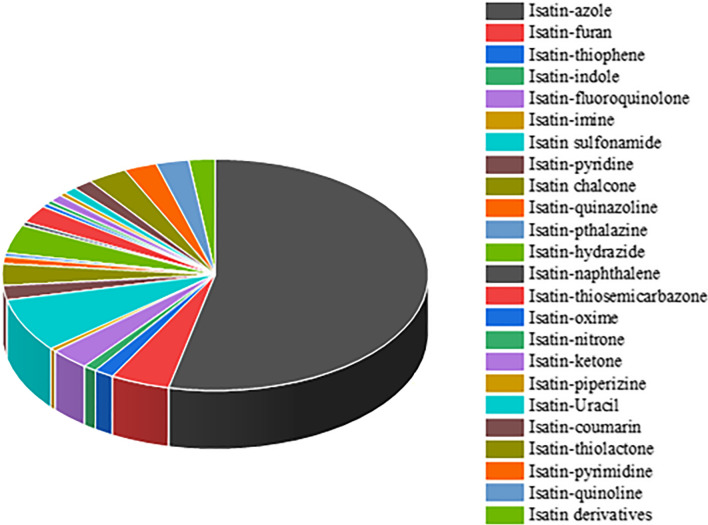
Recently, some isatin-containing compounds have been approved for clinical trials (Sunitinib and Toceranib) [17] used in the treatment of tumors, while others (Nintedanib, Semaxinib, and Orantinib) are currently undergoing clinical trials for the evaluation of their therapeutic activities as anticancer agents [2]. This triple angiokinase inhibitor, Nintedanib, indicated for the treatment of idiopathic pulmonary fibrosis, systemic sclerosis-associated interstitial lung disease, and in combination with docetaxel for non-small cell lung cancer is a hybrid of indole and piperazine pharmacophores. Sunitinib, a receptor tyrosine kinase inhibitor, and chemotherapeutic agent is used for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) is a hybrid of isatin and pyrrole pharmacophores. The development of a single hybrid compound by combining two or more pharmacophores has been proven to be a promising approach in the development of new drugs that have the potential to overcome drug resistance and possess improved activity when compared to parent drugs [39]. It is therefore plausible that the molecular hybridization of the isatin moiety with other pharmacophores has the potential to generate new and more effective therapeutic candidates [8]. There exist several isatin hybrid molecules generated by the combination of isatin moiety with other useful pharmacophores that have outstanding biological activities. Some of these hybrids include Isatin-Azole hybrids [8–10, 14, 23, 40–45], Isatin-furan hybrids [9, 18, 40, 42, 46], Isatin-thiophene hybrids [8, 47], Isatin-indole hybrids [9, 48], Isatin-fluoroquinolone hybrids [9, 17, 49], Isatin-Imine hybrids[9], Isatin-sulfonamide hybrids [2, 9, 21, 50, 51], Isatin-pyridine hybrids [52–55], Isatin-chalcone hybrids [56], Isatin-quinazoline hybrids [57, 58], Isatin-phthalazine hybrids [57], Isatin-hydrazide hybrids [9, 40, 42, 55], Isatin-naphthalene hybrids [14], isatin-thiosemicarbazone hybrid[9, 20], Isatin-oxime hybrids [59], Isatin-nitrone hybrids [59], Isatin-ketone hybrids [60], Isatin-piperazine hybrids [61], Isatin-uracil hybrids [62], Isatin-coumarin hybrids [63], Isatin-thiolactone hybrids [64], and Isatin-pyrimidine hybrids. Figure 2 presents a pie chart illustrating how these isatin hybrids are distributed (further details on the structures of these hybrids can be found in the supplementary data) [17]. The purpose of this review is to set up the direction for the design and development of isatin hybrids with tailored biological properties as effective therapeutic candidates inspired by nature.
Fig. 2.
Distribution of isatin hybrids
Isatin-azole hybrids
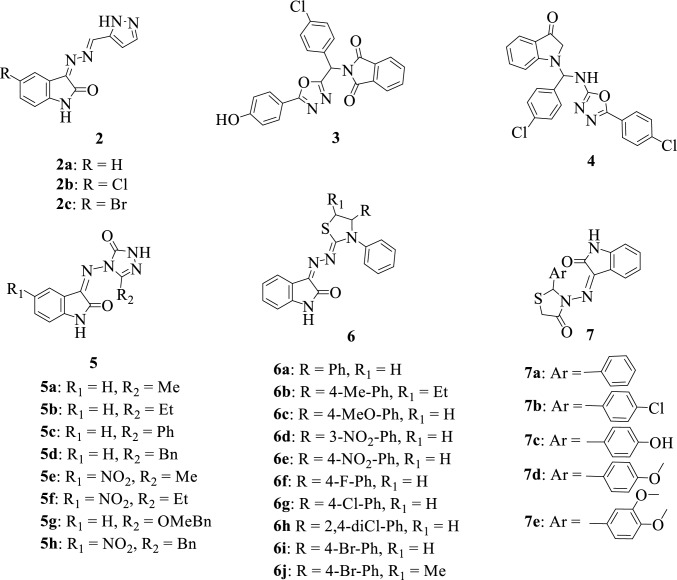
Azole, a privileged scaffold of choice when designing novel therapeutic agents, is mainly found as core structure in several natural products and synthesized compounds that are used by pharmaceutical or agrochemical industries [65]. Most azole compounds are used as antifungal drugs [66, 67] and some of its derivatives possess a variety of biological properties such as anticancer [7, 66], antibacterial [67, 68], and antitubercular properties [17, 69]. Several isatin-azole hybrids have been synthesized [4, 17, 28, 70–75] and reported to possess diverse pharmacological properties. The chemical structures of these isatin-azole hybrids are presented in Fig. 3. Eldehna et al. in 2018 [76] reported the synthesis of the isatin-pyrazole hybrids 2a-c and evaluated their antiproliferative properties. The hybrid 2b was identified as the most active analog portraying broad-spectrum activity against breast, colon, and lung human cancer cell lines with an average IC50 value of 2.14 μM. SAR studies revealed that the 5-pyrazolyl moiety was crucial and played an important role in the enhanced activity of this compound.
Fig. 3.
Chemical structures of isatin-azole hybrids
With the outbreak of SARS-CoV-2 and the urgent need for the development of bioactive molecules, Badavath et al. in 2020 [14] conducted in silico studies, by making use of computer-aided drug design approaches to screen over 118 compounds. The molecular docking studies against Mpro protein revealed that the isatin-oxidiazole hybrids 3 and 4 possessed excessive interactions with Mpro with best docking scores of − 11.22 and − 11.15 kcal/mol, respectively. These hybrids were composed of a central carbon atom bearing three different ring systems permitting them to make multiple interactions with the binding pocket of Mpro. Thus, they could serve as starting points for the development of potential SARS-CoV-2 Mpro inhibitors. Özil et al. in 2011 [77] synthesized a series of isatin-1,2,4-triazole hybrids 5a-h and evaluated their antimicrobial properties against four bacterial strains Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis. Hybrid 5 g emerged with quite interesting antibacterial activity against Staphylococcus aureus and Bacillus subtilis bearing a minimal inhibitory concentration (MIC) value of 8 and 16 µg mL−1, respectively. Triazole derivatives which are known to possess antifungal activity in this case played an essential role in enhancing the antibacterial activity of this compound against the tested gram-positive bacteria. Neglected tropical diseases remain a global threat to health and thus there is a need for the development of new approaches and therapies to fight against these infections. Freitas et al. in 2021 [43] reported the synthesis and evaluation of the antiparasitic properties of some isatin-thiazolyl 6a-6j hybrids. The hybrids 6e, 6 h, 6i, and 6j were found to be the most potent compounds with anti-Trypanosoma cruzi activity for trypomastigote form having IC50 values of 4.43 μM, 2.05 μM, 4.12 μM, and 1.72 μM, respectively. Nikalje et al., 2015 [10] described the microwave-assisted synthesis of a series of novel isatin-thiazolidin-4-one hybrids 7a-e and analyzed their anticonvulsant activities in mice using maximal electroshock seizure (MES) and subcutaneous pentylenetetrazole (sc-PTZ)-induced seizure tests. Hybrids 7c and 7e with small electron-donating polar groups at the para-position of the phenyl ring exhibited potent protection against maximal electroshock seizure (MES) test cells thus indicating interesting anticonvulsant properties [28].
Isatin-furan hybrids
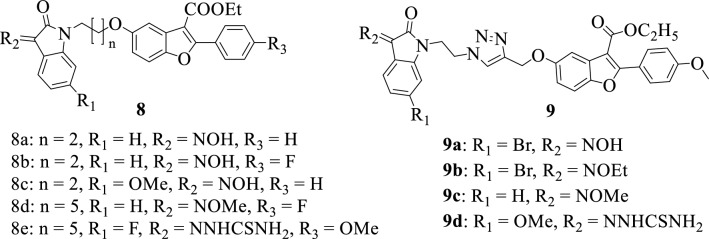
Furan is an important pharmacophore of natural origin with several biological properties (anticancer, antimalarial, antibacterial, and antifungal). It has been used as a starting material in the production of several industrial chemicals such as catalysts, resins, agrochemicals, and pharmaceuticals [78, 79]. The chemical structures of isatin-furan hybrids are presented in Fig. 4. The synthesis and antibacterial evaluation of a series of isatin-benzofuran hybrids 8a-e were reported by Gao et al., in 2019 [18]. The synthesized compounds were tested on a panel of gram-negative and gram-positive bacteria and the MIC values were obtained. The hybrid 8e was identified as the most promising compound with interesting antibacterial activity against majority of the tested pathogens (Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter aerogenes, Proteus mirabilis) with MIC values of < 1 μg/mL. SAR demonstrated that incorporating a thiosemicarbazide at position C-3 of the isatin moiety as well as an electron-withdrawing group at position C-5 enhanced the activity of the compound. In 2018, Gao et al., [80] reported the synthesis of some isatin-benzofuran hybrids 9a-d and evaluated their antimycobacterial activity against Mycobacterium tuberculosis (MTB H37Rv strain) and MDR-TB (Multidrug-Resistant Tuberculosis) strains. Among the synthesized compounds, the hybrid 9d was found to be the most active with over 128 folds effectiveness when compared to Rifampicin, a well-known antibiotic used in the treatment of tuberculosis having MIC values of 0.25 and 0.5 µg/mL against MTB H37Rv and MDR-TB strains, respectively. Results of SAR studies indicated that substituents at positions C-3 and C-5 of the isatin moiety play a vital role in the antimycobacterial activity of the compounds. The presence of an electron-donating group at C-5 and a hydrogen-bond donor group at C-3 accounts for the enhanced antimycobacterial activity of hybrid 9d [42].
Fig. 4.
Chemical structures of isatin-furan hybrids
Isatin-thiophene hybrids
Thiophene, one of the most abundantly found heterocyclic rings present in biological systems, has emerged as a potent scaffold in drug discovery. This moiety and its derivatives have found widespread applications in different fields of life such as the pharmaceutical and dye industries. Several pharmacological properties have been reported to be associated with this scaffold, some of which include anticancer, antimicrobial, and anti-inflammatory properties [81, 82]. Figure 5 shows some of the chemical structures of isatin-thiophene hybrids. Chen et al. in 2005 [83] synthesized some isatin derivatives 10a-b and 11a-f. The synthesized compounds were evaluated in vitro for their inhibitory activity against SARS coronavirus 3CL protease. Notably, some of the synthesized compounds exhibited potent inhibitory activity against the virus with hybrids 11a and 11e being the most active hybrids among the compounds having IC50 values of 0.98 µM and 0.95 µM, respectively. The SAR studies suggested that the bioactivity of these compounds was greatly influenced by the nature of the substituents on the isatin moiety and the sidechain [14, 47].
Fig. 5.
Chemical structures of isatin-thiophene hybrids
Isatin-indole hybrids
Indoles constitute an important subunit for the discovery of new drug candidates. It is widely distributed in natural products and bioactive molecules and is responsible for the fecal smell in human feces, scents of flowers, and the flowery smell of perfumes [84–86]. The indole moiety is a versatile molecule with several biological properties such as antifungal, antimicrobial, antiviral, and antitubercular properties [87]. Figure 6 presents some of the chemical structures of isatin-indole hybrids.
Fig. 6.
Chemical structures of isatin-indole hybrids
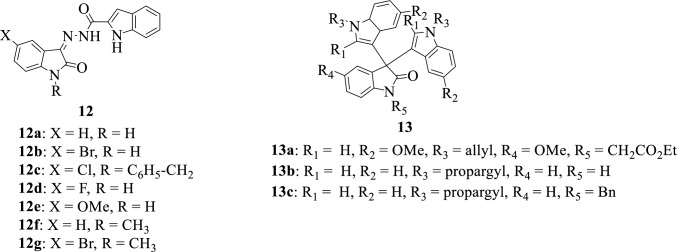
Al-wabli et al. in 2020 [48] reported the synthesis of some isatin-indole molecular hybrids 12a-g and evaluation of their properties as antiproliferative agents against human breast (ZR-75), colon (HT-29), and lung (A-549) tumor cell line. The hybrid 12c showed potent antiproliferative activity with an IC50 value of 1.17 µM which was approximately sevenfold greater than Sunitinib, a well-known anticancer medication. The SAR studies revealed that hybrids bearing N-benzyl moiety on isatin were more active with better antiproliferative activity. Some bis-isatin-indole hybrids 13a-c were synthesized and reported by Praveen in 2011 [88]. The anticonvulsant and antibacterial properties of the synthesized compounds were evaluated against Maxima Electroshock seizure (MES) model and two bacterial strains: Staphylococcus aureus and Escherichia coli, respectively. The hybrids 13b and 13c demonstrated excellent anticonvulsant activity and in addition, hybrid 13c revealed excellent antibacterial activity against Escherichia coli. Results from SAR demonstrated that the replacement of the N-allyl group (13a) with a propargyl group (13b and c) resulted in a remarkable improvement in the activity of these compounds [9].
Isatin-fluoroquinolone hybrids
Quinolone is an essential class of nitrogen-containing heterocycles widely used as a building block for medicinal agents. Fluoroquinolones possess a broad-spectrum activity and very good oral bioavailability, and as such are often used as antibacterial agents. Some fluoroquinolones which are currently available include Ciprofloxacin, Gemifloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, and Ofloxacin [19, 89–91]. Figure 7 presents some of the chemical structures of isatin-fluoroquinolone hybrids.
Fig. 7.
Chemical structures of isatin-fluoroquinolone hybrids
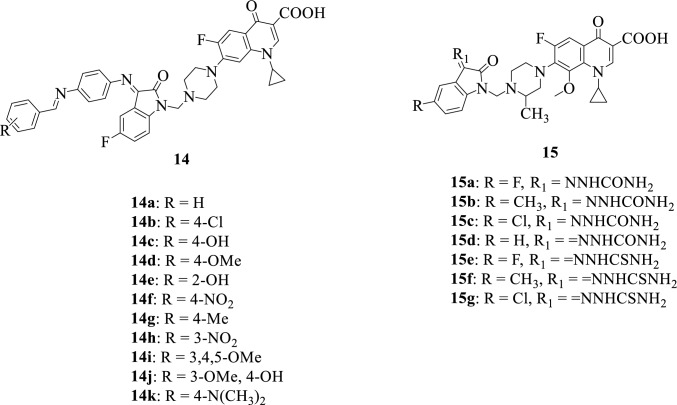
In 2013, to develop potential antimicrobials, Prakash et al. [92] reported the synthesis of a series of novel Ciprofloxacin-isatin hybrids 14a-k. Most of the compounds showed interesting in vitro antibacterial and antifungal activity against the investigated microbes. The hybrid 14c was identified as the most potent hybrid with better antibacterial activity against Staphylococcus. aureus, Escherichia coli, and Pseudomonas aeruginosa when compared to the parent drug Ciprofloxacin, and similar antifungal activity against Aspergillus fumigatus and Aspergillus niger when compared to Ketoconazole. The presence of the electron-donating substituent (-OH) plays a crucial role in improving the antibacterial activity of this compound [9].
Over one-third of the world’s population is potentially infected with tuberculosis (TB), a common infectious disease. In the quest for novel, effective, and fast-acting anti-TB drugs with low toxicity, Sriram et al. in 2006 [93] synthesized a series of Gatifloxacin-isatin 15a-g hybrids and evaluated their antimycobacterial activity. Hybrid 15d was shown to be the most potent with improved activity when compared to the parent drug Gatifloxacin with an IC50 value of 3.0 µg/mL. Fluoroquinolones play an essential role in the penetrative ability of compounds across cells leading to the assumption that penetration is pivotal for antimycobacterial activity of quinolones. Bearing this in mind, SAR studies illustrated that increasing the lipophilic character of the compounds at position C-7 resulted in an increase in activity [17].
Isatin-sulfonamide hybrids
Sulfonamides are naturally occurring structural motifs in medicinal chemistry with leading roles in novel drug design and development against complex infections [91]. They are highly versatile organo-sulfur compounds containing the -SO2NH2 and/or -SO2NH- groups and small chemical modifications often result in improved activity. Sulfonamides are generally used in the treatment of bacterial infections and possess several biological activities such as antifungal, anti-inflammatory, antioxidant, diuretic, and anticancer [98, 99]. The chemical structures of these isatin-sulfonamide hybrids are presented in Fig. 8.
Fig. 8.
Chemical structures of isatin-sulfonamide hybrids
In 2014, Farag [94] reported the synthesis and evaluation of antimicrobial activity of a series of 5-(morpholinosulfonyl)isatin hybrids 16 and 17a-h. The synthesized compounds were evaluated for their activity against gram + ve (Staphylococcus aureus, Staphylococcus epidermidis, and Bacillus subtilis), gram –ve (Proteus vulgaris, Klebsiella pneumonia, Shigella flexneri) bacteria, and fungi. Hybrid 16 revealed better antibacterial activity against all tested bacteria strains (MIC: 0.007–0.49 μg/Ml) when compared to Ampicillin B and fourfold antifungal potency against Aspergillus fumigatus when compared to Amphotricin B with an MIC value of 0.24 µg/mL. SAR revealed that the oxygen at position C-3 is crucial for activity and the replacement of oxygen with other substituents had detrimental impacts on the activity of the compounds.
Abo-Ashour et al., [95] with the main goal of developing novel isatin-based anticancer candidates targeting the tumor-associated hCA isoforms IX and XII, synthesized two series of isatin-sulfonamide hybrids 18 and 19a-h followed by the evaluation of their in vitro biological activity. All the synthesized compounds revealed potent inhibitory activities against the tested hCA isoforms and thus were further investigated for their antiproliferative activity against several cancer cell lines. Notably, the hybrids 19f and 19 h were the most active against the various cell lines inhibiting the cancer cells in a concentration-dependent manner [50].
Eldehna et al., 2018 [96] synthesized and evaluated the anticancer activity of a series of isatin-sulfonamide hybrids 20a-f against colorectal cancer (HCT-116) and breast cancer (MCF-7) cell lines. The most promising hybrid among the series 20e exhibited potent anticancer activity against colorectal cancer (HCT-116) cell lines with an IC50 value of 3.67 ± 0.33 µM. Inhibitory activities of these compounds were influenced by the nature of the substituent inserted at position C-5 of the isatin moiety. An improvement in activity was observed with compounds bearing electron-donating groups at that position, while those with electron-withdrawing groups at C-5 possessed slightly reduced activity.
Selvam and collaborators in 2010 [51] reported the synthesis of a series of isatin-sulfadimidine hybrids 21a-e and the determination of their antiviral activity against swine influenza A/California/07/2009 (H1N1) virus. The synthesized compounds revealed quite potent activity against the virus by blocking its adsorption to cells with the hybrids 21a and d being the most active among the synthesized compounds.
Isatin-pyridine hybrids
Pyridines are a class of heterocyclic nitrogenous compounds with tremendous applications in diverse fields of life. This moiety and its derivatives are naturally present in different molecules such as vitamins, co-enzymes, and alkaloids. Due to their wide range of pharmacological properties, pyridine-based compounds have found widespread applications in the field of drug design and discovery. It is widely used as a solvent for organic reactions, paints, and pharmaceuticals as well as intermediates in the manufacture of agrochemicals and pharmaceuticals [97]. The chemical structures of these isatin-pyridine hybrids are illustrated in Fig. 9.
Fig. 9.
Chemical structures of isatin-pyridine hybrids
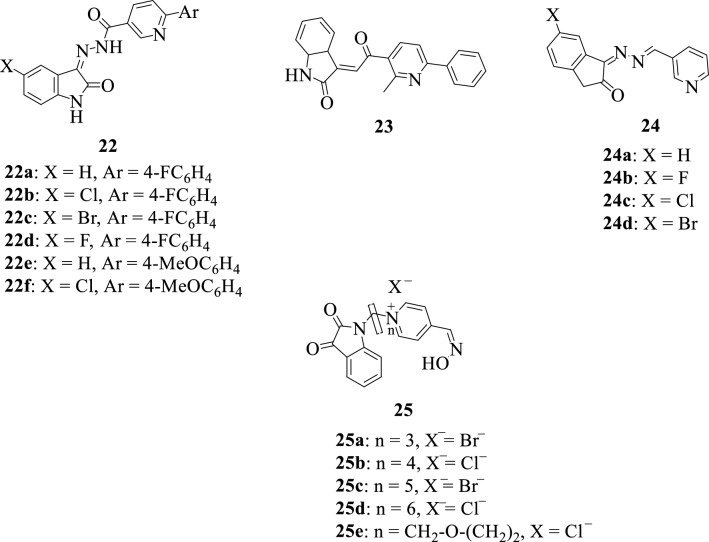
Adopting a hybrid-pharmacophore approach, Eldehna et al. in 2014 [52] designed, synthesized, and evaluated the antiproliferative activity of a series of isatin-pyridine hybrids 22–24 against HepG2, A549 (lung), and MCF-7 (breast) cancer cell lines. Notably, hybrid 23 was identified as the most active compound with an over 2.7-fold increase in activity against HepG2 cell line when compared to Doxorubicin, a known anticancer medication. Quantitative structure–activity relationship studies revealed that the introduction of a more lipophilic and the bulky chlorine atom resulted in a tremendous increase in activity thus making hybrid 24c the most active against A549 (lung) and MCF-7 (breast) cancer cell lines.
Kitagawa et al. in 2021 [53], in an attempt to combat organophosphorus poisoning caused by some pesticides and nerve agents, designed and synthesized a series of isatin-pyridine oxime hybrids 25a-e and analyzed their properties as acetylcholinesterase reactivators. All the synthesized compounds demonstrated reactivation properties with hybrids 25c and 25e showing the highest percentage of reactivation even at low concentrations thus making them potential lead compounds. The SAR of hybrids 25a-e suggested that the linker 1,5-pentanediyl (25c) is vital and plays an important role in the interaction of the compounds with the AChE binding sites.
Isatin-chalcone hybrids
Chalcones are one of the most important classes of natural products derived from plants with widespread distribution in vegetables, teas, fruits, and many others [135, 136]. They are a group of plant-derived polyphenolic compounds, known to be biogenetic precursors of flavonoids and isoflavonoids with several medicinal and pharmaceutical applications some of which include antihypertensive, antibacterial, antiobesity, antimalarial, antiretroviral, anticancer, fungicidal, germicidal, herbicidal, and insecticidal [100, 101]. Figure 10 shows some of the chemical structures of isatin-chalcone hybrids.
Fig. 10.
Chemical structures of isatin-chalcone hybrids
Fayed et al., in 2021 [56] reported the synthesis and screening of a series of isatin-chalcone hybrids 26–28 for their anticancer activities against MCF-7 (breast), HepG-2 (liver), and HCT-116 (colon) human cell lines. All the synthesized compounds demonstrated quite interesting antitumor properties with the hybrid 27 showing very high anticancer activity against HepG-2 cell line with an IC50 value of 5.33 µM/mL when compared to Imatinib.
Isatin-quinazoline hybrids
Quinazoline scaffold is a vital class of biologically active nitrogen-containing heterocycles with unique properties such as ease of synthetic accessibility and flexible structural modification, which have motivated the exploitation of their biological activities [102]. This scaffold has attracted significant attention over the past years due to its diverse pharmacological activities such as antimalarial, anticancer, anticonvulsant, and anti-inflammatory properties [103]. The chemical structures of some selected isatin-quinazoline hybrids are displayed in Fig. 11.
Fig. 11.
Chemical structures of isatin-quinazoline hybrids
Implementing a molecular hybridization approach, Fares et al., 2015 [58] designed and synthesized a series of isatin-quinazoline hybrids 29a-f. The synthesized compounds were tested for their in vitro anticancer activity against liver, breast, and colon cancer cell lines. It is worth noting that, the hybrids 29a, 29c, and 29f were the most active with the ability to induce apoptosis in liver HepG2 cells having IC50 values of 1.0 ± 0.2, 1.8 ± 0.4, and 2.4 ± 0.4 µM, respectively.
Eldehna et al. in 2017 [57] with the primary goal of developing potent antiproliferative agents capable of targeting triple-negative breast cancer (TNBC) MDA-MB-231 cell lines synthesized a series of isatin-quinazoline hybrids 30a-e. The hybrid 30e was found to be the most potent against MDA-MB-231 cell lines with over a 2.37-fold increase in activity when compared to 5-Fluorouracil, the reference drug. The anticancer SAR of hybrids 30 indicated that the introduction of the 2,6-dichloro substituent (30e) was beneficial for activity and could be assigned to its ability to increase the lipophilic character of the compound.
Isatin-phthalazine hybrids
Phthalazines are essential nitrogen-containing heterocyclic compounds with interesting chemical, industrial, and pharmacological properties such as anticancer, anticonvulsant, anti-inflammatory, antifungal, and antibacterial properties. Different drug molecules are presently available in the market which contain the phthalazine pharmacophore some of which include Hydralazine, Budralazine, Vatalanib, Olaparib, and Azelastine. Owing to its broad application in the treatment of diverse infections, the phthalazine scaffold has received much attention in the area of drug discovery. Phthalazines are used as starting materials for the development of new medications and as an intermediary in the synthesis of chemicals [104]. Figure 12 shows some of the chemical structures of isatin-phthalazine hybrids.
Fig. 12.
Chemical structures of isatin-phthalazine hybrids
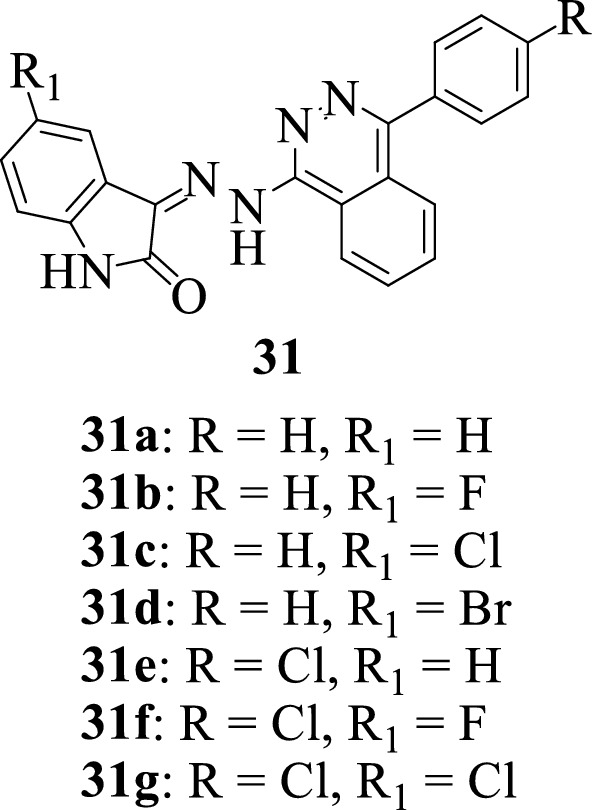
Exploring the potentials in the hybrid-pharmacophore approach, Eldehna et al., 2017 [57] reported the synthesis of a series of isatin-phthalazine hybrids 31a-g and evaluated their activity as antiproliferative agents against triple-negative breast cancer (TNBC) MDA-MB-231 cell lines. Notably, the hybrid 31 g showed improved activity against MDA-MB-231 (breast) cell lines with over a 2.44-fold increase in activity when compared to 5-Fluorouracil, the reference drug. SAR studies revealed that inserting substituents at the 4-phenyl group of the hybrids resulted in compounds with improved activity when compared to the unsubstituted compounds.
Isatin-hydrazide hybrids
Hydrazides represent an important class of organic compounds that contain the azomethine functional group connected to a carbonyl group. These functionalities accord the pharmacophore its unique pharmacological properties thus making it a key intermediate and vital starting material for the development of novel bioactive compounds. Several drugs are currently in use that contain the hydrazide moiety some of which include Isoniazid (antituberculosis), Nifuroxazide (antibiotic), Isocarbazide (antidepressant), Iproniazid (antituberculosis), and Galavit (anti-inflammatory) [105, 106]. The chemical structures of these isatin-hydrazide hybrids are presented in Fig. 13.
Fig. 13.
Chemical structures of isatin-hydrazide hybrids
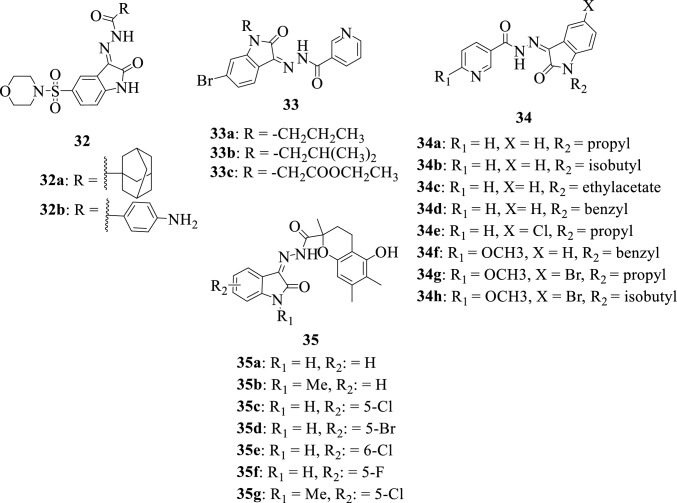
In 2020, Salem et al. reported the synthesis of some isatin-carbohydrazide hybrids 32a-b and further evaluated their in vitro antimicrobial activity [107]. The compounds were tested on some strains of both gram-positive and gram-negative bacteria and the hybrid 32b was found to possess the most potent antibacterial activity among the synthesized compounds with its activity comparable to that of Norfloxacin and Tetracycline. Antibacterial SAR evaluation of the hybrids demonstrated that the presence of the p-amino benzoic acid moiety in 32b greatly influenced the increase in bioactivity of this hybrid.
Elsayed et al., 2021 [55] reported the synthesis of a series of isatin-nicotinohydrazide hybrids 33a-c and 34a-h followed by the evaluation of their activities as antitubercular and antibacterial agents. Among the synthesized compounds, the hybrids 34 g and 34 h were found to be the most potent antitubercular agents demonstrating broad-spectrum antibacterial activity against the tested strains, and SAR indicated that N-benzylation/methylation of the isatin moiety plays a pivotal role in exertion of the biological properties [42].
Rawat et al., 2016 [108] reported the synthesis of a series of isatin-carbohydrazide hybrids 35a-g and the evaluation of their antimicrobial activity against different bacterial and fungal strains. Most of the synthesized compounds revealed interesting antimicrobial activities with the hybrids 35c and 35d being the most potent against the bacterial strain Escherichia coli, while hybrids 35a and 35b revealed very potent antifungal activity against Candida albicans.
Isatin-thiosemicarbazone hybrids
Thiosemicarbazones are an important class of ligands generally obtained as condensation products from the reaction of thiosemicarbazide with aldehydes and ketones. Over the years, thiosemicarbazones have gained so much interest owing to their metal-chelating properties, wide range of biological properties, and structural flexibility [109]. Figure 14 shows some of the chemical structures of isatin-thiosemicarbazone hybrids.
Fig. 14.
Chemical structures of isatin-thiosemicarbazone hybrids
To discover novel anti-methicillin-resistant Staphylococcus aureus (MRSA) agents, Zhang et al., 2015 [137] synthesized a series of isatin-β-thiosemicarbazones hybrids 36a-36i. The synthesized compounds were evaluated for their antibacterial activity against gram-positive bacterial strains: Staphylococcus aureus (ATCC 6538) and Bacillus subtilis (ATCC 6633). All tested compounds exhibited interesting antibacterial activity with 36b being the most active with a minimum inhibitory concentration (MIC) value of ≤ 1.56 mg/L against the tested strains, and SAR studies revealed that the insertion of a halogen at position-7 of isatin is an essential structural modification required to obtain favorable antibacterial activity.
Thanh et al., 2016 [20] reported the synthesis and evaluation of the in vivo antioxidant/in vitro antimicrobial activity of a series of isatin-thiosemicarbazone hybrids 37a-f. The in vitro antimicrobial activity was conducted against different bacterial (Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus epidermidis, Bacillus subtilis, Enterobacter aerogenes) and fungal strains (Aspergillus niger, Candida albicans, Fusarium oxysporum, Saccharomyces cerevisiae), while in vivo antioxidant activity was determined by evaluating the superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) activities of the compounds. The synthesized compounds revealed quite promising activities and the hybrid 37d was identified as the most potent antioxidant, antibacterial, and antifungal agent.
Conducting pharmacophoric modeling studies on non-nucleoside reverse transcriptase inhibitors (NNRTIs), a series of isatin-β-thiosemicarbazone hybrids 38–40 were synthesized and evaluated for their anti-HIV activity. The synthesized hybrids were found to possess interesting anti-HIV activity with hybrid 39 being the most active among the synthesized compounds with an EC50 value of 2.62 µM [15].
Isatin-oxime hybrids
Oximes are an essential class of nitrogen-containing compounds usually obtained as condensation products from the reaction of hydroxyl amines with aldehydes or ketones. This pharmacophore has found widespread use in different fields of life such as in industries, some oxime-containing compounds are used as artificial sweeteners. A good number of marketed drugs contain the oxime moiety some of which include Pyraloxime methiodine: a cholinesterase inhibitor and Ceftobiprole [110, 111]. Furthermore, oxime-containing chemicals have been reported to possess antiviral properties against influenza virus A and HIV-1 virus as well as anticancer properties against human breast and colon adenocarcinoma cell lines [112, 113]. The chemical structures of these isatin-oxime hybrids are presented in Fig. 15.
Fig. 15.

Chemical structures of isatin-oxime hybrids
To meet the demand for orally active inhibitors of respiratory syncytial virus (RSV) replication, Sin et al., 2009 [59] synthesized a series of isatin hybrids 41a–41i. The tested compounds revealed potent antiviral activities with the hybrids 41b–41g bearing methyl, ethyl, and fluoroethyl substituents being the most active hybrids, and SAR studies revealed these small oxime substituents are preferable for antiviral activity.
Isatin-nitrone hybrids
Nitrones are organic species that react with, “trap” and stabilize free radicals for identification and characterization purposes [114]. They are potent antioxidant molecules capable of reducing oxidative stress as well as suppressing signal transduction processes suggesting potential anti-inflammatory and antiapoptotic activities [115–117]. The chemical structures of these isatin-nitrone hybrids are shown in Fig. 16.
Fig. 16.

Chemical structures of isatin-nitrone hybrids
Sin et al., 2009 [59] reported the synthesis of a series of isatin-nitrone hybrids 42a–42c and the evaluation of their inhibitory activity against respiratory syncytial virus (RSV). The synthesized compounds revealed moderate antiviral activity with the hybrid 42c being the most potent.
Isatin-piperazine hybrids
Piperazine is a vital heterocyclic scaffold found in several biologically active compounds. This scaffold is present in some antiviral agents such as Delavirdine and Indinavir, which are used in HIV treatment. It is considered a privileged scaffold for drug design and widely used due to its unique properties some of which include solubility, basicity, chemical reactivity, and conformational properties [118, 119]. This ring is present in several commercially available drugs and its derivatives are known to possess a broad spectrum of therapeutic properties such as antidepressant, anticancer, antimalarial, anticonvulsant, antifungal, and antitubercular properties [120]. The chemical structure of an isatin-piperazine hybrid is shown in Fig. 17.
Fig. 17.

Chemical structure of an isatin-piperazine hybrid
In 2021, Omar et al. [61] in the quest for possible SARS-CoV-2 Protease Enzyme inhibitors synthesized the isatin-piperazine hybrid 43 and evaluated its physicochemical, bioactivity scores, and pharmacokinetic properties using in silico computational tools. Molecular docking studies were conducted to predict the inhibitory activity of the ligand against the SARS-CoV-2 main protease enzyme. Based on the study, the piperazine ligand made strong hydrogen bonding interactions with the SARS-CoV-2 Protease with a negative dock energy thus suggesting it could be a good lead for the design of new drug candidates.
Isatin-uracil hybrids
Uracil, a naturally occurring pyrimidine nucleobase, is a major component of nucleic acid. Oxidative degradation of uracil yields urea and maleic acid in the presence of hydrogen peroxide and ferrous ions. It has widespread applications in different fields of life such as medicine, pesticide, and chemical synthesis. Uracil is commonly used as a starting material in the synthesis of many pyrimidine-based herbicides and the design and application of medicine [62]. The chemical structures of some isatin-uracil hybrids are shown in Fig. 18.
Fig. 18.

Chemical structures of isatin-uracil hybrids
Kumar et al., 2012 [62] reported the synthesis of a series of isatin-uracil hybrids 44a–44 l and evaluation of their cytotoxic activity against three human cancer cell lines HeLa (cervix), MCF-7 (breast), and DU145 (prostate). Among the synthesized compounds, the hybrids 44g and 44k were found to be active against DU145 (prostate) cancer cell lines at low concentrations. Notably, most of the compounds were inactive against the HeLa (cervix) cell line except for hybrids 44d and 44h bearing electron-withdrawing substituents. SAR studies identified two key factors that influence the activity of these hybrids, the presence of a halogen atom on Uracil and increasing the chain length from n = 2 to n = 3.
Isatin-coumarin hybrids
Coumarin represents a privileged scaffold for medicinal chemists with unique physicochemical properties that undergo easy synthetic transformations [121, 122]. It is found extensively in nature and its derivatives have been found to demonstrate interesting pharmacological activities (antibacterial, antifungal, antimalarial, and anticancer activities). Coumarins are widely used in perfumes, hand soap, detergents, and lotions where they function as fragrance enhancers or stabilizers [123, 124]. Figure 19 presents some of the chemical structures of isatin-coumarin hybrids.
Fig. 19.
Chemical structures of isatin-coumarin hybrids
Considering the availability of limited and unsatisfactory antileishmanial chemotherapeutics, Khatoon et al., in 2021 [63] synthesized a series of isatin-coumarin hybrids 45a–45i. The synthesized compounds were evaluated for their in silico and in vitro activities against Leishmaniasis. Notably, hybrids 45f, 45h, and 45i were found to be the most active at macro molar concentrations against Leishmania tropica promastigotes and amastigotes.
In 2019, Diao et al. [125] reported the design and synthesis of a series of isatin-coumarin hybrids 46a–46l, and evaluation of their in vitro anticancer activities against HepG2 (liver carcinoma), Hela (cervical cancer), A549 (lung adenocarcinoma), DU145 (prostatic cancer), SKOV3 (ovarian carcinoma), MCF-7 (breast cancer), and drug-resistant MCF-7/DOX (doxorubicin-resistant MCF-7) human cancer cell lines. The compounds revealed weak to moderate anticancer activities, and as such can be considered as starting points for further research. The anticancer SAR studies demonstrated that the nature of the substituents at positions C-3 and C-5 are vital for activity, as electron-donating substituents at C-5 enhanced activity while hydrogen-bond donor groups at C-3 are important for activity.
Huang et al., 2019 [126] reported the design, synthesis, and evaluation of the in vitro antitubercular activity of a series of isatin-coumarin hybrids 47a–47d against MTB H37Rv. The compounds, however, were inactive but could serve as good starting points for the development of anti-TB molecules.
Isatin-thiolactone hybrids
Thiolactone is an essential class of heterocyclic scaffold with the extensive use of their cores as synthetic intermediates for the generation of ligands required for applications in catalysis and medicinal chemistry. They are often referred to as latent thiols and have been reported to possess anticancer, antibacterial, and anti-Alzheimer activity [127]. Figure 20 presents some of the chemical structures of isatin-thiolactone hybrids.
Fig. 20.

Chemical structures of isatin-thiolactone hybrids
Hans et al., 2011 [64] synthesized and evaluated the antiplasmodial activity of a series of isatin-thiolactone hybrids 48a–f against chloroquine-resistant (W2) strain of Plasmodium falciparum. Notably, none of the compounds revealed potent antimalarial activity. However, it was observed that the activity of some of the compounds was enhanced because of hybridization and could be a starting point for further investigation.
Isatin-pyrimidine hybrids
Pyrimidines represent one of the most active classes of compounds with a wide spectrum of biological activities that can be exploited for drug discovery [128]. Substituted pyrimidines are widely distributed in nature and are one of the first compounds that were studied by organic chemists. They can be found in both natural products (Vitamin B1) and synthetic compounds (Barbituric acid and Veranal) used as hypnotics [129]. The chemical structures of some isatin-pyrimidine hybrids are presented in Fig. 21.
Fig. 21.
Chemical structures of isatin-pyrimidine hybrids
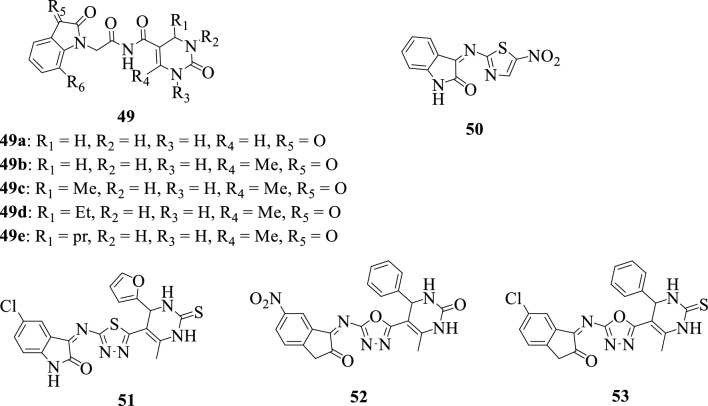
In 2016, Devale et al. [54] reported the synthesis of a series of isatin-pyrimidine hybrids 49a–e. These compounds were screened for their in vitro Reverse Transcriptase (RT) inhibitory activity against the HIV-1 virus, resulting in the identification of two hybrids 49c and 49d with higher RT inhibitory activity when compared to Rilpivirine, a reference drug. SAR studies revealed that the presence of aliphatic substituents rather than aromatic substituents at position R1 greatly favored the inhibitory activity of the compounds.
Akhaja et al., 2012 [130] reported the synthesis and in vitro evaluation of some isatin-pyrimidine hybrids 50–53 as antitubercular agents. Most of the synthesized compounds revealed moderate activity with the hybrids 50 and 51 being the most active against MTB H37Rv. Notably, hybrids 52 and 53 were found to completely inhibit MTB H37Rv by 99% at an MIC of 3.10–3.12 mg/mL.
Isatin-quinoline hybrids
The quinoline moiety, a nitrogen-containing heterocyclic compound, can be found in several natural compounds. It is one of the most recognized fragments in bioactive compounds and is found in different pharmaceutically important alkaloids such as quinine and cinchonine. Pharmacological studies of quinoline have reported a broad spectrum of activities associated with this moiety [121, 122]. Figure 22 shows some of the chemical structures of isatin-quinoline hybrids.
Fig. 22.
Chemical structures of isatin-quinoline hybrids
Raj et al., 2014 [131] reported the synthesis and evaluation of antimalarial activity of two isatin-chloroquinoline hybrids 54 and 55 against chloroquine-resistant W2 strain of Plasmodium falciparum. The synthesized compounds were not as potent as standard antimalarial drugs. However, the most potent compound revealed activity that is comparable to that of Chloroquine thus suggesting these compounds could be a starting point for further research.
Isatin-thioacetazone hybrids
Thioacetazone is a bacteriostatic drug used in combination with other antimycobacterial agents to treat tuberculosis. However, the dermatological side effects associated with its use by AIDS patients have limited its exploitation. Thioacetazone has weak activity against MTB and is never used on its own. It is useful in preventing resistance to more powerful drugs like Isoniazid and Rifampicin [138]. The chemical structures of some isatin-thioacetazone hybrids are shown in Fig. 23.
Fig. 23.
Chemical structures of isatin-thioacetazone hybrids
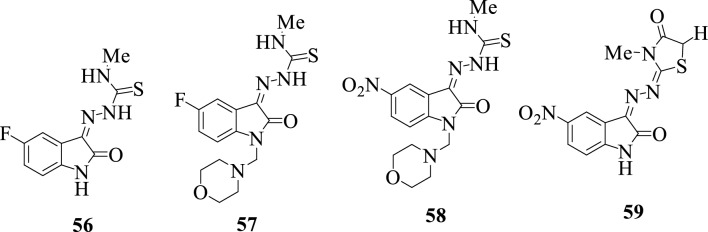
To develop new and more potent antitubercular agents, a series of thioacetazone-isatin hybrids 56–59 were synthesized. Hybrid 57 revealed quite interesting inhibitory activity against MTB H37Rv, while hybrid 58 was found to be the least potent, and SAR revealed that halogenation at position C-5, as well as the insertion of a substituent at the N-1, influenced the antitubercular activity of the compounds [17].
Other isatin hybrids
A series of isatin-imine 60a-60e analogs were successfully synthesized and evaluated for their antibacterial and antifungal activities against certain microbes by Debnath et al., 2015. Some of the compounds portrayed quite interesting properties with 60d being the most potent against the investigated microbes having the highest docking score. Structure–activity relationship studies revealed that the introduction of 2,5-dimethyl substituent at position R2 improved the activity of the compound [132]. Figure 24 presents some of the chemical structures of other isatin hybrids.
Fig. 24.
Chemical structures of other isatin hybrids
In 2018, Xu et al. [133] reported the synthesis of a series of ethylene tethered bis isatin derivatives 61a-i. The synthesized compounds were evaluated for their in vitro antimycobacterial activities against MTB H37Rv and MDR-TB. All tested compounds revealed interesting antimycobacterial properties with 61i being the most potent, and SAR illustrated that NNHCSNH2 at position C-3 and insertion of a halogen at C-5 greatly boosted the activity of this compound.
Teng et al., 2015 [134] reported the design and synthesis of a series of di- and tri-substituted isatin derivatives 62a-g and 63a-d, as well as the evaluation of their in vitro anticancer properties against human T-lymphocyte Jurkat cells. The compound 63a was found to be the most potent compound capable of inhibiting the proliferation of Jurkat cells by inducing apoptosis with an IC50 value of 0.03 µΜ. SAR studies demonstrated that the combination of a 1-benzyl and 5-[trans-2-(methoxycarbonyl)ethen-1-yl] substitution results in improved cytotoxic activity.
Wang et al., 2018 [60] while attempting to exploit the potentials in molecular hybridization for the development of anticancer drugs synthesized some novel isatin-α,β-unsaturated ketone hybrids 64a-64 k and 65a-65d. Most of the synthesized compounds revealed potent antiproliferative properties in the tested cell line, and SAR revealed that the inhibition activity of the compounds greatly depended on the electron-withdrawing substituent on the benzyl ring. The hybrid 65a was identified as the most potent hybrid which can be a promising lead compound for the development of anticancer agents.
Conclusions
The isatin privileged scaffold can be found in a broad range of natural and synthetically derived pharmacologically active compounds having antibacterial, antifungal, antiviral, anticancer, anti-inflammatory, anticonvulsant, antitubercular, antiparasitic, and antioxidant properties. This review compiles published data on the synthesis and biological properties of some isatin hybrids as potential drug targets in an active area of medicinal chemistry. The literature survey demonstrated that the N‐1, C‐3, C‐4, C‐5, and C‐7 positions of the isatin scaffold can be modified, and the N‐1, C‐3, and C‐5 positions are much more favorable for modifications. In addition, the introduction of electron-withdrawing groups at positions 5, 6, and 7 of the indole rings can greatly increase the activities of the hybrids in comparison with isatin. However, the mono-substitution at the C-5 position can be considered most favorable since it is beneficial to control the electronic effect, lipophilicity, and physicochemical properties. For the N‐1 position, N-alkyl, -aryl, and -acyl substitutions are possible including azole. For C‐3 position, imine, hydrazone, and spiro‐ring are most common, but other pharmacophores, such as azole, are also tolerated. Among the isatin hybrids in this review, hybrids 2b, 12c, and 20e showed interesting anticancer properties with IC50 values 2.14, 1.17 µM, and 3.67 ± 0.33 µM, respectively. Hybrids 5 g and 8e possessed promising antibacterial properties with MIC (minimal inhibition concentration) values 8 and < 1 µg/mL, respectively. Hybrids 7 and 13b with the isatin moiety substituted at C-3 position expressed interesting anticonvulsant properties. Summarily, hybrids 6j and 45f showed antiparasitic properties, hybrids 9d and 15d antimycobacterial properties, hybrids 11e and 21e antiviral property, and hybrids 14c and 16 antifungal properties. The compounds discussed in this review could serve as a starting point for further research on promising therapeutic drug candidates. Therefore, the concept of molecular hybridization with the possible modifications on the isatin moiety at the N-1, C-3, and C-5 positions can result in an array of compounds with diverse biological properties.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- SAR
Structure–activity relationship
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- IC50
Half maximal inhibitory concentration
- MES
Maxima electroshock seizure
- sc-PTZ
Subcutaneous pentylenetetrazole
- hCA
Human carbonic anhydrase
- AChE
Acetylcholinesterase
- SOD
Superoxide dismutase
- GSH-Px
Glutathione peroxidase
- CAT
Catalase
- NNRTIs
Non-nucleoside reverse transcriptase inhibitors
- RSV
Respiratory syncytial virus
- MTB H37Rv
Mycobacterium tuberculosis Strain H37Rv
- MDR-TB
Multidrug-resistant tuberculosis
- MIC
Minimum inhibitory concentration
- RT
Reverse transcriptase
Author contributions
Conceptualization: S.V.A., D.B.E, and F.N.-K.; methodology: S.V.A., D.B.E, and F.N.-K.; investigation: S.V.A. and D.B.E.; resources: D.B.E and F.N.-K.; data curation: S.V.A., D.B.E, and F.N.-K.; writing—original draft preparation: S.V.A. and D.B.E; writing—review and editing: S.V.A., D.B.E, and F.N.-K.; supervision: D.B.E and F.N.-K.; project administration: D.B.E and F.N.-K.; funding acquisition: F.N.-K. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge financial support from the Bill & Melinda Gates Foundation through the Calestous Juma Science Leadership Fellowship awarded to Fidele Ntie-Kang (grant award number: INV-036848 to University of Buea). FNK also acknowledges joint funding from the Bill & Melinda Gates Foundation (award number: INV-055897) and LifeArc (grant ID: 10646) under the African Drug Discovery Accelerator program. FNK acknowledges further funding from the Alexander von Humboldt Foundation for a Research Group Linkage project.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Donatus Bekindaka Eni, Email: donatus.bekindaka@ubuea.cm.
Fidele Ntie-Kang, Email: fidele.ntie-kang@ubuea.cm.
References
- 1.Medvedev A, Buneeva O, Gnedenko O, Ershov P, Ivanov A (2018) Isatin, an endogenous nonpeptide biofactor; a review of its molecular targets, mechanisms of actions, and their biomedical implications. BioFactors 44:95–108 [DOI] [PubMed] [Google Scholar]
- 2.Shalini SC, Arora A, Kumar V (2022) A mini review on isatin, an anticancer scaffold with potential activities against neglected tropical diseases (NTDs). Pharmaceuticals 15:536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagnou M, Mavroidi B, Kaminari A, Boukos N, Pelecanou M (2020) Novel isatin thiosemicarbazone derivatives as potent inhibitors of β-amyloid peptide aggregation and toxicity. ACS Chem Neurosci 11:2266–2276 [DOI] [PubMed] [Google Scholar]
- 4.Rezki N, Almehmadi MA, Ihmaid S, Shehata AM, Omar AM, Ahmed HEA, Aouad MA (2020) Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1,2,3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors; synthesis, antitumor and mechanistic analyses. Bioorg Chem 103:104133 [DOI] [PubMed] [Google Scholar]
- 5.Pakravan P, Kashanian S, Khodaei MM, Harding FA (2013) Biochemical and pharmacological characterization of isatin and its derivatives; from structure to activity. Pharmacol Rep 65:313–335 [DOI] [PubMed] [Google Scholar]
- 6.Chauhan G, Pathak DP, Ali F, Bhutani R, Kapoor G, Khasimbi S (2021) Advances in synthesis, derivatization and bioactivity of isatin; a review. Curr Org Synth 18:37–74 [DOI] [PubMed] [Google Scholar]
- 7.Ding Z, Zhou M, Zeng C (2020) Recent advances in isatin hybrids as potential anticancer agents. Arch Pharm 353:e1900367 [DOI] [PubMed] [Google Scholar]
- 8.Hou Y, Shang C, Wang H, Yun J (2020) Isatin–azole hybrids and their anticancer activities. Arch Pharm 353:e1900272 [DOI] [PubMed] [Google Scholar]
- 9.Guo H (2019) Isatin derivatives and their anti-bacterial activities. Eur J Med Chem 164:678–688 [DOI] [PubMed] [Google Scholar]
- 10.Nikalje AP, Ansari A, Bari S, Ugale V (2015) Synthesis, biological activity, and docking study of novel isatin coupled thiazolidin-4-one derivatives as anticonvulsants. Arch Pharm 348:433–445 [DOI] [PubMed] [Google Scholar]
- 11.Smitha S, Pandeya SN, Stables JP, Ganapathy S (2008) Anticonvulsant and sedative-hypnotic activities of n-acetyl/methyl isatin derivatives. Sci Pharm 76:621–636 [Google Scholar]
- 12.Chahal V, Nirwan S, Kakkar R (2019) Isatin and its derivatives; a survey of recent syntheses, reactions, and applications. Med Chem Commun 10:351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motiwale M, Yadav NS, Kumar S, Kushwaha T, Choudhir G, Sharma S, Singour PK (2022) Finding potent inhibitors for COVID-19 main protease (Mpro); an in silico approach using SARSCoV-3CL protease inhibitors for combating CORONA. J Biomol Struct Dyn 40:1534–1545 [DOI] [PubMed] [Google Scholar]
- 14.Badavath VN, Kumar A, Samanta PK, Maji S, Das A, Blum G, Jha A, Sen A (2022) Determination of potential inhibitors based on isatin derivatives against SARS-CoV-2 main protease (Mpro); a molecular docking, molecular dynamics and structure-activity relationship studies. J Biomol Struct Dyn 40:3110–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bal TR, Anand B, Yogeeswari P, Sriram D (2005) Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorg Med Chem Lett 15:4451–4455 [DOI] [PubMed] [Google Scholar]
- 16.Sriram D, Yogeeswari P, Meena K (2006) Synthesis, anti-HIV and antitubercular activities of isatin derivatives. Pharmazie 61:274–277 [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Zhang S, Gao C, Fan J, Zhao F, Lv Z, Feng L (2017) Isatin hybrids and their anti-tuberculosis activity. Chin Chem Lett 28:159–167 [Google Scholar]
- 18.Gao F, Ye L, Wang Y, Kong F, Zhao S, Xiao J, Huang G (2019) Benzofuran-isatin hybrids and their in vitro anti-mycobacterial activities against multi-drug resistant Mycobacterium tuberculosis. Eur J Med Chem 183:111678 [DOI] [PubMed] [Google Scholar]
- 19.Gao F, Chen Z, Ma L, Fan Y, Chen L, Lu G (2019) Synthesis and biological evaluation of moxifloxacin-acetyl-1,2,3–1Htriazole-methylene-isatin hybrids as potential anti-tubercular agents against both drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. Eur J Med Chem 180:648–655 [DOI] [PubMed] [Google Scholar]
- 20.Thanh ND, Giang NTK, Quyen TH, Huong DT, Toan VN (2016) Synthesis and evaluation of in vivo antioxidant, in vitro antibacterial, MRSA and antifungal activity of novel substituted isatin N-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)thiosemicarbazones. Eur J Med Chem 123:532–543 [DOI] [PubMed] [Google Scholar]
- 21.Akdemir A, Güzel-Akdemir Ö, Karali N, Supuran CT (2016) Isatin analogs as novel inhibitors of Candida spp. beta-carbonic anhydrase enzymes. Bioorg Med Chem 24:1648–1652 [DOI] [PubMed] [Google Scholar]
- 22.Susithra E, Rajkumar S, Pansare SKW, Praveena S, Arun PVPS (2022) Design, synthesis, antimicrobial and anticancer activity of some novel benzoxazole-isatin conjugates. Biointerface Res Appl Chem 12:2392–2403 [Google Scholar]
- 23.Tangadanchu VKR, Sui Y, Zhou C (2021) Isatin-derived azoles as new potential antimicrobial agents; design, synthesis and biological evaluation. Bioorg Med Chem Lett 41:128030 [DOI] [PubMed] [Google Scholar]
- 24.Muglu H, Cavus MS, Bakir T, Yakan H (2019) Synthesis, characterization, quantum chemical calculations and antioxidant activity of new bis-isatin carbohydrazone and thiocarbohydrazone derivatives. J Mol Struct 1196:819–827 [Google Scholar]
- 25.Wakchaure ND (2012) Review on common methods to synthesize substituted 1H-indole-2,3-dione (isatin) derivatives and their medicinal significance. Am J Pharmtech Res v:289–310 [Google Scholar]
- 26.Nisha JG, Rosenthal PJ, Kumar V (2014) β-amino-alcohol tethered 4-aminoquinoline-isatin conjugates; synthesis and antimalarial evaluation. Eur J Med Chem 24:566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raj R, Gut J, Rosenthal PJ, Kumar V (2014) 1H–1,2,3-Triazole-tethered isatin-7-chloroquinoline and 3-hydroxy-indole-7-chloroquinoline conjugates; synthesis and antimalarial evaluation. Bioinorg Med Chem Lett 24:756–759 [DOI] [PubMed] [Google Scholar]
- 28.Sharma PK, Balwani S, Mathur D, Malhotra S, Singh BK, Prasad AK, Len C, Eycken EVV, Ghosh B, Richards NG, Parmar VS (2016) Synthesis and anti-inflammatory activity evaluation of novel triazolyl-isatin hybrids. J Enzyme Inhib Med Chem 31:1520–1526 [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim MM, Elsaman T, Al-Nour MY (2018) Synthesis, anti-inflammatory activity, and in silico study of novel diclofenac and isatin conjugates. Int J Med Chem 2018:9139786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravindar L, Hasbullah SA, Rakesh KP, Raheem S, Agustar HK, Ismail N, Ling LY, Hassan NI (2024) Exploring diverse frontiers: advancements of bioactive 4-aminoquinoline-based molecular hybrids in targeted therapeutics and beyond. Eur J Med Chem 264:116043. 10.1016/j.ejmech.2023.116043 [DOI] [PubMed] [Google Scholar]
- 31.Ravindar L, Hasbullah SA, Rakesh KP, Hassan NI (2023) Triazole hybrid compounds: a new frontier in malaria treatment. Eur J Med Chem 259:115694. 10.1016/j.ejmech.2023.115694 [DOI] [PubMed] [Google Scholar]
- 32.Ravindar L, Hasbullah SA, Rakesh KP, Hassan NI (2023) Recent developments in antimalarial activities of 4-aminoquinoline derivatives. Eur J Med Chem 256:115458. 10.1016/j.ejmech.2023.115458 [DOI] [PubMed] [Google Scholar]
- 33.Sridhara MB, Rakesh KP, Manukumar HM, Shantharam CS, Vivek HK, Kumara HK, Mohammed YHE, Gowda DC (2020) Synthesis of dihydrazones as potential anticancer and DNA binding candidates: a validation by molecular docking studies. Anticancer Agents Med Chem 20(7):845–858. 10.2174/1871520620666200225104558 [DOI] [PubMed] [Google Scholar]
- 34.Moku B, Ravindar L, Rakesh KP, Qin HL (2019) The significance of N-methylpicolinamides in the development of anticancer therapeutics: synthesis and structure-activity relationship (SAR) studies. Bioorg Chem 86:513–537. 10.1016/j.bioorg.2019.02.030 [DOI] [PubMed] [Google Scholar]
- 35.Rakesh KP, Darshini N, Manukumar HM, Vivek HK, Eissa MYH, Prasanna DS, Mallesha N (2018) Xanthone conjugated amino acids as potential anticancer and DNA binding agents: molecular docking, cytotoxicity and SAR studies. Anticancer Agents Med Chem 18(15):2169–2177. 10.2174/1871520618666180903105256 [DOI] [PubMed] [Google Scholar]
- 36.Rakesh KP, Vivek HK, Manukumar HM, Shantharam CS, Bukhari SNA, Qin HL, Sridhara MB (2018) Promising bactericidal approach of dihydrazone analogues against bio-film forming Gram-negative bacteria and molecular mechanistic studies. RSC Adv 8(10):5473–5483. 10.1039/c7ra13661g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdulrahmana SH, Al-healya FM, Ali WK (2021) A review on computational study of tribulin compound and its derivatives; QSAR studies. Ann Romanian Soc Cell Biol 25:8725–8735 [Google Scholar]
- 38.Ma T, Chen R, Xue H, Miao Z, Chen L, Zhang H, Shi X (2019) Di-isatin heteronuclear compounds and their antibacterial activity. J Heterocycl Chem 57:503–509 [Google Scholar]
- 39.Xu J-H, Fan YL, Zhou J (2018) Quinolone–triazole hybrids and their biological activities. J Heterocycl Chem 55:1854–1862 [Google Scholar]
- 40.Song F, Li Z, Bian Y, Huo X, Fang J, Shao L, Zhou M (2020) Indole/isatin-containing hybrids as potential antibacterial agents. Arch Pharm 353:e2000143 [DOI] [PubMed] [Google Scholar]
- 41.M RK, Gideon DA, Mariadasse R, Nirusimhan V, A SR, Edward JC, Jeyaraman J, Dhayabaran V (2022) In silico evaluation of isatin-based derivatives with RNA-dependent RNA polymerase of the novel coronavirus SARS-CoV-2. J Biomol Struct Dyn 40:6710–6724 [DOI] [PubMed] [Google Scholar]
- 42.Afroz M, Vasanthi R, Fathima A (2021) A review on medicinal importance of isatin scaffolds with anti-mycobacterial activity. J Cardiovasc Dis Res 12:1155–1170 [Google Scholar]
- 43.Freitas LAB, Santos ACS, Silva GDC, Albuquerque FNN, Silva ED, Simone CA, Pereira VRA, Alves LC, Gomes PATM (2021) Structural improvement of new thiazolyl-isatin derivatives produces potent and selective trypanocidal and leishmanicidal compounds. Chem Biol Interact 345:109561 [DOI] [PubMed] [Google Scholar]
- 44.Babita A, Khan NS, Khan P, Queen A, Hussain A, Rehman MT, Alajmi MF, El-seedi H, Ali S, Hassan MI, Abid M (2019) Design and development of Isatin-triazole hydrazones as potential inhibitors of microtubule affinity-regulating kinase 4 for the therapeutic management of cell proliferation and metastasis. Eur J Med Chem 163:840–852 [DOI] [PubMed] [Google Scholar]
- 45.Jiang Y, Qian A, Li Y (2019) 1H–1,2,3-Triazole tethered isatin-moxifloxacin; design, synthesis and in vitro anti-mycobacterial evaluation. Arch Pharm 352:1900040 [DOI] [PubMed] [Google Scholar]
- 46.Gao F, Wang T, Gao M, Zhang X, Liu Z, Zhao S, Lv Z, Xiao J (2019) Benzofuran-isatin-imine hybrids tethered via different length alkyl linkers; design, synthesis and in vitro evaluation of anti-tubercular and anti-bacterial activities as well as cytotoxicity. Eur J Med Chem 165:323–331 [DOI] [PubMed] [Google Scholar]
- 47.Liang P-H (2006) Characterization and inhibition of SARS-coronavirus main protease. Curr Top Med Chem 6:361–376 [DOI] [PubMed] [Google Scholar]
- 48.Al-Wabli RI, Almomen AA, Almutari MS, Keeton AB, Piazza GA, Attia MI (2020) New isatin–indole conjugates; synthesis, characterization, and a plausible mechanism of their in vitro antiproliferative activity. Drug Des Dev Ther 14:483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Zhao S, Lv Z, Gao F, Wang Y, Zhang F, Bai L, Deng J (2019) Fluoroquinolone-isatin hybrids and their biological activities. Eur J Med Chem 162:396–406 [DOI] [PubMed] [Google Scholar]
- 50.Limpachayaporn P, Wagner S, Kopka K, Schober MS, Haufe G (2014) Synthesis of 7-halogenated isatin sulfonamides; nonradioactive counterparts of caspase-3/-7 inhibitor-based potential radiopharmaceuticals for molecular imaging of apoptosis. J Med Chem 57:9383–9395 [DOI] [PubMed] [Google Scholar]
- 51.Selvam P, Chandramohan M, Hurst B, Smee DF (2010) Activity of isatine-sulfadimidine derivatives against 2009 pandemic H1N1 influenza virus in cell culture. Antiviral Chem Chemother 20:143–145 [DOI] [PubMed] [Google Scholar]
- 52.Eldehna WM, Altoukhy A, Mahrous H, Abdel-Aziz HA (2015) Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential antiproliferative agents. Eur J Med Chem 90:684–694 [DOI] [PubMed] [Google Scholar]
- 53.Kitagawa DAS, Rodrigues RF, Silva TN, Santos WV, Rocha VCV, Almeida JSFD, Bernardo LB, Carvalho-Silva T, Nepovimova E, Kuca K, Franca TCC, Cavalcante SFA (2021) Design, synthesis, in silico studies and in vitro evaluation of isatin-pyridine oximes hybrids as novel acetylcholinesterase reactivators. J Enzyme Inhib Med Chem 36:1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devale TL, Parikh J, Miniyar P, Sharma P, Shrivastava B, Murumkar P (2017) Dihydropyrimidinone-isatin hybrids as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg Chem 70:256–266 [DOI] [PubMed] [Google Scholar]
- 55.Elsayed ZM, Eldehna WM, Abdel-Aziz M, Hassab MAE, Elkaeed EB, Al-Warhi T, Abdel-Aziz H, Abou-seri S, Mohammed ER (2021) Development of novel isatin–nicotinohydrazide hybrids with potent activity against susceptible/resistant Mycobacterium tuberculosis and bronchitis causing–bacteria. J Enzyme Inhib Med Chem 36:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fayed EA, Eldin RRE, Mehany ABM, Bayoumi AH, Ammar YA (2021) Isatin-Schiff’s base and chalcone hybrids as chemically apoptotic inducers and EGFR inhibitors; design, synthesis, anti-proliferative activities and in silico evaluation. J Mol Struct 1234:130159 [Google Scholar]
- 57.Eldehna WM, Almahli H, Al-Ansary GH, Ghabbour HA, Aly MH, Ismael OE, Al-Dhfyan A, Abdel-Aziz HA (2017) Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosisinducing agents. J Enzyme Inhib Med Chem 32:600–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fares M, Eldehna WM, Abou-Seri SM, Abdel-Aziz HA, Aly MH, Tolba MF (2015) Design, synthesis and in vitro antiproliferative activity of novel isatin-quinazoline hybrids. Arch Pharm 348:144–154 [DOI] [PubMed] [Google Scholar]
- 59.Sin N, Venables BL, Combrink KD, Gulgeze HB, Yu K, Civiello RL, Thuring J, Wang XA, Yang Z, Zadjura L, Marino A, Kadow KF, Cianci CW, Clarke J, Genovesi EV, Medina I, Lamb L, Krystal M, Meanwell NA (2009) Respiratory syncytial virus fusion inhibitors: part 7; structure–activity relationships associated with a series of isatin oximes that demonstrate antiviral activity in vivo. Bioorg Med Chem Lett 19:4857–4862 [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Yun D, Yao J, Fu W, Huang F, Chen L, Wei T, Yu C, Xu H, Zhou X, Huang Y, Wu J, Qui P, Li W (2018) Design, synthesis and QSAR study of novel isatin analogues inspired Michael acceptor as potential anticancer compounds. Eur J Med Chem 144:493–503 [DOI] [PubMed] [Google Scholar]
- 61.Omar AZ, Mosa TW, El-Sadany SK, Hamed EA, El-Atawy M (2021) Novel piperazine based compounds as potential inhibitors for SARS-CoV-2 protease enzyme; synthesis and molecular docking study. J Mol Struct 1245:131020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar K, Sagar S, Esau L, Kaur M, Kumar V (2012) Synthesis of novel 1H–1,2,3-triazole tethered C-5 substituted uracile isatin conjugates and their cytotoxic evaluation. Eur J Med Chem 58:153–159 [DOI] [PubMed] [Google Scholar]
- 63.Khatoon S, Aroosh A, Islam A, Kalsoom S, Ahmad F, Hameed S, Abbasi SW, Yasinzai M, Naseer MM (2021) Novel coumarin-isatin hybrids as potent antileishmanial agents; synthesis, in silico and in vitro evaluations. Bioorg Chem 110:104816 [DOI] [PubMed] [Google Scholar]
- 64.Hans RH, Wiid IJF, Helden PDV, Wan B, Franzblau SG, Gut J, Rosenthal PJ, Chibale K (2011) Novel thiolactone–isatin hybrids as potential antimalarial and antitubercular agents. Bioorg Med Chem Lett 21:2055–2058 [DOI] [PubMed] [Google Scholar]
- 65.Bozorova K, Zhaoa J, Aisa HA (2019) 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry; a recent overview. Bioorg Med Chem 27:3511–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bennani FE, Doudach L, Cherrah Y, Ramli Y, Karrouchi K, Ansar M, Faouzi MEA (2020) Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg Chem 97:103470 [DOI] [PubMed] [Google Scholar]
- 67.Rostom SAF, Ashour HMA, Razik HAAE, Fattah AEFHAE, El-Din NN (2009) Azole antimicrobial pharmacophore-based tetrazoles; synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorg Med Chem 17:2410–2422 [DOI] [PubMed] [Google Scholar]
- 68.Gao F, Wang T, Xiao J, Huang G (2019) Antibacterial activity study of 1,2,4-triazole derivatives. Eur J Med Chem 173:274–281 [DOI] [PubMed] [Google Scholar]
- 69.Shu Zhang ZX, Xu Z, Gao C, Ren Q, Lv LCZ, Feng L (2017) Triazole derivatives and their anti-tubercular activity. Eur J Med Chem 138:501–513 [DOI] [PubMed] [Google Scholar]
- 70.Ding Z, Hou P, Liu B (2019) Gatifloxacin-1,2,3-triazole-isatin hybrids and their antimycobacterial activities. Arch Pharm 352:e1900135 [DOI] [PubMed] [Google Scholar]
- 71.Solomon VR, Hu C, Lee H (2009) Hybrid pharmacophore design and synthesis of isatin–benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem 17:7585–7592 [DOI] [PubMed] [Google Scholar]
- 72.Javida MT, Rahim F, Taha M, Nawaz M, Wadood A, Ali M, Mosaddik A, Shah SAA, Farooq RK (2018) Synthesis, SAR elucidations and molecular docking study of newly designed isatin based oxadiazole analogs as potent inhibitors of thymidine phosphorylase. Bioorg Chem 79:323–333 [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim HS, Abou-seri SM, Tanc M, Elaasser MM, Abdel-Aziz HA, Supuran CT (2015) Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumorassociated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 103:583–593 [DOI] [PubMed] [Google Scholar]
- 74.El-Naggar M, Eldehna WM, Almahli H, Elgez A, Fares M, Elaasser MM, Abdel-Aziz HA (2018) Novel thiazolidinone/thiazolo[3,2-a] benzimidazolone-isatin conjugates as apoptotic anti-proliferative agents towards breast cancer; one-pot synthesis and in vitro biological evaluation. Molecules 23:1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eldehna WM, El Hassab MA, Abo-Ashour MF, Al-Warhi T, Elaasser MM, Safwat NA, Suliman H, Ahmed M, Al-Rashood ST, Abdel-Aziz HA, El-Haggar R (2021) Development of isatin-thiazolo[3,2-a]benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity; synthesis, biological and molecular dynamics investigations. Bioorg Chem 110:104748 [DOI] [PubMed] [Google Scholar]
- 76.Eldehna WM, Al-Wabli RI, Almutairi MS, Keeton AB, Piazza GA, Abdel-Aziz HA, Attia MI (2018) Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent anti-proliferative agents. J Enzyme Inhib Med Chem 33:867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Özil M, Menteşe E, Yılmaz F, İslamoğlu F, Kahveci B (2011) Synthesis of novel triazol compounds containing isatin as potential antibacterial and antifungal agents by microwave and conventional methods. J Chem Res 35:268–271 [Google Scholar]
- 78.Xiang P, Cao Q, Dong Q, Yang X, Tang J, Bai H (2018) Furan-site transformations of obacunone as potent insecticidal agents. Heliyon 4:e01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ansari MF, Siddiqui SM, Ahmad K, Avecilla F, Dharavath S, Gourinath S, Azam A (2016) Synthesis, antiamoebic and molecular docking studies of furan-thiazolidinone hybrids. Eur J Med Chem 124:393–406 [DOI] [PubMed] [Google Scholar]
- 80.Gao F, Yang H, Lu T, Chen Z, Ma L, Xu Z, Schaffer P, Lu G (2018) Design, synthesis and anti-mycobacterial activity evaluation of benzofuran-isatin hybrids. Eur J Med Chem 159:277–281 [DOI] [PubMed] [Google Scholar]
- 81.Archna SP, Chawla PA (2020) Thiophene-based derivatives as anticancer agents; An overview on decade’s work. Bioorg Chem 101:104026 [DOI] [PubMed] [Google Scholar]
- 82.Schaper K, Müller TJJ (2018) thiophene syntheses by ring forming multicomponent reactions. Top Curr Chem 376:38 [DOI] [PubMed] [Google Scholar]
- 83.Chen L-R, Wang Y-C, Lin YW, Chou S-Y, Chen S-F, Liu LT, Wu Y-T, Kuo C-J, Chen TS-S, Juang S-H (2005) Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg Med Chem Lett 15:3058–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumari A, Singh RK (2019) Medicinal chemistry of indole derivatives; current to future therapeutic prospectives. Bioorg Chem 89:103021 [DOI] [PubMed] [Google Scholar]
- 85.Han Y, Dong W, Guo Q, Li X, Huang L (2020) The importance of indole and azaindole scaffold in the development of antitumor agents. Eur J Med Chem 203:112506 [DOI] [PubMed] [Google Scholar]
- 86.Patil R, Patil SA, Beaman KD, Patil SA (2016) Indole molecules as inhibitors of tubulin polymerization; potential new anticancer agents, an update (2013–2015). Future Med Chem 8:1291–1316 [DOI] [PubMed] [Google Scholar]
- 87.Sharma V, Kumar P, Pathak D (2010) Biological importance of the indole nucleus in recent years; a comprehensive review. J Heterocycl Chem 47:491–502 [Google Scholar]
- 88.Praveen C, Ayyanar A, Perumal PT (2011) Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorg Med Chem Lett 21:4072–4077 [DOI] [PubMed] [Google Scholar]
- 89.Mohammed AAM, Suaifan GARY, Shehadeh MB, Okechukwu PN (2020) Design, synthesis and antimicrobial evaluation of novel glycosylated-fluoroquinolones derivatives. Eur J Med Chem 202:112513 [DOI] [PubMed] [Google Scholar]
- 90.Patel MM, Patel LJ (2014) Design, synthesis, molecular docking, and antibacterial evaluation of some novel flouroquinolone derivatives as potent antibacterial agent. Sci World J 2014:897187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vu TH, Ha-Duong NT, Aubry A, Capton E, Fechter P, Plésiat P, Verbeke P, Serradji N (2019) In vitro activities of a new fluoroquinolone derivative highly active against Chlamydia trachomatis. Bioorg Chem 83:180–185 [DOI] [PubMed] [Google Scholar]
- 92.Prakash CR, Raja S (2013) Synthesis, characterization and in vitro antimicrobial activity of some novel 5-substituted Schiff and Mannich base of isatin derivatives. J Saudi Chem Soc 17:337–344 [Google Scholar]
- 93.Sriram D, Aubry A, Yogeeswari P, Fisher LM (2006) Gatifloxacin derivatives; synthesis, antimycobacterial activities, and inhibition of Mycobacterium tuberculosis DNA gyrase. Bioorg Med Chem Lett 16:2982–2985 [DOI] [PubMed] [Google Scholar]
- 94.Farag AA (2014) Synthesis and antimicrobial activity of 5-(morpholinosulfonyl)isatin derivatives incorporating a thiazole moiety. Drug Res 65:373–379 [DOI] [PubMed] [Google Scholar]
- 95.Abo-Ashour MF, Eldehna WM, Nocentini A, Bonardi A, Bua S, Ibrahim HS, Elaasser MM, Kryštof V, Jorda R, Gratteri P, Abou-Seri SM, Supuran CT (2019) 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity; synthesis, in vitro biological evaluation and in silico insights. Eur J Med Chem 184:111768 [DOI] [PubMed] [Google Scholar]
- 96.Eldehna WM, Nocentini A, Al-Rashood ST, Hassan GS, Alkahtani HM, Almehizia AA, Reda AM, Abdel-Aziz HA, Supuran CT (2018) Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in vitro anticancer activity towards colon cancer. Bioorg Chem 81:425–432 [DOI] [PubMed] [Google Scholar]
- 97.Albratty M, Alhazmi HA (2022) Novel pyridine and pyrimidine derivatives as promising anticancer agents; a review. Arab J Chem 15:103846 [Google Scholar]
- 98.Kumar VS, Verma R, Xue F, Kumar TP, Girish YR, Rakesh KP (2020) Antibacterial activities of sulfonyl or sulfonamide containing heterocyclic derivatives and its structure-activity relationships (SAR) studies; a critical review. Bioorg Chem 105:104400 [DOI] [PubMed] [Google Scholar]
- 99.Wan Y, Fang G, Chen H, Deng X, Tang Z (2021) Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur J Med Chem 226:113837 [DOI] [PubMed] [Google Scholar]
- 100.Mahapatra DK, Bharti DSK, Asati V (2015) Chalcone scaffolds as anti-infective agents; structural and molecular target perspectives. Eur J Med Chem 101:496–524 [DOI] [PubMed] [Google Scholar]
- 101.Sahu NK, Balbhadra SS, Choudhary J, Kohli DV (2012) Exploring pharmacological significance of chalcone scaffold; a review. Curr Med Chem 19:209–225 [DOI] [PubMed] [Google Scholar]
- 102.Hameeda A, Al-Rashida M, Uroos M, Ali SA, Arshia MI, Khan KM (2018) Quinazoline and quinazolinone as important medicinal scaffolds; a comparative patent review. Expert Opin Therap Patents 28:281–297 [DOI] [PubMed] [Google Scholar]
- 103.Khan I, Ibrar A, Abbas N, Saeed A (2014) Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds; synthetic approaches and multifarious applications. Eur J Med Chem 76:193–244 [DOI] [PubMed] [Google Scholar]
- 104.Bayoumi WA, Barghash AM, Gineinah MM, Massoud MA, Abdelal AM (2014) Design, synthesis and antioxidant evaluation of certain new phthalazine derivatives. Der Pharma Chemica 3:89–102 [Google Scholar]
- 105.Popiołek Ł (2017) Hydrazide–hydrazones as potential antimicrobial agents; overview of the literature since 2010. Med Chem Res 26:287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Angelova VT, Valcheva V, Vassilev NG, Buyukliev R, Momekov G, Dimitri I, Saso L, Djukic M, Shivachev B (2017) Antimycobacterial activity of novel hydrazide-hydrazone derivatives with 2H chromene and coumarin scaffold. Bioinorg Med Chem Lett 27:223–227 [DOI] [PubMed] [Google Scholar]
- 107.Salem MA, Ragab A, El-Khalafawy A, Makhlouf AH, Askar AA, Ammar YA (2020) Design, synthesis, in vitro antimicrobial evaluation and molecular docking studies of indol-2-one tagged with morpholinosulfonyl moiety as DNA gyrase inhibitors. Bioorg Chem 96:103619 [DOI] [PubMed] [Google Scholar]
- 108.Rawat P, Verma SM (2016) Synthesis and pharmacological evaluation of 6-hydroxy-2,5,7,8-tetramethylN’-(2-oxoindolin-3-ylidene)chroman-2-carbohydrazide derivatives as antimicrobial agents. J Chem Pharm Res 8:149–154 [Google Scholar]
- 109.Kalinowski DS, Quach P, Richardson DR (2009) Thiosemicarbazones; the new wave in cancer treatment. Future Med Chem 1:1143–1151 [DOI] [PubMed] [Google Scholar]
- 110.Yang Y, Pannecouque C, Clercq ED, Zhuang C, Chen F (2020) Privileged scaffold inspired design of novel oxime-biphenyl-DAPYs in treatment of HIV-1. Bioinorg Chem 99:103825 [DOI] [PubMed] [Google Scholar]
- 111.Moodie LWK, Cervin G, Trepos R, Labriere C, Hellio C, Pavia H, Svenson J (2018) Design and biological evaluation of antifouling dihydrostilbene oxime hybrids. Mar Biotechnol 20:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gornostaev LM, Tsvetkov VB, Markova AA, Lavrikova TI, Khalyavina YG, Kuznetsova A, Kaluzhny DN, Shunayev AV, Tsvetkova MV, Glazunova VA, Chernyshev V, Shtil AA (2017) The oxime derivatives of 1-R-1H-naphtho[2,3-d][1,2,3]triazole-4,9-dione 2-oxides; synthesis and properties. Anticancer Agents Med Chem 17:1814–1823 [DOI] [PubMed] [Google Scholar]
- 113.Ma C-M, Nakamura N, Hattori M, Kawahata T, Otake T (2002) Inhibitory effects of triterpene-azidothymidine conjugates on proliferation of human immunodeficiency virus type 1 and its protease. Chem Pharm Bull 50:877–880 [DOI] [PubMed] [Google Scholar]
- 114.Floyd RA, Kopke RD, Choi C-H, Foster SB, Doblas S, Towner RA (2008) Nitrones as therapeutics. Free Radical Biol Med 45:1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosselin M, Poeggeler B, Durand G (2017) Nitrone derivatives as therapeutics; from chemical modification to specific-targeting. Curr Top Med Chem 17:2006–2022 [DOI] [PubMed] [Google Scholar]
- 116.Firuzi O, Miri R, Tavakkoli M, Saso L (2011) Antioxidant therapy; current status and future prospects. Curr Med Chem 18:3871–3888 [DOI] [PubMed] [Google Scholar]
- 117.Marco-Contelles J (2020) Recent advances on nitrones design for stroke treatment. J Med Chem 63:13413–13427 [DOI] [PubMed] [Google Scholar]
- 118.Kant R, Maji S (2021) Recent advances in synthesis of piperazine based ligands, metal complexes and their applications. Dalton Trans 50:785–800 [DOI] [PubMed] [Google Scholar]
- 119.Jain A, Chaudhary J, Khaira H, Chopra B, Dhingra A (2021) Piperazine; a promising scaffold with analgesic and anti-inflammatory potential. Drug research 71:62–72 [DOI] [PubMed] [Google Scholar]
- 120.Shaquiquzzaman M, Verma G, Marella A, Akhter M, Akhtar W, Khan MF, Tasneem S, Alam MM (2015) Piperazine scaffold; a remarkable tool in generation of diverse pharmacological agents. Eur J Med Chem 102:487–529 [DOI] [PubMed] [Google Scholar]
- 121.Zhang L, Xu Z (2019) Coumarin-containing hybrids and their anticancer activities. Eur J Med Chem 181:111587 [DOI] [PubMed] [Google Scholar]
- 122.Feng D, Zhang A, Yang Y, Yang P (2020) Coumarin-containing hybrids and their antibacterial activities. Arch Pharm 353:e1900380 [DOI] [PubMed] [Google Scholar]
- 123.Stefanachi A, Leonetti F, Pisani L, Catto M, Carotti A (2018) Coumarin; a natural, privileged and versatile scaffold for bioactive compounds. Molecules 23:250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katerinopoulos HE (2004) The coumarin moiety as chromophore of fluorescent ion indicators in biological systems. Curr Pharm Des 10:3835–3851 [DOI] [PubMed] [Google Scholar]
- 125.Diao Q-P, Guo H, Wang G-Q (2019) Design, synthesis, and in vitro anticancer activities of diethylene glycol tethered isatin-1,2,3-triazole-coumarin Hybrids. J Heterocycl Chem 5:1667–1671 [Google Scholar]
- 126.Huang G-C, Xu Y, Xu Z, Lv Z-S, Zhang J, Guo H-Y, Hu Y-Q, Liu M-L, Guan J, Lu Y (2018) Propylene-1H-1,2,3-triazole-4-methylene-tethered isatin-coumarin hybrids; design, synthesis, and in vitro anti-tubercular evaluation. J Heterocycl Chem 55:830–835 [Google Scholar]
- 127.Beck B, Khoury KK, Herdtweck E, Domling A (2010) One-pot multicomponent synthesis of two novel thiolactone scaffolds. Mol Diversity 14:479–491 [DOI] [PubMed] [Google Scholar]
- 128.Kumar S, Deep A, Narasimhan B (2015) Pyrimidine derivatives as potential agents acting on central nervous system. Cent Nerv Syst Agents Med Chem 15:5–10 [DOI] [PubMed] [Google Scholar]
- 129.He ZX, Zhao TQ, Gong YP, Zhang X, Ma LY, Liu HM (2020) Pyrimidine; A promising scaffold for optimization to develop the inhibitors of ABC transporters. Eur J Med Chem 200:112458 [DOI] [PubMed] [Google Scholar]
- 130.Akhaja TN, Raval JP (2012) Design, synthesis, in vitro evaluation of tetrahydropyrimidine-isatin hybrids as potential antibacterial, antifungal and anti-tubercular agents. Chinese Chem Lett 23:446–449 [Google Scholar]
- 131.Raj R, Biot C, Carrere-Kremer S, Kremer L, Guerardel Y, Gut J, Rosenthal PJ, Forge D, Kumar V (2014) 7-Chloroquinoline–isatin conjugates; antimalarial, antitubercular, and cytotoxic evaluation. Chem Biol Drug Des 83:622–629 [DOI] [PubMed] [Google Scholar]
- 132.Debnath B, Ganguly S (2015) Synthesis, biological evaluation, in silico docking and virtual ADME studies of novel isatin analogs as promising antimicrobial agents. Anti-Infect Agents 13:139–153 [Google Scholar]
- 133.Xu Y, Guan J, Xu Z, Zhao S (2018) Design, synthesis and in vitro anti-mycobacterial activities of homonuclear and heteronuclear bis-isatin derivatives. Fitoterapia 127:383–386 [DOI] [PubMed] [Google Scholar]
- 134.Teng YO, Zhao H-Y, Wang J, Liu H, Gao M-L, Zhou Y, Han KL, Fan Z-C, Zhang Y-M, Sun H, Yu P (2016) Synthesis and anti-cancer activity evaluation of 5-(2-carboxyethenyl)-isatin derivatives. Eur J Med Chem 112:145–156 [DOI] [PubMed] [Google Scholar]
- 135.Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z (2017) Chalcone; a privileged structure in medicinal chemistry. Chem Rev 117:7762–7810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ouyang Y, Li J, Chen X, Fu X, Sun S, Wu Q (2021) Chalcone derivatives; role in anticancer therapy. Biomolecules 11:894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang X-M, Guo H, Li ZS, Song F-H, Wang W-M, Dai H-Q, Wang J-G (2015) Synthesis and evaluation of isatin-β-thiosemicarbazones as novel agents against antibiotic-resistant Gram-positive bacterial species. Eur J Med Chem 101:419–430 [DOI] [PubMed] [Google Scholar]
- 138.Alahari A, Trivelli X, Guérardel Y, Dover LG, Besra GS, Sacchettini JC, Reynolds RC, Coxon GD, Kremer L (2007) Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria. PLoS ONE 2:e1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raj R, Singh P, Haberkern NT, Faucher RM, Patel N, Land KM, Kumar V (2013) Synthesis of 1H–1,2,3-triazole linked beta-lactameisatin bi-functional hybrids and preliminary analysis of in vitro activity against the protozoal parasite Trichomonas vaginalis. Eur J Med Chem 63:897–906 [DOI] [PubMed] [Google Scholar]
- 140.Nagarsenkar A, Guntuku L, Guggilapu SD, Bai KD, Srinivasulu G, Naidu VGM, Babu BN (2016) Synthesis and apoptosis inducing studies of triazole linked 3-benzylidene isatin derivatives. Eur J Med Chem 124:782–793 [DOI] [PubMed] [Google Scholar]
- 141.Abo-Ashour MF, Eldehna WM, Nocentini A, Ibrahim HS, Bua S, Abou-Seri SM, Supuran CT (2018) Novel hydrazido benzenesulfonamides-isatin conjugates; Synthesis, carbonic anhydrase inhibitory activity and molecular modeling studies. Eur J Med Chem 157:28–36 [DOI] [PubMed] [Google Scholar]
- 142.Guo H (2018) Design, synthesis, and in vitro anti-mycobacterial activities of propylene tethered benzofuran–isatin hybrids. J Heterocycl Chem 58:338–342 [Google Scholar]
- 143.Kumar S, Saha ST, Gu L, Palma G, Perumal S, Singh-Pillay A, Singh P, Anand A, Kaur M, Kumar V (2018) 1H–1,2,3-Triazole tethered nitroimidazole−isatin conjugates; synthesis, docking, and anti-proliferative evaluation against breast cancer. ACS Omega 3:12106–12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.