Abstract
The order Holosporales is a broad and ancient lineage of bacteria obligatorily associated with eukaryotic hosts, mostly protists. Significantly, this is similar to other evolutionary distinct bacterial lineages (e.g. Rickettsiales and Chlamydiae). Here, we provide a detailed and comprehensive account on the current knowledge on the Holosporales. First, acknowledging the up-to-date phylogenetic reconstructions and recent nomenclatural proposals, we reevaluate their taxonomy, thus re-ranking them as a suborder, i.e. Holosporineae, within the order Rhodospirillales. Then, we examine the phylogenetic diversity of the Holosporineae, presenting the 20 described genera and many yet undescribed sub-lineages, as well as the variety of the respective environments of provenance and hosts, which belong to several different eukaryotic supergroups. Noteworthy representatives of the Holosporineae are the infectious intranuclear Holospora, the host manipulator ‘Caedimonas’, and the farmed shrimp pathogen ‘Candidatus Hepatobacter’. Next, we put these bacteria in the broad context of the whole Holosporineae, by comparing with the available data on the least studied representatives, including genome sequences. Accordingly, we reason on the most probable evolutionary trajectories for host interactions, host specificity, and emergence of potential pathogens in aquaculture and possibly humans, as well as on future research directions to investigate those many open points on the Holosporineae.
Keywords: Paramecium, Acanthamoeba, R-body, Bacterial endosymbionts, Protists, Rickettsiales, Holospora, Caedibacter/Caedimonas, Intranuclear bacteria, Killer trait, Holosporaceae, Holosporales, Holosporineae
Overview and Purposes
The Holosporales are an alphaproteobacterial order that was taxonomically described in the last decade [1], but research on their members has a long-lasting tradition in microbiology, in particular on two noteworthy representatives, namely the eponym Holospora and ‘Caedimonas’, both living intracellularly in ciliate protists. Holospora spp. are highly infectious bacteria inhabiting the nucleus of Paramecium hosts [2, 3], and their description dates back to the seminal work by Mardukhey Wolf-Vladimir Hafkin in the late nineteenth century [4]. ‘Caedimonas varicaedens’ confers its Paramecium hosts the so-called ‘killed trait’, similarly to the gammaproteobacterium Caedibacter taeniospiralis, and was indeed ascribed to the Caedibacter genus before molecular phylogenies showed their unrelatedness [5–7]. Long before understanding that the causative agents were actually bacteria, the killer trait was identified as early as 1938 in the pioneering investigations by Tracy Sonneborn [8].
Over the last three decades, extensive molecular surveys of host-associated bacteria showed that Holospora and ‘Caedimonas’ are phylogenetically close and form a conspicuous lineage together with several other bacteria [5, 9–12]. Currently, this lineage is formally described at the order rank (Holosporales) (https://lpsn.dsmz.de/order/holosporales) following Szokoli and co-authors [1]. As motivated in details below, here we will propose to rank it as a suborder (i.e. Holosporineae). Until the dedicated section, here, we will use taxonomic terms according to Szokoli and co-authors unless otherwise specified.
The Holosporales are likely very ancient (over 1 billion years) [13], and their extant members characterised so far are solely host-associated, with the vast majority of such hosts being unicellular eukaryotes, i.e. protists [14]. The potential host association status is unknown for additional uncharacterised members of the Holosporales, known only by their DNA sequences obtained in environmental screening and/or metagenomic studies (e.g. [15–27]), in several cases with more or less strong indications of association to eukaryotic hosts, e.g. [28–37]. Accordingly, it seems probable that the association with protists (and other eukaryotes) dates back to the last common ancestor of the Holosporales.
Their evolutionary antiquity, diversity breadth, and ancient host-association make the Holosporales a noteworthy subject of study and research, bringing them together with other well-known bacterial lineages with ancient host-association, such as Rickettsiales, Chlamydiae, and Legionellales [38–40]. The common features of those lineages (including the Holosporales) led some authors to define them as ‘professional symbionts’, underlining their ability to adapt to varied hosts and in some way presumably take control of the interactions [41, 42]. Therefore, the study of Holosporales is particularly relevant for comparisons with other professional symbionts, in order to highlight evolutionary convergent or lineage-specific traits. For example, the evolutionary origin and possible conservation of functional features of the killer trait of ‘Caedimonas’ has a broad significance, as it may be seen as a host addictive manipulation phenomenon [43]. Last but not least, differently from other professional symbiont lineages [44–46], the Holosporales do not encompass renowned human pathogens, but they do include the members of the genus ‘Candidatus Hepatobacter’ [10, 47, 48], which affect farmed shrimps and other crustaceans, causing necrotising hepatopancreatitis (NHP), a disease recognised by the World Organization for Animal Health [49].
The research interest on the Holosporales is shown by numerous research papers produced in recent years (e.g. [14, 34, 50–58]). However, to our best knowledge, there is not yet a dedicated review piece focused on the Holosporales as a whole and on their evolutionary and functional features, possibly due to their relatively recent recognition as a high-rank and independent lineage [1, 59]. Here, we aim to fill this gap, dealing with the following subjects:
Taxonomical overview and proposed revisions
Diversity of representatives and the respective hosts
Evolution of the lineage with a focus on the interactions with the hosts, informed by genomics
The Taxonomy of the Holosporales: Historical Overview and Proposed Revisions
The phylogenetic reconstructions of the Holosporales and the consequent taxonomic views have changed multiple times across recent years. Although not all taxonomic proposals were formally validated, this inevitably led to some incongruence among different studies published within short timings from one to another, each referring to a different taxonomical version, with a substantial risk to mislead non-specialists. Here, we will review in chronological order the main changes in the taxonomy of Holosporales, showing in particular the current ambiguities and the reasons that led us to propose here a novel revision that, in our view, should settle the issue for long.
The taxonomic affiliation of Holospora was initially uncertain [60]. Early molecular phylogenies based on 16S rRNA gene sequences indicated its relatedness to the order Rickettsiales [61], forming a distinct early-diverging lineage together with other bacteria associated with ciliates and other protists (including ‘Caedimonas’) [5, 9, 11, 62, 63], as well as with metazoans [10]. Accordingly, Görtz and Schmidt created a novel family for these bacteria within the Rickettsiales, namely the Holosporaceae [64], including also few other symbionts of ciliates that were not yet characterised molecularly. Curiously, molecular phylogenies later disproved the affiliation to the Holosporaceae of some of the latter, namely Lyticum and Pseudolyticum, both actually belonging to the ‘Candidatus Midichloriaceae’ (Rickettsiales) [65, 66], while molecular data are still lacking for others (Pseudocaedibacter and Tectibacter) [67, 68], making their actual affiliation uncertain to date.
Later on, based on the strong synapomorphies in the morphology and life cycle among Holospora and Holospora-like bacteria (HLB) [3, 4], Boscaro and colleagues informally proposed to restrict the Holosporaceae only to those bacteria, while treating all other Holosporaceae sensu Görtz and Schmidt as incertae sedis within the Rickettsiales [69].
In the same years, the phylogeny and taxonomy of these bacteria were influenced by multiple studies. On one hand, multiple other members of the Holosporaceae sensu Görtz and Schmidt were identified, e.g. [29, 70–75], with Hess and colleagues formally proposing to elevate at the family rank a sublineage including multiple symbionts of amoebas, namely the ‘Candidatus Paracaedibacteraceae’ [75]. Moreover, seminal phylogenetic studies with extended molecular markers [76–78] cast significant doubt on the actual affiliation of the Holosporaceae sensu Görtz and Schmidt to the Rickettsiales, which had relied on the 16S rRNA gene. Specifically, Ferla and colleagues made a first informal proposal to elevate them at the order rank [77].
Accounting for the above findings, Szokoli and colleagues formally re-organised the taxonomy and systematics of these bacteria [1], re-describing the Holosporales as an order, with four families, which is the taxonomy formally accepted to date (https://lpsn.dsmz.de/order/holosporales). These families are the ‘Candidatus Paracaedibacteraceae’ sensu Hess and co-authors, the Caedibacter/Nucleicultrix clade (soon after described as ‘Caedimonadaceae’ by Schrallhammer and colleagues, jointly with the description of ‘Caedimonas’ as a distinct genus from Caedibacter [7]), the Holosporaceae (revised in order to include HLB and their close relatives, thus narrower than sensu Görtz and Schmidt, but broader than the proposal by Boscaro and colleagues), and the newly described ‘Candidatus Hepatincolaceae’. The latter encompass mostly arthropod-associated bacteria [1, 79, 80] but have been recently shown to be phylogenetically unrelated to the Holosporales [81]. Below, we will propose a systematic revision for them as well.
Soon afterwards, the phylogenetic placement of the Holosporales was reassessed by Muñoz-Gómez and co-authors, who provided substantial evidence that the placement within Rickettsiales was an artefact due to compositional biases and that the Holosporales are actually related to, and possibly nested within, another broad and ancient alphaproteobacterial order, namely the Rhodospirillales [59]. Accordingly, they proposed to down-rank the Holosporales at the family level (to be named once again Holosporaceae) within the Rhodospirillales and to down-rank their families recognised at the time as subfamilies (i.e. Holosporodeae, ‘Candidatus Paracaedibacteriodeae’, and ‘Candidatus Hepatincolodeae’).
While the taxonomic revision proposed by Muñoz-Gómez and co-authors was not formally validated, it was adopted by a number of successive studies, e.g. [54, 82], in parallel to the version by Szokoli and co-authors in others, e.g. [55–57]. The presence of these two alternative versions is quite unfortunate, especially when considering that both use the term Holosporaceae with different meanings.
This taxonomic scenario is further complicated by the revisions recently proposed by Chuvochina and co-authors based on the Genome Taxonomy Database (GTDB) [83]. These consist in subdividing the members of the Holosporales into three orders roughly corresponding to the three families accounted by Szokoli and co-authors, namely Holosporales, ‘Candidatus Paracaedibacterales’, and ‘Caedimonadales’, and in proposing two additional families, namely the ‘Candidatus Hepatobacteraceae’ (Holosporales) and ‘Candidatus Nucleicultricaceae’ (‘Caedimonadales’). Those novel proposals are currently treated as non-standing heterotypic synonyms (https://lpsn.dsmz.de/). The discrepancies with respect to other classifications are partly due to the fact that the inference of the underlying phylogenetic backbone did not counteract the known artefacts evidenced by Muñoz-Gómez and co-authors, thus resulting in incorrect placing and splitting of the Holosporales (sensu Szokoli et al.). However, they are also due to different and more stringent thresholds used to delineate taxonomic ranks. It seems worth to consider that, given such thresholds, Chuvochina and co-authors also proposed to elevate at the order rank several lineages that are affiliated to the Rhodospirillales according to the currently validated taxonomy [84], namely Acetobacterales, Azospirillales, Geminicoccales, Oceanibaculales, Reyranellales, Thalassobaculales, and Tistrellales [83].
We believe that the current taxonomic ambiguities on the Holosporales should be settled by an evolutionarily and biologically meaningful proposal that meets the following criteria: (i) consistency with most up-to-date phylogenetic reconstructions, (ii) consistency and compatibility with most up-to-date (and ideally also foreseen) taxonomic classification schemes of Alphaproteobacteria, (iii) compliance to the formal standards for validation. The most credible phylogenetic backbone for the Holosporales is the one obtained by Muñoz-Gómez and co-authors, namely phylogenetically nested within the Rhodospirillales sensu Hördt et al., as confirmed by successive studies [40, 85]. While the taxonomic proposal by Muñoz-Gómez and co-authors accounts for this phylogeny, it also implies the down-ranking of the families of Holosporales as subfamilies, which does not account for the great phylogenetic diversity of those, the same that led Chuvochina and co-authors to rank them as separate orders. We believe that the most suitable solution is intermediate between those extremes. Accordingly, we formally propose to down-rank the Holosporales sensu Szokoli et al. (corresponding to the Holosporaceae sensu Gortz and Schmidt and sensu Muñoz-Gómez et al.) as a suborder within the Rhodospirillales sensu Hördt et al., namely Holosporineae. Accordingly, the rank and composition of the included families should be kept unchanged as per Szokoli et al. This novel taxonomic proposal has the additional advantage that it would suit well to potential future revisions of high-order taxonomy of the Alphaproteobacteria, including subdividing the Rhodospirillales into multiple orders, should a ‘splitter’ view similar to the one by Chuvochina and co-authors eventually prevail. Indeed, in such a case, the Holosporineae could be meaningfully re-elevated at the order rank, but, differently from other previous proposals, without the need of further changes in their inner subdivisions, thus simplifying revisions and favouring nomenclatural consistency over time. From now on, here, we will refer to this lineage as Holosporineae. Moreover, accounting for the most recent phylogenetic evidence of an independent branching of the ‘Candidatus Hepatincolaceae’ and Holosporineae within the Rhodospirillales [81], here, we formally propose to move this family out of the Holosporineae. Those taxonomic revisions are presented at the end of the text.
Diversity of the Holosporineae and Their Hosts
All the characterised Holosporineae (i.e. Holosporales sensu Szokoli et al.) were consistently retrieved as intracellular bacteria in multiple eukaryotic hosts, predominantly protists [1, 3, 7, 14, 41, 54, 57, 59, 75]. Therefore, here, we will assume that all the Holosporineae are host-associated and will treat those representatives sequenced in environmental screening studies as putatively associated with unknown hosts. Accordingly, their environmental provenance would actually reflect the one of the respective hosts, notwithstanding the possibility of detecting temporarily free transmission forms of the bacteria, such as the infectious forms of Holospora [2, 3]. Among the Holosporineae putatively associated with Metazoa, only the members of the ‘Candidatus Hepatobacter’ genus were investigated in detail and confirmed as intracellular in the host cells [10, 47, 48]. On the other hand, all the others were sequenced from samples coming from the host gut [28, 32, 36, 86–90] or skin [91–94]. In those cases, a hypothetical intracellular association with the target host is still to be verified, while an alternative association with microbial eukaryotes, possibly ingested by the animal or part of its stable gut community, seems also plausible [95].
In this section, we will review the phylogenetic diversity of the Holosporineae, with a focus on experimentally characterised and taxonomically described species over specimens known only from metagenomic screenings. The Holosporineae encompass three families, namely Holosporaceae, ‘Caedimonadaceae’, and ‘Candidatus Paracaedibacteraceae’ (Fig. 1). Most phylogenetic inference studies, both on the 16S rRNA gene or by phylogenomics, indicate that Holosporaceae and ‘Caedimonadaceae’ are sister groups [1, 14, 50, 54, 58, 69, 74, 75, 78, 95].
Fig. 1.
Cladogram showing the phylogenetic relationships among the Holosporineae, based on a combination of published reference studies, with inconsistent/unresolved relationships represented as polytomies [1, 14, 54, 57, 58, 75]. For presentation purposes, the branches leading to the ‘fast-evolving Holosporaceae’ are shown longer than the others. For each organism/clade, the most typical hosts are shown on the right-hand side, with name labels on the first occurrence of each host (see main text for further details). Branches without text labels indicate unnamed bacteria found through environmental screening studies in association with the shown putative hosts. Dotted branches indicate the grouping, for space constraints, of organisms that descend from the previous node in the tree, but with unconfirmed reciprocal phylogenetic relationships. The three families of the Holosporineae are shown by coloured backgrounds, and two subgroups of the Holosporaceae are encircled by dashed rectangular shapes. “Ca.” is an abbreviation for ‘Candidatus’, while ‘HLB’ for ‘Holospora-like bacteria’
The Holosporaceae are mostly associated with ciliates and other protists [96] but also with arthropods, and encompass 13 described genera (Table 1). Among them, the monophyletic lineage made up of Holospora and the HLB (three other described genera, namely ‘Candidatus Goertzia’, ‘Preeria’, and ‘Candidatus Hafkinia’) is prominent (highlighted in Fig. 1) [3, 69, 97, 98]. These bacteria are associated with ciliates, prevalently of the genus Paramecium, and are characterised by distinctive apomorphies, including morpho-functional differentiations linked with an infectious lifecycle that involves invasion of the host nuclei [2]. Such peculiarities allowed Hafkin to notice these bacteria for the first time over 100 years ago [4] and consented a reliable assignment of new specimens to this lineage based on microscopy observations [60, 68]. Eventually, molecular data revealed the phylogenetic breadth of this genus, with five species accordingly recognised to date, namely H. obtusa, H. undulata, H. curviuscula, H. acuminata, and ‘Candidatus Holospora parva’, hosted by Paramecium caudatum, P. bursaria, P. aurelia, and P. chlorelligerum (Table 1) [61, 69, 99–101]. Molecular phylogenies are not always fully consistent with morphology, leading to a recent revision of H. undulata to encompass also the former species H. elegans and H. recta [102] and to the re-classification of the former H. caryophila into a novel genus as ‘Preeria caryophila’, being more distant from Holospora than the other HLB genera (Fig. 1) [97]. On the other hand, ‘Candidatus Hafkinia simulans’ is the closest relative of Holospora spp. but is hosted by the brackish water peniculid ciliate Frontonia salmastra rather than by its close relative Paramecium [98]. ‘Candidatus Goertzia’ includes three species, namely ‘Candidatus Goertzia infectiva’, ‘Candidatus Goertzia shahrazadae’, and ‘Candidatus Goertzia yakutica’, respectively hosted by P. jenningsi, P. multimicronucleatum, and P. putrinum (Table 1) [69, 103, 104]. Further, Holospora/HLB are possibly awaiting to be fully characterised in association with other ciliates (e.g. Trithigmastoma cucullulus and Prorodon teres) [4]. Most of Holospora and HLB species were found infecting a single host species [4], but this species-specificity is not always so sharp (e.g. ‘Preeria’ found in species of P. aurelia complex as well as in P. caudatum) (Table 1) [97], consistent with the incongruence between bacterial and host phylogenies [96, 98]. Uncharacterised Holospora-related bacteria were also detected in the gut of the freshwater fish Panaque nigrolineatus [105] and in biofilm [106] while ‘Candidatus Goertzia’-like ones in the microbiome of cladocerans [35].
Table 1.
List of described and molecularly validated Holosporineae species
| Species | Family | Host(s) | Location(s) | Genome available | Reference |
|---|---|---|---|---|---|
| Holospora undulata | Holosporaceae | Paramecium caudatum | Intranuclear and infectious | Yes | [60] |
| Holospora obtusa | Holosporaceae | Paramecium caudatum | Intranuclear and infectious | Yes | [60] |
| ‘Holospora curviuscula’ | Holosporaceae | Paramecium bursaria | Intranuclear and infectious | Yes | [307] |
| ‘Holospora acuminata’ | Holosporaceae | Paramecium bursaria | Intranuclear and infectious | No | [308] |
| ‘Candidatus Holospora parva’ | Holosporaceae | Paramecium chlorelligerum | Intranuclear and infectious | No | [100] |
| ‘Candidatus Goertzia infectiva’ | Holosporaceae | Paramecium jenningsi | Intranuclear and infectious | No | [69] |
| ‘Candidatus Goertzia shahrazadae’ | Holosporaceae | Paramecium multimicronucleatum | Intranuclear and infectious, occasionally cytoplasmic | No | [103] |
| ‘Candidatus Goertzia yakutica’ | Holosporaceae | Paramecium putrinum | Intranuclear and infectious | No | [104] |
| ‘Candidatus Hafkinia simulans’ | Holosporaceae | Frontonia salmastra | Intranuclear and infectious | No | [98] |
| ‘Preeria caryophila’ | Holosporaceae | Paramecium biaurelia, Paramecium octaurelia, Paramecium novaurelia, Paramecium caudatum, Paramecium sp. | Intranuclear and infectious | No | [97] |
| ‘Candidatus Fujishimia apicalis’ | Holosporaceae | Euplotes octocarinatus | Cytoplasm, mostly apical | No | [52] |
| ‘Candidatus Hydrogenosomobacter endosymbioticus’ | Holosporaceae | Cyclidium-like scuticociliate | Cytoplasm, close to hydrogenomes | Yes | [53] |
| ‘Candidatus Paraholospora nucleivisitans’ | Holosporaceae | Paramecium sexaurelia | Cytoplasm and nucleus | No | [71] |
| ‘Candidatus Mystax nordicus’ | Holosporaceae | Paramecium nephridiatum | Cytoplasm, sometimes aggregating with mitochondria | No | [56] |
| ‘Candidatus Gromoviella agglomerans’ | Holosporaceae | Paramecium polycaryum | Cytoplasm, sometimes forming aggregates | Yes | [58] |
| ‘Candidatus Cytomitobacter primus’ | Holosporaceae | Diplonema japonicum | Cytoplasm, occasionally possibly inside mitochondria | Yes | [50] |
| ‘Candidatus Cytomitobacter indipagum’ | Holosporaceae | Diplonema aggregans | Cytoplasm, occasionally possibly inside mitochondria | Yes | [50] |
| ‘Candidatus Cytomitobacter rhynchopi’ | Holosporaceae | Rhynchopus asiaticus | Cytoplasm | No | [108] |
| ‘Candidatus Ignotibacter abundans’ | Holosporaceae | Diplonema aggregans | Cytoplasm | Yes | [54] |
| ‘Candidatus Hepatobacter penaei’ | Holosporaceae | Litopenaeus vannamei and other crustaceans | Cytoplasm | Yes | [47] |
| ‘Candidatus Hepatobacter paralithodis’ | Holosporaceae | Paralithodes platypus | Cytoplasm | No | [48] |
| ‘Candidatus Bealeia paramacronuclearis’ | Holosporaceae | Paramecium biaurelia | Cytoplasm, in proximity of the macronucleus | Yes | [1] |
| ‘Caedimonas varicaedens’ | ‘Caedimonadaceae’ | Paramecium biaurelia, Paramecium novaurelia, Paramecium caudatum, Paramecium duboscqui, Spirostomum ambiguum, Euplotes sp., Peridinium cinctum | Cytoplasm or nucleus, depending on the host species | Yes | [7] |
| ‘Candidatus Paracaedimonas acanthamoebae’ | ‘Caedimonadaceae’ | Acanthamoeba sp. | Cytoplasm | Yes | [7] |
| ‘Candidatus Nucleicultrix amoebiphila’ | ‘Caedimonadaceae’ | Hartmanella sp. | Intranuclear and infectious | Yes | [74] |
| ‘Candidatus Paracaedibacter symbiosus’ | ‘Candidatus Paracaedibacteraceae’ | Acanthamoeba sp. | Cytoplasm | Yes | [11] |
| ‘Candidatus Odyssella thessalonicensis’ | ‘Candidatus Paracaedibacteraceae’ | Acanthamoeba sp. | Cytoplasm | Yes | [62] |
| ‘Candidatus Odyssella acanthamoebae’ | ‘Candidatus Paracaedibacteraceae’ | Acanthamoeba sp. | Cytoplasm | Yes | [11] |
| ‘Candidatus Finniella lucida’ | ‘Candidatus Paracaedibacteraceae’ | Orciraptor agilis | Cytoplasm | No | [75] |
| ‘Candidatus Finniella inopinata’ | ‘Candidatus Paracaedibacteraceae’ | Viridiraptor invadens | Cytoplasm | Yes | [75] |
| ‘Candidatus Finniella dimorpha’ | ‘Candidatus Paracaedibacteraceae’ | Euplotes daidaleos, Euplotes eurystomus, Euplotes octocarinatus | Cytoplasm | No | [52] |
| ‘Candidatus Intestinibacterium nucleariae’ | ‘Candidatus Paracaedibacteraceae’ | Nuclearia delicatula | Cytoplasm | No | [148] |
| ‘Candidatus Intestinibacterium parameciiphilum’ | ‘Candidatus Paracaedibacteraceae’ | Paramecium biaurelia | Cytoplasm | No | [95] |
| ‘Candidatus Captivus acidiprotistae’ | ‘Candidatus Paracaedibacteraceae’ | Unnamed protists from acidic mine drainage | Cytoplasm | No | [63] |
| ‘Candidatus Parafinniella ignota’ | ‘Candidatus Paracaedibacteraceae’ | Euplotes sp. | Cytoplasm | No | [52] |
| ‘Candidatus Bodonicaedibacter vickermanii’ | ‘Candidatus Paracaedibacteraceae’ | Bodo saltans | Cytoplasm, in proximity of the nucleus | Yes | [57] |
Holospora and HLB display long branches in molecular phylogenies, indicative of fast sequence evolution, and their close relatives display quite long branches as well. Accordingly, the members of whole clade, including Holospora and HLB, have been defined as ‘fast-evolving’ Holosporaceae (highlighted in Fig. 1) [58], which include the majority of the characterised Holosporaceae. Besides holosporas, several fast-evolving Holosporaceae live in association with ciliates as well (Table 1) [96, 107], namely ‘Candidatus Fujishimia apicalis’, symbiont of Euplotes octocarinatus [52], ‘Candidatus Hydrogenosomobacter endosymbioticus’, symbiont of an anaerobic Cyclidium-like scuticociliate [53], ‘Candidatus Paraholospora nucleivisitans’, symbiont of Paramecium sexaurelia [71], ‘Candidatus Mystax nordicus’, symbiont of Paramecium nephridiatum [56], and ‘Candidatus Gromoviella agglomerans’, symbiont of Paramecium polycaryum [58]. Other members of this clade were characterised in association with marine diplonemids (Table 1), namely ‘Candidatus Cytomitobacter primus’, symbiont of Diplonema japonicum [50], ‘Candidatus Cytomitobacter rhynchopi’, symbiont of Rhynchopus asiaticus [108], as well as ‘Candidatus Cytomitobacter indipagum’ and ‘Candidatus Ignotibacter abundans’ (formerly ‘Candidatus Nesciobacter abundans’), symbionts of the same strain of Diplonema aggregans [54]. Uncharacterised fast-evolving Holosporaceae were found in association with the prairie dog flea Oropsylla hirsuta [29], in the gut of the hemipteran Pyrrhocoris apterus [89], and in the microbiome of the marine bryozoon Bugula neritina [33]. Other members of this clade were detected in multiple environments and sources, such as freshwater lakes [17, 22, 24, 109], hypersaline microbial mat [18], activated sludge [110], acidic mine drainage [25], ocean depths [111], soil [112], and hospital dental units [113].
The genus ‘Candidatus Hepatobacter’ is phylogenetically proximate to the fast-evolving Holosporaceae (Fig. 1). ‘Candidatus Hepatobacter penaei’ thrives inside the epithelial cells of the hepatopancreas of the marine shrimp Litopenaeus vannamei (Table 1) [10, 47, 49] and possibly other akin crustaceans, such as L. setiferus, L. stylirostris, Farfantepenaeus aztecus, and F. californiensis [114]. On the other hand, ‘Candidatus Hepatobacter paralithodis’ was found in the hepatopancreatic epithelium of the crab Paralithodes platypus [48]. Phylogenetically close bacteria were found in possible association with amphipods (Melita plumosa) [115], sponges (Xestospongia muta) [31], and oribatid mites (Achipteria coleoptrata) [34]. Thus, notwithstanding the uncertainties on the actual hosts for the latter bacteria, it is possible to wonder whether the members of this subclade could present a marked preference towards arthropod and other metazoan hosts rather than protists (Fig. 1), being an exception among the whole Holosporineae.
Early diverging Holosporaceae are characterised by overall shorter branches in molecular phylogenies. The only described species is ‘Candidatus Bealeia paramacronuclearis’, found as symbiont of two P. biaurelia strains (Fig. 1; Table 1), in one case in coexistence with the Rickettsiales bacterium ‘Candidatus Fokinia cryptica’ [1]. ‘Candidatus Bealeia’-allied bacteria were found in freshwater particles associated with blooms of dinoflagellate Alexandrium monilatum [116]. Other early-diverging Holosporaceae were detected from various origins, including the stomach of the catfish Pelteobagrus fulvidraco [90], the intestine of zebrafish [88], aquatic moss [117], soil [118–122], mine drainage and water [16, 123], freshwater and marine sediments [124–126], and volcanic cinder deposit [127].
Further sequences assigned to Holosporaceae-related bacteria were found in the microbiota of Daphnia cf. pulex [37] and in olive oil production pomace [128].
The ‘Caedimonadaceae’ are hosted by diverse protists, such as ciliates, dinoflagellates, and amoebas [7, 11, 74, 129]. This is the least rich family in terms of described genera and species, including only three monotypic genera (Fig. 1; Table 1). ‘Caedimonas varicaedens’ is the most-studied member. This bacterium was re-described recently [7], but it was known for decades, being previously placed within the gammaproteobacterial genus Caedibacter, due to shared traits such as R-bodies and killer trait [7]. Former species now part of ‘Caedimonas varicaedens’ were Caedibacter varicaedens, Caedibacter caryophilus, and ‘Caedibacter macronucleorum’ [67, 130, 131]. ‘Caedimonas’ was found as symbiont of multiple Paramecium species, in particular, P. caudatum, members of the P. aurelia complex such as P. biaurelia and P. novaurelia, and P. duboscqui, being the causative agent of the killer effect [6, 67, 131–133]. On the other hand, it was also detected as symbiont of other ciliates (Table 1), such as Spirostomum ambiguum [134] and Euplotes sp. [52], as well as in the dinoflagellate Peridinium cinctum [6, 129], though without evidence of a killer effect. Further relatives of ‘Caedimonas’ were retrieved from various origins, such as urban aerosol [135] and sludge [136]. ‘Candidatus Paracaedimonas acanthamobae’ (formerly ‘Candidatus Caedibacter acanthamoebae’ [11]) is phylogenetically close to ‘Caedimonas’ and was originally described as symbiont of Acanthamoeba [11]. ‘Candidatus Paracaedimonas’ bacteria were repeatedly detected in acanthamoebas worldwide [137, 138] and were also found in soil [139] and bioreactors [140]. Moreover, sequences of bacteria more distantly allied to ‘Caedimonas’ and ‘Candidatus Paracaedimonas’ were retrieved from drinking water [15].
The other and more diverging described representative of the ‘Caedimonadaceae’ is ‘Candidatus Nucleicultrix amoebiphila’ (Fig. 1; Table 1), symbiont of the amoebozoan Hartmanella sp. [74]. This bacterium displays an infectious cycle, as was able to invade Acanthamoeba castellanii in laboratory experiments. Sequences of bacteria that are phylogenetically allied to ‘Candidatus Nucleicultrix’ were found in soil [141, 142] and in a lake [143].
Further members of the ‘Caedimonadaceae’ were detected in the gut of the beetle Harpalus pensylvanicus [86], as well as in lakes [26], seawater [144], marine sediments [145, 146], and drinking water [147].
The family ‘Candidatus Paracaedibacteraceae’ displays a high phylogenetic diversity, with seven genera and 11 species described (Fig. 1; Table 1). The breadth of their collective host range is comparable to the Holosporaceae and possibly even wider in terms of relative frequency of each main host lineage. Ascertained hosts are protists belonging to various lineages, including amoebas, cercozoans, ciliates, nucleariids, and euglenozoans [11, 57, 62, 69, 75, 95, 148]. The most long-term known representatives of the family belong to the genus ‘Candidatus Paracaedibacter’ and are found intracellularly in Acanthamoeba, including clinical isolates [11, 149–152], which led some authors wondering about their role in pathogenicity for the eye of the amoebas. ‘Candidatus Paracaedibacter’ is paraphyletic with respect to ‘Candidatus Odyssella thessalonicensis’, which is a symbiont of Acanthamoeba as well [62] and is more closely related to ‘Candidatus Paracaedibacter acanthamoebae’ than the two of them to ‘Candidatus Paracaedibacter symbiosus’ (Fig. 1) [1, 75]. Specifically, ‘Candidatus Paracaedibacter acanthamoebae’ and ‘Candidatus Odyssella thessalonicensis’ share a 16S rRNA gene identity of 97.8%, while, respectively, having 92.5% and 91.8% identity with ‘Candidatus Paracaedibacter symbiosus’ [1]. Considering the commonly accepted genus threshold for the 16S rRNA gene (94.5%) [153], and that the original description did not designate any type species for the genus ‘Candidatus Paracaedibacter’ [11], we propose to elect ‘Candidatus Paracaedibacter symbiosus’ as type and to move ‘Candidatus Paracaedibacter acanthamoebae’ into the genus ‘Candidatus Odyssella’ as ‘Candidatus Odyssella acanthamoebae’ comb. nov. (Table 1) and we will be referr to this bacterium as such from now on (see taxonomic revision at the end). Relatives of ‘Candidatus Paracaedibacter’/ ‘Candidatus Odyssella’ were detected in many environments and sources, namely lakes [22, 24], soil [154, 155], groundwater [156, 157], drinking water [23, 158], sludge [159, 160], wastewater [161], acidic mine drainage [25], textiles [162], human faeces [163], hospital dental units [113], and hydrocarbon [164].
The ‘Candidatus Paracaedibacteraceae’ includes several other clades with respective phylogenetic relationships not yet fully and consistently resolved (Fig. 1). ‘Candidatus Finniella’ bacteria belong to one of those clades and are hosted by cercozoans, namely ‘Candidatus Finniella lucida’ symbiont of Orciraptor agilis and ‘Candidatus Finniella inopinata’ of Viridiraptor invadens [75], and by ciliates, namely ‘Candidatus Finniella dimorpha’ symbiont of multiple Euplotes spp. (Table 1) [52]. Relatives of ‘Candidatus Finniella’ were retrieved in a possible association with Hydra vulgaris [70] and from multiple additional sources, such as lakes/rivers [21, 24, 27, 165], drinking water [23, 166], acidic mine drainage [16, 25], and electronic waste aerosol [167].
Another clade of the ‘Candidatus Paracaedibacteraceae’ is the one encompassing the genus ‘Candidatus Intestinibacterium’ (formerly ‘Candidatus Intestinusbacter’) (Fig. 1; Table 1), with the described species ‘Candidatus Intestinibacterium nucleariae’ symbiont of the opisthokont Nuclearia delicatula [148] and ‘Candidatus Intestinibacterium parameciiphilum’ symbiont of P. biaurelia [95]. Members of the same clade were retrieved from several, prevalently aquatic, sources, such as lakes [17, 19–21, 24, 26, 27, 168, 169], a river [170], seawater [143], a sulphidic spring [171], subsurface water [172], drinking water [173], wastewater [161], biofilm [157, 174], microbial mat [175], and even human skin [91, 94]. Moreover, bacteria related to both ‘Candidatus Intestinibacterium’ and ‘Candidatus Finniella’ were retrieved in an aquaculture after applying silver nanoparticles [176].
‘Candidatus Captivus acidiprotistae’ is another representative of the family (Table 1), found as endosymbiont in unnamed protists from an acidic mine drainage [63]. A close relative is an unnamed symbiont of the euglenozoan Petalomonas sphagnophila (Fig. 1) [72]. Further relatives were found in soil [177–179], sediment [180], acidic mine drainage [181], and an acidic pit lake [169]. The other two described members of the ‘Candidatus Paracaedibacteraceae’ are ‘Candidatus Parafinniella ignota’, symbiont of Euplotes ciliates [52] in coexistence with other intracellular bacteria [182], and ‘Candidatus Bodonicaedibacter vickermanii’ (formerly ‘Candidatus Bodocaedibacter vickermanii’), symbiont of the free-living kinetoplastid Bodo saltans (Fig. 1; Table 1) [57].
Another quite conspicuous clade of ‘Candidatus Paracaedibacteraceae’ includes only uncharacterised bacteria, frequently derived from the gut of various animals (Fig. 1), in particular termites (e.g. Reticulitermes speratus, R. santonensis, and Coptotermers curvignatus) [28, 32, 183–188], as well as the sea cucumber Apostichopus japonicus [36], the fish Ctenochaetus striatus [189], and springbok antelopes [87].
Further ‘Candidatus Paracaedibacteraceae’ bacteria were retrieved from human skin [93], the blowhole of the bottlenose dolphin Tursiops truncatus [92], the epithelium of Hydra magnipapillata [30], lakes [21, 24, 190], a river [191], biofilm [192, 193], drinking water [147, 194], groundwater [195], and wastewater [196, 197].
Host Interactions, Genomics, and Evolution
The interactions between the Holosporineae and their hosts, both in terms of mechanisms and effects, are overall still poorly understood, also due to the inherent experimental limitations in handling host-associated bacteria. In the last 15 years, a growing number of Holosporineae genomes have been sequenced, belonging to each of the three families, namely Holosporaceae, three ‘Caedimonadaceae’, and five ‘Candidatus Paracaedibacteraceae’ (Table 2). While many genus-level sublineages are not yet sequenced, available genomes have represented a major advance, allowing a deeper understanding of the functional features of the Holosporineae and of their evolution. Leveraging on the available observational, experimental, and genomic data, below, we will present a comprehensive account of the current knowledge on those subjects, focusing in particular on the most-studied cases, namely Holospora, ‘Caedimonas’, and the NHP determinant ‘Candidatus Hepatobacter penaei’.
Table 2.
List of Holosporineae with assembled genomes, and respective assembly statistics
| Species | Host | Size | GC% | Number of contigs/scaffolds | N50 | Plasmid number | Accession | Reference |
|---|---|---|---|---|---|---|---|---|
| Family Holosporaceae | ||||||||
| Holospora undulata subsp. undulata | Paramecium caudatum | 1.4 Mbp | 36 | 203 | 10.9 kbp | Not determined | GCA_000388175.3 | [226, 309] |
| Holospora undulata subsp. elegans | Paramecium caudatum | 1.3 Mbp | 36 | 152 | 13 kbp | Not determined | GCA_000648275.1 | [226] |
| Holospora obtusa | Paramecium caudatum | 1.3 Mbp | 35 | 91 | 24.4 kbp | Not determined | GCA_000469665.2 | [226] |
| ‘Holospora curviuscula’ | Paramecium bursaria | 1.7 Mbp | 37.5 | 152 | 40.5 kbp | Not determined | GCA_002930195.1 | [101] |
| ‘Candidatus Hydrogenosomobacter endosymbioticus’ | Cyclidium-like scuticociliate | 826.7 kbp | 41.5 | 1 | 826.7 kbp | Absent | GCA_021654655.1 | [310] |
| ‘Candidatus Cytomitobacter primus’ | Diplonema japonicum | 622.4 kbp | 30 | 1 | 622.4 kbp | Absent | GCA_008189405.1 | [54] |
| ‘Candidatus Cytomitobacter indipagum’ | Diplonema aggregans | 628 kbp | 29.5 | 1 | 628 kbp | Absent | GCA_008189285.1 | [54] |
| ‘Candidatus Ignotibacter abundans’ | Diplonema aggregans | 616.1 kbp | 30 | 1 | 616.1 kbp | Absent | GCA_008189525.1 | [54] |
| ‘Candidatus Gromoviella agglomerans’ | Paramecium polycaryum | 590 kbp | 32 | 1 | 590 kbp | Absent | GCA_021065005.1 | [58] |
| ‘Candidatus Hepatobacter penaei’ | Litopenaeus vannamei | 1.1 Mbp | 50 | 5 | 334.2 kbp | Not determined | GCA_000742475.1 | [78] |
| ‘Candidatus Bealeia paramacronuclearis’ | Paramecium biaurelia | 1.9 Mbp | 43 | 1 | 1.9 Mbp | 6 | GCA_036670005.1 | [14] |
| Family ‘Caedimonadaceae’ | ||||||||
| ‘Caedimonas varicaedens’ | Paramecium biaurelia | 1.7 Mbp | 42 | 142 | 20.2 kbp | Not determined | GCA_001192655.1 | [311] |
| ‘Candidatus Paracaedimonas acanthamoebae’ | Acanthamoeba sp. | 1.7 Mbp | 38 | 1 | 1.7 Mbp | 5 | GCA_000743035.1 | [78] |
| ‘Candidatus Nucleicultrix amoebiphila’ | Hartmanella sp. | 1.8 Mbp | 39.5 | 1 | 1.8 Mbp | Absent | GCA_002117145.1 | Schulz et al., unpublished |
| Family ‘Candidatus Paracaedibacteraceae’ | ||||||||
| ‘Candidatus Paracaedibacter symbiosus’ | Acanthamoeba sp. | 2.7 Mbp | 41 | 50 | 1.7 Mbp | 2 | GCA_000757605.1 | [78] |
| ‘Candidatus Odyssella thessalonicensis’ | Acanthamoeba sp. | 2.8 Mbp | 42 | 20 | 388.1 kbp | Not determined | GCA_000190415.2 | [76] |
| ‘Candidatus Odyssella acanthamoebae’ | Acanthamoeba sp. | 2.5 Mbp | 41 | 1 | 2.5 Mbp | 1 | GCA_000742835.1 | [78] |
| ‘Candidatus Finniella inopinata’ | Viridiraptor invadens | 1.8 Mbp | 44 | 28 | 174.7 kbp | Not determined | GCA_004210305.1 | [59] |
| ‘Candidatus Bodonicaedibacter vickermanii’ | Bodo saltans | 1.4 Mbp | 40.5 | 1 | 1.4 Mbp | Absent | GCA_014896945.1 | [57] |
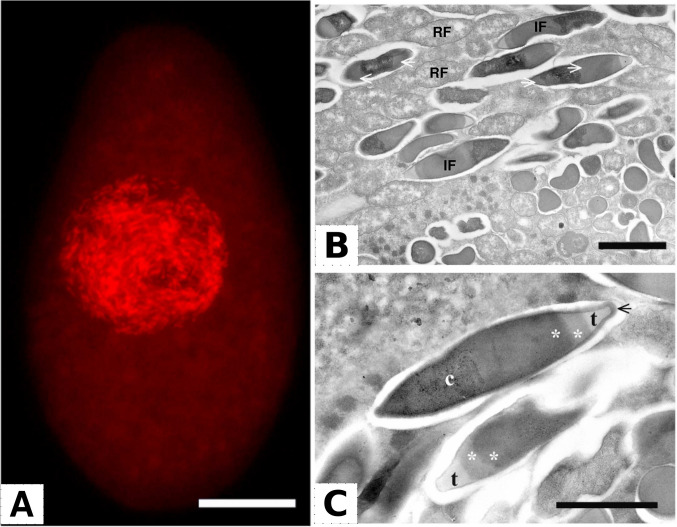
Holospora and HLB are probably the most deeply investigated among the Holosporineae and have been subject of dedicated extensive reviews over the years [2–4]. Indeed, these bacteria present a distinct set of apomorphic features, which allow them to engage in a dimorphic infectious life cycle interacting with their ciliate hosts, which in most of the characterised cases, though not all [98], belong to genus Paramecium [4, 69, 97, 100, 104]. The reproductive form of the typical holosporas is shaped as a short Gram-negative rod [69, 198, 199] and actively multiplies within the host nucleus, which may be the micronucleus or, more frequently, macronucleus of the ciliate, depending on the bacterial and host species (Fig. 2) [2, 4]. Under starvation or other conditions, which may be linked to the arrest of host protein synthesis [2, 200], the reproductive form differentiates into an elongated infectious form, which presents an extended electron-dense periplasm with a translucent tip (Fig. 2) [4, 69, 198, 200, 201]. The infectious forms do not divide and are typically released into the external medium through exocytic vesicles [202–206], formed by different mechanisms according to the species [207]. In extreme cases, infectious forms may cause the host cell lysis, possibly as a consequence of the deliverance of lipopolysaccharide, thus leading to their own release [2]. The infectious form is able to survive apart from the host for several days [208]. If ingested by a novel host cell, it gets activated by the acidification of the host digestive vacuole and is able to escape from the vacuole with the periplasmic tip ahead and then interacting with the host membrane trafficking systems and cytoskeleton, in order to reach its target nucleus [209–214]. It can enter the nucleus without causing its disruption and therein will produce back novel reproductive forms, thus fuelling the Holospora life cycle [2, 3, 215, 216].
Fig. 2.
Subcellular location and ultrastructure of ‘Candidatus Holospora parva’, symbiont of the ciliate P. chlorelligerum, modified from [100]. A Densely packed bacteria inside the macronucleus of the host, stained with a red fluorescent probe specific for ‘Candidatus Holospora parva’. B, C Ultrastructure of the bacteria. In B, both reproductive forms (RF) and infectious forms (IF) are shown, with the white arrows indicating the enlarged periplasm of the latter form. In C, a magnification of the details of an infection form is presented, namely the cytoplasm (c), the periplasm (asterisks), and its apical tip (t). The black arrow indicates fine fibrous material that may be present on the surface of some infectious forms of this bacterium. Scale bars: A 20 μm; B 2 μm; C 1.5 μm
The molecular determinants of the infection are only partly understood. Some studies, mainly on H. obtusa, were aimed at the characterisation of stage-specific proteins and their possible involvement in different infection phases, such as binding to actin or to the macronucleus [212, 217–224]. The identified proteins have little homology with those of other organisms and were labelled by their molecular weight (e.g. 89 kDa, 63 kDa, or 5.4 kDa proteins), thus preventing more extensive comparative studies to date.
As described above, Holospora and HLB present the typical traits of specialised parasites, namely an infectious life cycle with the possibility to harm their hosts, including hampering sexual processes [206, 225]. An ongoing parasitic interaction is also supported by genomic evidences of quite pronounced scavenging of metabolites by the bacteria from their hosts [101, 226], as well as by the presence of host resistance mechanisms of the ciliates against the infection [227–231]. As such, Holospora/HLB and Paramecium have been repeatedly used as experimental models for the evolution of host-parasite interactions, including plasticity and trade-offs between transmission modes, infectivity, and virulence in the parasite [232–235], as well as between resistance and fitness in the host [236]. Further studies investigated local adaptation [237–239], effect of environmental variations [240–243], impact of host growth and lifespan on the growth, infectivity and virulence of the parasite [244, 245], reciprocal effects of parasite traits and host dispersal [246–249], and competition among parasites [250]. At the same time, the effect of Holospora and the interplay with its host are more complex than a purely parasitic interaction. Indeed, in particular as a reproductive form, it was shown to induce positive effects on the host, namely protection against environmental stress, such as temperature, salinity, and osmotic variations [2, 251–253]. This has been tentatively linked to an enhanced heat-shock protein expression both of the host and the bacterium, which could make the host more reactive to stressors [254–256]. These findings could explain the observation of a higher frequency of bacteria in hosts sampled from brackish with respect to freshwater environments [134].
In any case, the remarkable traits of the interaction between Holospora/HLB and Paramecium/other ciliates are suggestive of a significant (co)evolutionary specialisation, which however can be difficult to trace by comparative analyses, considering the sharp differences with respect to the other closely-related Holosporineae. Nevertheless, it seems interesting to notice two different cases, which show somehow intermediate traits and could thus be seen as potentially reminiscent of some ancestral steps in the evolution of the peculiar infectious nuclear tropism. The first case is the HLB ‘Candidatus Goertzia sharazharadae’, which, besides being resident within the host macronucleus as typical for this bacterial lineage, was recurringly found free (i.e. without being enclosed in host-derived membranes) in the host cytoplasm [103]. The other instance is the one of ‘Candidatus Paraholospora nucleivisitans’, a fast-evolving Holosporaceae bacterium that is closely related to Holospora and HLB clade, although not its direct sister lineage. ‘Candidatus Paraholospora’ was observed in the host cytoplasm or, alternatively, in the nucleus, otherwise more rarely in both locations in the same host cell [71].
It is worth considering that many other Holosporaceae and Holosporineae in general display some relation with host organelles including the nucleus, up to being intranuclear as well. The early-diverging Holosporaceae bacterium ‘Candidatus Bealeia paramacronuclearis’ is loosely co-localised with the host macronucleus from its outside [1], similarly to the position of the ‘Candidatus Paracaedibacteraceae’ bacterium ‘Candidatus Bodonicaedibacter vickermanii’ with respect to its host nucleus [57]. On the other hand, the fast-evolving Holosporaceae bacteria ‘Candidatus Cytomitobacter primus’ and ‘Candidatus Mystax nordicus’ were found in proximity or even aggregation with host mitochondria [50, 56], with the former bacterium possibly able to enter within these organelles. Moreover, both ‘Candidatus Mystax’ and ‘Candidatus Gromoviella agglomerans’ (also a member of fast-evolving Holosporaceae) can form bacterial aggregates within the respective host cells [56, 58], the latter also with potential lethal division effects on the host. Finally, ‘Candidatus Hydrogenosomobacter endosymbioticus’ was found in association with host hydrogenosomes [53].
The endonuclear localisation is not exclusive of the Holosporaceae among the Holosporineae, as the same condition is typical also of some members of the ‘Caedimonaceae’. For instance, the intracellular localisation of ‘Caedimonas’ appears to be correlated with the host species, being intramacronuclear in Paramecium caudatum and Paramecium duboscqui [130, 131], while cytoplasmic in the species of the P. aurelia complex [7, 132]. The case of ‘Candidatus Nucleicultrix amoebiphila’ is even more distinctive, since this bacterium presents a complex infectious life cycle that is highly reminiscent of the one of Holospora and HLB [74]. Similarly to the latter, the effect of ‘Candidatus Nucleicultrix’ on the host is variable and was shown to be negligible for its natural host, the amoeba Hartmanella, but lethal for experimentally infected Acanthamoeba castellanii. Overall, it seems legitimate to speculate that the ability for interacting with host nuclei could have been ancestral in the Holosporaceae + ‘Caedimonadaceae’ lineage, being successively lost (or remaining unnoticed to date) in some of the descendants. Alternatively, multiple parallel evolutionary events of this trait are also possible, considering its independent evolution in other phylogenetically unrelated bacteria [257]. On the other hand, and remarkably, it seems more parsimonious to infer that the highly specialised infectious life cycles of Holospora + HLB and of ‘Candidatus Nucleicultrix’ have most likely arisen independently from such a hypothetical ‘permissive’ ancestral ability to colonise host nuclei.
‘Caedimonas varicaedens’, the other deeply investigated member of the Holosporineae, has been studied particularly for the distinctive killer trait that it confers to its Paramecium hosts, similarly to the gammaproteobacterium Caedibacter taeniospiralis [5–7]. A decades-long history of investigations was focused on the killer trait and its determinants, also before the discovery that ‘Caedimonas’ and Caedibacter were phylogenetically apart. As such, these two bacteria were treated jointly in many studies and in reviews on the subject [6, 258, 259], which will be summarised below. Part of the intracellular bacterial population ceases to divide and produce proteinaceous coiled ribbons (Fig. 3) that are light-refractile and thus called R-bodies [260, 261]. The bacteria bearing R-bodies can be discharged extracellularly through their host cytoproct [262] and, when ingested by a novel host, are lysed in the digestive vacuoles leading to the release of the R-bodies, which under those acidic conditions unroll and break the vacuolar membrane (Fig. 3) [261, 262]. The unrolling of R-bodies ultimately leads to host death, with different lethal symptoms according to the bacterial genotype, including reversion of the normal rotation direction of the cell, formation of large vacuoles, or paralysis [258]. Although required for the killer trait [263], the R-bodies are not directly toxic, rather their role is in the disruption of the host vacuolar membrane, allowing the delivery of the actual toxin produced by the bacterium [264–266]. This toxin is probably a protein but was yet not conclusively identified [6, 259]. The potential toxic activity of ‘Caedimonas’ towards eukaryotes other than Paramecium has been only seldom explored until now, with still inconclusive evidence [267]. On the other hand, paramecia hosting ‘Caedimonas’ are resistant, probably thanks to an antidote produced by the bacterium. Interestingly, in both ‘Caedimonas’ and Caedibacter, the determinants of R-bodies (as well as probably toxin and antidote) are encoded on plasmids and/or linked to phage particles and prophage induction [260, 268–272]. As such, the multipartite interactions involving ciliates, bacteria, and plasmids/phages were meaningfully defined as involving ‘extrachromosomal elements of extrachromosomal elements of Paramecium’ [273]. Being encoded on mobile elements, R-bodies and other determinants of the killer trait are likely horizontally transmissible, which would explain their presence in unrelated bacteria, such as Caedibacter and ‘Caedimonas’, as well as others (see below).
Fig. 3.
Ultrastructure of R-bodies, modified from [306]. A Coiled R-bodies (black arrows) within the bacterial symbiont cells in the Paramecium cytoplasm. B Isolated R-body in the process of unrolling in a telescopic fashion. Scale bars: A 100 nm; B 1 μm
Thanks to the killer trait, the paramecia bearing ‘Caedimonas’ display competitive advantages [133, 274], but at the same time, the bacteria can be parasitic for taking ATP for energy and other metabolites from their hosts [130, 274, 275], somehow comparably to Holospora. Besides, the peculiar effect of the killer trait was tentatively envisioned as a phenomenon of addictive manipulation, namely a way by which the bacterium indirectly prevents the host from getting rid of it, by ‘punishing’ it thanks to the action of bacteria released by still-infected neighbouring host cells [43]. This is posited to be analogous to the reproductive manipulation of arthropods exerted by Wolbachia and other bacteria, in particular cytoplasmic incompatibility, by which infected males sterilise the crosses with uninfected females, thus favouring the reproduction of infected females, the only ones that transmit the bacteria to the progeny [276]. Following these lines of thought, it is worth to consider that, although R-bodies and their genetic determinants are best studied in Caedibacter and ‘Caedimonas’, they are found in a wide range of phylogenetically unrelated bacteria [258, 277], which can employ their R-bodies in interaction with eukaryotes [278–281]. Interestingly, these other bacteria equipped with R-body genes include two other Holosporineae, affiliated to different families, namely ‘Candidatus Bealeia paramacronuclearis’ (Holosporaceae) and ‘Candidatus Finniella inopinata’ (‘Candidatus Paracaedibacteraceae’) [14]. R-bodies were not observed in either of these bacteria [1, 75], suggesting that their expression is conditional, and leaving still open which is their role (if any) in the interaction with the respective hosts, in particular, a possible addictive manipulation alike to the one exerted by ‘Caedimonas’ through the killer trait. Herein, it seems worthwhile to consider the potential implications in the evolution of Holosporineae as a whole, since phylogenetic reconstructions of the R-body genes are compatible both with a vertical inheritance from the ancestor of this lineage (followed by losses in other representatives) or with a recent exchange between the equipped members [14].
The other more investigated case study among the Holosporineae is ‘Candidatus Hepatobacter penaei’ (Holosporaceae), known for being the causative agent of NHP [49]. NHP is mostly known in the Pacific white shrimp L. vannamei, which is the main farmed shrimp worldwide [282], but the disease (or its causative agent) was detected also in other shrimp species, such as L. setiferus, L. stylirostris, F. aztecus, F. californiensis, F. duorarum, Penaeus monodon, Fenneropenaeus merguensis, and Melicertus marginatus, as well as the American lobster Homarus americanus [49, 114]. ‘Candidatus Hepatobacter penaei’ resides and multiplies exclusively inside the tubular epithelial cells of the host hepatopancreas [283], and its involvement in NHP was experimentally demonstrated [284]. This bacterium is pleomorphic, being observed as a coccoid/short rod-shaped form, and as a long helical rod with eight long periplasmic flagella [285, 286], which might be involved in bacterial motility, adherence to host cells, and virulence [287]. The bacterial infection heavily damages the hepatopancreas, causing detachment of tubular cells, melanisation, and necrosis of the tubules, strong intracellular haemocytosis, and oedema [49]. The disease ultimately affects multiple organs and functions, as the observed signs include, among many others, anorexia, lethargy, abdominal muscular atrophy, soft exoskeleton, decreased growth rate, empty intestines, erosion of appendages, darkening, and lesions in the cuticle [288, 289]. NHP chronically causes mortalities of up to 50 − 95% in affected postlarval stages and well as in juveniles and broodstock [290], with significant economical impacts in terms of production losses and management costs [291]. At the appropriate progression stages, it can be effectively counteracted by antibiotic treatments [292]. The transmission of the NHP is not entirely clarified but can occur rapidly in densely populated farms [49], and a possible role of microalgae, other crustaceans (Artemia), or zooplankton as vectors was hypothesised [293, 294]. The disease is common especially in the south of the USA, as well as in Central and South America, and was shown to occur typically after persistently high water temperature (29–35 °C) and salinity (30–40‰) during summer, with the bacterium reaching over 15% prevalence in farms [295], but less than 1% in the wild [282]. Available molecular diagnostic tools include a multilocus sequence analysis [114], as well as a qPCR on flgE flagellar gene [290].
It is noteworthy that the recently described relative ‘Candidatus Hepatobacter paralithodis’ also affects a crustacean, namely the blue king crab P. platypus, although with very low prevalence in the wild [48]. Several traits are in common with NHP, as this bacterium as well is localised only within the epithelial cells of the host hepatopancreas, mostly free from host vacuoles. Two main morphotypes are present, namely rounded forms, often found in chains, and elongated rods devoid of flagella. The hepatopancreas structure and function are impaired by the bacteria, with hypertrophy and desquamation of infected cells, softening of tubules, granuloma, and necrosis. However, very little external signs of the disease were observed, besides lethargy. The geographical and climatic pattern is different from NHP, as ‘Candidatus Hepatobacter paralithodis’ was found in a much colder area of the Northern Hemisphere (Sea of Okhotsk), but seasonal patterns in the disease emergence were suspected as well [48].
Taking into account the more deeply investigated cases presented above and the available data on other representatives, it is thus possible to summarise the available knowledge into an evolutionary scenario for the interactions between the Holosporineae and their hosts. In quite ancient times, the free-living ancestors of this bacterial lineage acquired the ability to interact with eukaryotic hosts, which were most likely unicellular aquatic ones, and, based on relative environmental frequency of the current available representatives and its phylogenetic patterns, may have been more specifically freshwater [95]. It seems likely that, analogous to other professional symbionts [296–299], secretion systems and effectors have likely played a pivotal role in the establishment and successive development of these interactions. In the case of the Holosporineae, the most credible candidate is the type VI secretion system, conserved in almost all the genomes sequenced, including the smallest ones, with the exception of Holospora spp. Despite the lack of experimental data on its functioning and on possible secreted molecules, this high conservation is highly indicative of the key role of this apparatus in the lifestyle of Holosporineae. The type VI secretion system of Holosporineae is probably a non-standard one, considering the apparent lack of genes for some main components (outer membrane complex TssD and inner tube TssJ) [54, 58]. The case of Holospora could be explained by the markedly specialised infectious life cycle of these bacteria [2, 3], which likely involves equally specialised effector molecules [212, 220, 222, 224], possibly making the ‘canonical’ ones among the Holosporineae superfluous.
As a matter of fact, while only few members of the Holosporineae were experimentally shown to invade novel hosts [3, 74, 284], indirect evidence from compared host and symbiont phylogenies clearly indicates the recurrent ability of these bacteria of host transfer and host species shift along their evolutionary history, e.g. [1, 14, 52, 54, 56, 75]. Flagella likely play an important role in transmission and invasion of novel hosts, as hypothesised for other professional symbionts [40, 300–302], potentially working also as an additional secretion system [303]. As described above, a role of flagella in host invasion could be the case for ‘Candidatus Hepatobacter penaei’, which is the only Holosporineae bacterium observed bearing flagella but likely applies also for several other representatives equipped with flagellar genes, which could express them conditionally, similarly to what many Rickettsiales are thought to do [40, 300]. From an evolutionary perspective, a still relevant open point is the transition from putative ancestral protist hosts to Metazoans. This is a common trait observed among professional symbionts, having significant medical or veterinary impacts, as it can pave the way for the emergence of dangerous pathogens [44–46]. In the case of the Holosporineae, this has occurred relatively rarely, with the only ascertained documented case being the ‘Candidatus Hepatobacter’ lineage [47, 48]. It is yet to be determined whether this is only somehow accidental, or whether the molecular genetic set of the Holosporineae is for any reason less permissive than other professional symbiont lineages for such transition.
Comparative genomics quite sharply indicates metabolic dependence of the Holosporineae on their hosts, particularly in terms of obtaining metabolic precursors, such as amino acids or nucleotides, and cofactors [14]. With respect to their free-living ancestors, all the Holosporineae appear to have experienced variable degrees of genome reduction, from relatively large sizes (2.5–3 Mb) in certain ‘Candidatus Paracaedibacteraceae’ [76, 78], down to less than 600 kbp in some fast-evolving Holosporaceae [54, 58]. This suggests that the reduction was probably rather gradual and/or recent, with possible lineage-specific patterns, with the larger gene repertoires suggestive of more complex yet uncharacterised regulatory mechanisms and interaction mechanisms with the hosts. Reductive trends are particularly marked among the Holosporaceae, likely with concurrent specialisation towards certain life cycles and/or hosts, in particular in the extremely reduced fast-evolving members. Indications of possible multiple losses of a biosynthetic ability (i.e. for biotin) as a consequence of the independent acquisition of the respective transporters in different sub-lineages of Holosporineae were obtained [14], reminiscently of the Rickettsiales [40]. Nevertheless, the data available so far suggest that the major evolutionary steps which resulted in host-dependence occurred only once in the common ancestral evolution of all Holosporineae, rather than independently in the different sub-lineages.
Regarding interaction mechanisms and effects on the host, the presence in other Holosporineae of the genes for the R-bodies, involved in the killer trait conferred by ‘Caedimonas’ to its hosts, raises the question of whether similar mechanisms might be more pervasive among the Holosporineae and whether they might also have some implications in earlier evolutionary steps of this lineage [14]. This is even more so if we consider such phenomena as instances of addictive manipulation exerted by the bacteria on their hosts [43] and if we also take into account other cases among Holosporineae which could indicate addiction. These pertain ‘Candidatus Cytomitobacter spp.’ (Holosporaceae) and ‘Candidatus Bodonicaedibacter vickermanii’ (‘Candidatus Paracaedibacteraceae’), which, while devoid of R-bodies, could not be eliminated by antibiotics or cause the death of the host as well if removed, respectively [50, 57].
Finally, considering that multiple Holosporineae belonging to different families are pleomorphic, including the abovementioned Holospora/HLB, ‘Caedimonas’, and ‘Candidatus Hepatobacter’, as well as ‘Candidatus Finniella’ species [52, 75], it is interesting to wonder whether these are purely secondary lineage-specific adaptations or rely also on an ancestral morpho-functional ‘flexibility’ in the lifestyle of the ancestral Holosporineae.
Final Remarks and Perspectives
Here, we provide an in-depth review of the literature on a broad, diverse, and ancient alphaproteobacterial lineage living in obligate association with eukaryotic hosts, mostly protists, aiming for a comprehensive dedicated resource for interested researchers. With the purpose of offering a common and stable nomenclature ground, we also propose a taxonomic revision based on phylogenetic and taxonomic considerations. Specifically, while recently these bacteria were commonly referred to either as an order (Holosporales) or a family (Holosporaceae) [1, 59], we move them as a suborder within the Rhodospirillales, namely the Holosporineae, keeping the internal taxonomic substructure of the Holosporales sensu Szokoli et al. [1].
We put forward that the knowledge on the diversity, host range, environmental, and geographical spread of the Holosporineae, though growing steadily through the years with continuous novel reports, is probably still an underestimate to date. Indeed, their most frequent hosts, protists, are still neglected in studies on associations with bacteria as compared to multicellular eukaryotes [41] and, despite being ecologically and geographically widespread [304], have little individual biomass, which may frequently hinder the detection of their associated bacteria, Holosporineae included, in environmental metagenomic screening studies.
Even in the most studied Holosporineae Holospora and ‘Caedimonas’, the interactions with the hosts, including mechanisms and reciprocal effects, are still insufficiently understood and almost entirely unknown in most of the other members of this lineage. This is quite unfortunate, not only for the sake of scientific and evolutionary curiosity on this neglected lineage, but also for several other relevant reasons. One of those is the still underappreciated, but well-plausible, impact that Holosporineae may have on aquatic ecosystems, based on the marked effects they have on certain widespread hosts, such as the competitive advantages conferred to the ‘killer’ paramecia by ‘Caedimonas’ [6]. Moreover, we underline the still not well-exploited convenience of using Holosporineae in comparative studies with other more renowned bacterial lineages sharing lifestyle features, such as the other professional symbionts [41]. This applies in particular to the Rickettsiales [40, 299], as these two lineages are independent instances of evolutionarily long-lasting associations with eukaryotes among the Alphaproteobacteria, thus offering a way to discern traits that are common, and thus more likely fundamental, from lineage-specific ones.
It is also worthwhile to consider the direct economical impact of those Holosporineae that affect farmed crustaceans [49, 291]. Additionally, we should not overlook that some Holosporineae are frequently associated with human pathogenic amoebas [11, 62, 137, 138, 149, 150, 152] and that DNA of others was found associated to humans [91, 93, 94, 163]. The latter findings may indicate a yet-to-be-confirmed direct association with human cells or the presence of undetected skin protists/fungi intracellularly hosting the bacteria. All these studies suggest a hypothetical role of the bacteria in the diseases [11], with potential analogies with the Wolbachia symbionts of filarial nematodes [305], and thus deserving further targeted investigations.
To sum up, accounting for evolutionary, ecological, economical, and possibly sanitary reasons, we highlight the need for future investigations to reveal the diversity of the Holosporineae and elucidate their functional interactions with eukaryote, which may be hopefully fostered and sustained by the recent and possible future increased availability of genomic sequences of these bacteria.
Taxonomic Proposals
Description of the Species ‘Candidatus Odyssella acanthamoebae’ comb. nov.
‘Candidatus Odyssella acanthamoebae’ (O.dys.sel’la. a.can.tha.moe’bae, N.L. fem. pl. n.). This corresponds to the description of ‘Candidatus Paracaedibacter acanthamoebae’ [11], with the following modifications. Phylogenetic position, family ‘Candidatus Paracaedibacteraceae’; intracellular symbiont of Acanthamoeba sp. UWC9 and other Acanthamoeba strains [11, 149–151].
Emended Description of the Genus ‘Candidatus Paracaedibacter’ Horn et al. 1999
‘Candidatus Paracaedibacter’ (Pa.ra.cae.di.bac’ter, N.L. masc. s. n.). The genus contains only one described species, namely ‘Candidatus Paracaedibacter symbiosus’ [11]. Is the type genus of the family ‘Candidatus Paracaedibacteraceae’ [75].
Emended Description of the Family ‘Candidatus Hepatincolaceae’ Szokoli et al. 2016
‘Candidatus Hepatincolaceae’ (He.pat.in.co.la’ce.ae, N.L. fem. pl. n.). The description of ‘Candidatus Hepatincolaceae’ [1] is emended as follows. The family currently contains three genera, ‘Candidatus Hepatincola’ [12], ‘Candidatus Tenuibacter’ [79], and ‘Candidatus Tardigradibacter’ [81], and is affiliated to the order Rhodospirillales. The type genus is ‘Candidatus Hepatincola’.
Description of the Suborder Holosporineae subord. nov.
Holosporineae (Ho.lo.spo.ri’ne.ae, N. L. fem. n., Holospora type genus of the suborder; suff. -ineae ending to denote a suborder; N.L. fem. pl. n. Holosporineae, the suborder of the genus Holospora). The description is the same as that given previously for Holosporales [1], with some modifications. Defined by phylogenetic analyses based on SSU rRNA gene sequences and on concatenated conserved protein-coding ortholog genes. The suborder contains three families (Holosporaceae, ‘Caedimonadaceae’, and ‘Candidatus Paracaedibacteraceae’). The suborder Holosporineae is a member of the order Rhodospirillales.
Author Contribution
M.C. and G.P. planned and organised the work. M.C. performed the literature search, wrote the main manuscript text and prepared the figures. All authors reviewed the manuscript.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Highlights
• The Holosporineae (until now named Holosporales) are a lineage of bacteria living in association with a wide variety of eukaryotic hosts, mostly protists.
• The Holosporineae originated anciently (possibly over 1 bya) from within the Rhodospirillales and likely became soon host-dependent.
• Many Holosporineae display distinctive subcellular locations in their hosts, such as externally and internally associated with nuclei and mitochondria/hydrogenosomes.
• Some Holosporineae are highly infectious (and intranuclear). Most of the other Holosporineae as well are likely able of horizontal transmission and host-species shift, as testified by the incongruence between host and symbiont phylogenies.
• Some Holosporineae are able to selectively kill uninfected hosts, thus providing a competitive advantage to infected cells and at the same time creating a barrier against their own loss by the host (addictive manipulation).
• Some Holosporineae are pathogenic for farmed crustaceans, with relevant economical impacts, while others are associated with human parasitic amoebae.
• The mechanisms and dynamics of interactions between the Holosporineae and their hosts, as well as the consequent effects, are still poorly understood.
• The type VI secretion system, widespread in the Holosporineae, is a probable candidate for delivering key molecules for modulating host interactions.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Szokoli F, Castelli M, Sabaneyeva E et al (2016) Disentangling the taxonomy of Rickettsiales and description of two novel symbionts (“Candidatus Bealeia paramacronuclearis” and “Candidatus Fokinia cryptica”) sharing the cytoplasm of the ciliate protist Paramecium biaurelia. Appl Environ Microbiol 82:7236–7247. 10.1128/AEM.02284-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujishima M (2009) Infection and maintenance of Holospora species in Paramecium caudatum. In: Fujishima M (ed) Endosymbionts in Paramecium. Springer, Berlin, Heidelberg, pp 201–225 [Google Scholar]
- 3.Schrallhammer M, Potekhin A (2020) Epidemiology of nucleus-dwelling Holospora: infection, transmission, adaptation, and interaction with Paramecium. Results Probl Cell Differ 69:105–135. 10.1007/978-3-030-51849-3_4 [DOI] [PubMed] [Google Scholar]
- 4.Fokin SI, Görtz H-D (2009) Diversity of Holospora bacteria in Paramecium and their characterization. In: Fujishima M (ed) Endosymbionts in Paramecium. Springer, Berlin, Heidelberg, pp 161–199 [Google Scholar]
- 5.Beier CL, Horn M, Michel R et al (2002) The genus Caedibacter comprises endosymbionts of Paramecium spp. related to the Rickettsiales (Alphaproteobacteria) and to Francisella tularensis (Gammaproteobacteria). Appl Environ Microbiol 68:6043–6050. 10.1128/AEM.68.12.6043-6050.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrallhammer M, Schweikert M (2009) The killer effect of Paramecium and its causative agents. In: Fujishima M (ed) Endosymbionts in Paramecium. Springer, Berlin, Heidelberg, pp 227–246 [Google Scholar]
- 7.Schrallhammer M, Castelli M, Petroni G (2018) Phylogenetic relationships among endosymbiotic R-body producer: bacteria providing their host the killer trait. Syst Appl Microbiol 41:213–220. 10.1016/j.syapm.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Sonneborn TM (1938) Mating types in Paramecium aurelia: diverse conditions for mating in different stocks; occurrence, number and interrelations of the types. Proc Am Philos Soc 79:411–434 [Google Scholar]
- 9.Springer N, Ludwig W, Amann R et al (1993) Occurrence of fragmented 16S rRNA in an obligate bacterial endosymbiont of Paramecium caudatum. Proc Natl Acad Sci U S A 90:9892–9895. 10.1073/pnas.90.21.9892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loy JK, Dewhirst FE, Weber W et al (1996) Molecular phylogeny and in situ detection of the etiologic agent of necrotizing hepatopancreatitis in shrimp. Appl Environ Microbiol 62:3439–3445. 10.1128/aem.62.9.3439-3445.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn M, Fritsche TR, Gautom RK et al (1999) Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryophilus. Environ Microbiol 1:357–367. 10.1046/j.1462-2920.1999.00045.x [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Stingl U, Anton-Erxleben F et al (2004) ’ Candidatus Hepatincola porcellionum’ gen. nov., sp. nov., a new, stalk-forming lineage of Rickettsiales colonizing the midgut glands of a terrestrial isopod. Arch Microbiol 181:299–304. 10.1007/s00203-004-0655-7 [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Luo H (2021) Dating Alphaproteobacteria evolution with eukaryotic fossils. Nat Commun 12:3324. 10.1038/s41467-021-23645-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannini M, Petroni G, Castelli M (2024) Novel evolutionary insights on the interactions of the Holosporales (Alphaproteobacteria) with eukaryotic hosts from comparative genomics. Environ Microbiol 26:e16562. 10.1111/1462-2920.16562 [DOI] [PubMed] [Google Scholar]
- 15.Poitelon J-B, Joyeux M, Welté B et al (2009) Assessment of phylogenetic diversity of bacterial microflora in drinking water using serial analysis of ribosomal sequence tags. Water Res 43:4197–4206. 10.1016/j.watres.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Brown JF, Jones DS, Mills DB et al (2011) Application of a depositional F acies model to an acid mine drainage site. Appl Environ Microbiol 77:545–554. 10.1128/AEM.01550-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jogler M, Siemens H, Chen H et al (2011) Identification and targeted cultivation of abundant novel freshwater sphingomonads and analysis of their population substructure. Appl Environ Microbiol 77:7355–7364. 10.1128/AEM.05832-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirk Harris J, Gregory Caporaso J, Walker JJ et al (2013) Phylogenetic stratigraphy in the Guerrero Negro hypersaline microbial mat. ISME J 7:50–60. 10.1038/ismej.2012.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavagutti VS, Andrei A-Ş, Mehrshad M et al (2019) Phage-centric ecological interactions in aquatic ecosystems revealed through ultra-deep metagenomics. Microbiome 7:135. 10.1186/s40168-019-0752-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavagutti VS, Bulzu P-A, Chiriac CM et al (2023) High-resolution metagenomic reconstruction of the freshwater spring bloom. Microbiome 11:15. 10.1186/s40168-022-01451-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rissanen AJ, Saarela T, Jäntti H et al (2021) Vertical stratification patterns of methanotrophs and their genetic controllers in water columns of oxygen-stratified boreal lakes. FEMS Microbiol Ecol 97:fiaa252. 10.1093/femsec/fiaa252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran PQ, Bachand SC, McIntyre PB et al (2021) Depth-discrete metagenomics reveals the roles of microbes in biogeochemical cycling in the tropical freshwater Lake Tanganyika. ISME J 15:1971–1986. 10.1038/s41396-021-00898-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vosloo S, Huo L, Anderson CL et al (2021) Evaluating de novo assembly and binning strategies for time series drinking water metagenomes. Microbiol Spectr 9:e0143421. 10.1128/Spectrum.01434-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiriac M-C, Bulzu P-A, Andrei A-S et al (2022) Ecogenomics sheds light on diverse lifestyle strategies in freshwater CPR. Microbiome 10:84. 10.1186/s40168-022-01274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Liu W, Liang J et al (2022) Mining of novel secondary metabolite biosynthetic gene clusters from acid mine drainage. Sci Data 9:760. 10.1038/s41597-022-01866-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancey CE, Smith DJ, Den Uyl PA et al (2022) Metagenomic and metatranscriptomic insights into population diversity of Microcystis blooms: spatial and temporal dynamics of mcy genotypes, including a partial operon that can be abundant and expressed. Appl Environ Microbiol 88:e0246421. 10.1128/aem.02464-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe AL, Bardot C, Le Jeune A-H et al (2023) Variable impact of geochemical gradients on the functional potential of bacteria, archaea, and phages from the permanently stratified Lac Pavin. Microbiome 11:14. 10.1186/s40168-022-01416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hongoh Y, Deevong P, Inoue T et al (2005) Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl Environ Microbiol 71:6590–6599. 10.1128/AEM.71.11.6590-6599.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RT, McCormick KF, Martin AP (2008) Bacterial communities of Bartonella-positive fleas: diversity and community assembly patterns. Appl Environ Microbiol 74:1667–1670. 10.1128/AEM.02090-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraune S, Abe Y, Bosch TCG (2009) Disturbing epithelial homeostasis in the metazoan Hydra leads to drastic changes in associated microbiota. Environ Microbiol 11:2361–2369. 10.1111/j.1462-2920.2009.01963.x [DOI] [PubMed] [Google Scholar]
- 31.Montalvo NF, Hill RT (2011) Sponge-associated bacteria are strictly maintained in two closely related but geographically distant sponge hosts. Appl Environ Microbiol 77:7207–7216. 10.1128/AEM.05285-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King JHP, Mahadi NM, Bong CFJ et al (2014) Bacterial microbiome of Coptotermes curvignathus (Isoptera: Rhinotermitidae) reflects the coevolution of species and dietary pattern. Insect Sci 21:584–596. 10.1111/1744-7917.12061 [DOI] [PubMed] [Google Scholar]
- 33.Miller IJ, Weyna TR, Fong SS et al (2016) Single sample resolution of rare microbial dark matter in a marine invertebrate metagenome. Sci Rep 6:34362. 10.1038/srep34362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konecka E, Olszanowski Z (2019) Detection of a new bacterium of the family Holosporaceae (Alphaproteobacteria: Holosporales) associated with the oribatid mite Achipteria coleoptrata. Biologia 74:1517–1522. 10.2478/s11756-019-00251-w [Google Scholar]
- 35.Samad MS, Lee HJ, Cerbin S et al (2020) Niche differentiation of host-associated pelagic microbes and their potential contribution to biogeochemical cycling in artificially warmed lakes. Front Microbiol 11. 10.3389/fmicb.2020.00582 [DOI] [PMC free article] [PubMed]
- 36.Zhao Z, Jiang J, Zheng J et al (2022) Exploiting the gut microbiota to predict the origins and quality traits of cultured sea cucumbers. Environ Microbiol 24:3882–3897. 10.1111/1462-2920.15972 [DOI] [PubMed] [Google Scholar]
- 37.Ichige R, Urabe J (2023) Divergence of the host-associated microbiota with the genetic distance of host individuals within a parthenogenetic Daphnia species. Microb Ecol 86:2097–2108. 10.1007/s00248-023-02219-5 [DOI] [PubMed] [Google Scholar]
- 38.Dharamshi JE, Tamarit D, Eme L et al (2020) Marine sediments illuminate Chlamydiae diversity and evolution. Curr Biol 30:1032-1048.e7. 10.1016/j.cub.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 39.Hugoson E, Guliaev A, Ammunét T, Guy L (2022) Host adaptation in Legionellales is 1.9 Ga, coincident with eukaryogenesis. Mol Biol Evol 39:msac037. 10.1093/molbev/msac037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castelli M, Nardi T, Gammuto L et al (2024) Host association and intracellularity evolved multiple times independently in the Rickettsiales. Nat Commun 15:1093. 10.1038/s41467-024-45351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husnik F, Tashyreva D, Boscaro V et al (2021) Bacterial and archaeal symbioses with protists. Curr Biol 31:R862–R877. 10.1016/j.cub.2021.05.049 [DOI] [PubMed] [Google Scholar]
- 42.Bontemps Z, Paranjape K, Guy L (2024) Host–bacteria interactions: ecological and evolutionary insights from ancient, professional endosymbionts. FEMS Microbiol Rev 48:fuae021. 10.1093/femsre/fuae021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castelli M, Nardi T, Giovannini M, Sassera D (2024) Addictive manipulation: a perspective on the role of reproductive parasitism in the evolution of bacteria–eukaryote symbioses. Biol Let 20:20240310. 10.1098/rsbl.2024.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elwell C, Mirrashidi K, Engel J (2016) Chlamydia cell biology and pathogenesis. Nat Rev Microbiol 14:385–400. 10.1038/nrmicro.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauhan D, Shames SR (2021) Pathogenicity and virulence of Legionella: intracellular replication and host response. Virulence 12:1122–1144. 10.1080/21505594.2021.1903199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salje J (2021) Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat Rev Microbiol 19:375–390. 10.1038/s41579-020-00507-2 [DOI] [PubMed] [Google Scholar]
- 47.Nunan LM, Pantoja CR, Gomez-Jimenez S, Lightner DV (2013) “Candidatus Hepatobacter penaei”, an intracellular pathogenic enteric bacterium in the hepatopancreas of the marine shrimp Penaeus vannamei (Crustacea: Decapoda). Appl Environ Microbiol 79:1407–1409. 10.1128/AEM.02425-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryazanova TV, Eliseikina MG, Kukhlevsky AD (2020) First record of new rickettsia-like organism in the blue king crab Paralithodes platypus from the Sea of Okhotsk: distribution, morphological evidence and genetic analysis. J Invertebr Pathol 170:107325. 10.1016/j.jip.2020.107325 [DOI] [PubMed] [Google Scholar]
- 49.Yu Y-B, Choi J-H, Kang J-C et al (2022) Shrimp bacterial and parasitic disease listed in the OIE: a review. Microb Pathog 166:105545. 10.1016/j.micpath.2022.105545 [DOI] [PubMed] [Google Scholar]
- 50.Tashyreva D, Prokopchuk G, Votýpka J et al (2018) Life cycle, ultrastructure, and phylogeny of new diplonemids and their endosymbiotic bacteria. mBio 9:e02447-17. 10.1128/mBio.02447-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vecchi M, Newton ILG, Cesari M et al (2018) The microbial community of tardigrades: environmental influence and species specificity of microbiome structure and composition. Microb Ecol 76:467–481. 10.1007/s00248-017-1134-4 [DOI] [PubMed] [Google Scholar]
- 52.Boscaro V, Husnik F, Vannini C, Keeling PJ (2019) Symbionts of the ciliate Euplotes: diversity, patterns and potential as models for bacteria-eukaryote endosymbioses. Proc Biol Sci 286:20190693. 10.1098/rspb.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeshita K, Yamada T, Kawahara Y et al (2019) Tripartite symbiosis of an anaerobic scuticociliate with two hydrogenosome-associated endosymbionts, a Holospora-related alphaproteobacterium and a methanogenic archaeon. Appl Environ Microbiol 85:e00854-e919. 10.1128/AEM.00854-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George EE, Husnik F, Tashyreva D et al (2020) Highly reduced genomes of protist endosymbionts show evolutionary convergence. Curr Biol 30:925-933.e3. 10.1016/j.cub.2019.12.070 [DOI] [PubMed] [Google Scholar]
- 55.Guidetti R, Vecchi M, Ferrari A et al (2020) Further insights in the Tardigrada microbiome: phylogenetic position and prevalence of infection of four new Alphaproteobacteria putative endosymbionts. Zool J Linn Soc 188:925–937. 10.1093/zoolinnean/zlz128 [Google Scholar]
- 56.Korotaev A, Benken K, Sabaneyeva E (2020) “Candidatus Mystax nordicus” aggregates with mitochondria of its host, the ciliate Paramecium nephridiatum. Diversity 12:251. 10.3390/d12060251 [Google Scholar]
- 57.Midha S, Rigden DJ, Siozios S et al (2021) Bodo saltans (Kinetoplastida) is dependent on a novel Paracaedibacter-like endosymbiont that possesses multiple putative toxin-antitoxin systems. ISME J 15:1680–1694. 10.1038/s41396-020-00879-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castelli M, Lanzoni O, Giovannini M et al (2022) “Candidatus Gromoviella agglomerans”, a novel intracellular Holosporaceae parasite of the ciliate Paramecium showing marked genome reduction. Environ Microbiol Rep 14:34–49. 10.1111/1758-2229.13021 [DOI] [PubMed] [Google Scholar]
- 59.Muñoz-Gómez SA, Hess S, Burger G et al (2019) An updated phylogeny of the Alphaproteobacteria reveals that the parasitic Rickettsiales and Holosporales have independent origins. Elife 8:e42535. 10.7554/eLife.42535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gromov BV, Ossipov DV (1981) Holospora (ex Hafkine 1890) nom. rev., a genus of bacteria inhabiting the nuclei of paramecia. Int J Syst Evol Microbiol 31:348–352. 10.1099/00207713-31-3-348 [Google Scholar]
- 61.Amann R, Springer N, Ludwig W et al (1991) Identification in situ and phylogeny of uncultured bacterial endosymbionts. Nature 351:161–164. 10.1038/351161a0 [DOI] [PubMed] [Google Scholar]
- 62.Birtles RJ, Rowbotham TJ, Michel R et al (2000) “Candidatus Odyssella thessalonicensis” gen. nov., sp. nov., an obligate intracellular parasite of Acanthamoeba species. Int J Syst Evol Microbiol 50:63–72. 10.1099/00207713-50-1-63 [DOI] [PubMed] [Google Scholar]
- 63.Baker BJ, Hugenholtz P, Dawson SC, Banfield JF (2003) Extremely acidophilic protists from acid mine drainage host Rickettsiales-lineage endosymbionts that have intervening sequences in their 16S rRNA genes. Appl Environ Microbiol 69:5512–5518. 10.1128/AEM.69.9.5512-5518.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Görtz H-D, Schmidt HJ (2005) Family III. Holosporaceae fam. nov. In: Bergey’s manual of systematic bacteriology, 2nd edition. Springer, pp 146–149
- 65.Boscaro V, Schrallhammer M, Benken KA et al (2013) Rediscovering the genus Lyticum, multiflagellated symbionts of the order Rickettsiales. Sci Rep 3:3305. 10.1038/srep03305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kursacheva E, Korotaev A, Benken K et al (2023) Phenotypic polymorphism in two endosymbiotic bacteria of the ciliate Paramecium: Pseudolyticum multiflagellatum and “Ca. Megaira venefica.” Diversity 15:924. 10.3390/d15080924 [Google Scholar]
- 67.Quackenbush RL (1978) Genetic relationships among bacterial endosymbionts of Paramecium aurelia. Microbiology 108:181–187. 10.1099/00221287-108-2-181 [Google Scholar]
- 68.Preer JR, Preer LB (1982) Revival of names of protozoan endosymbionts and proposal of Holospora caryophila nom. nov. Int J Syst Bacteriol 32:140–141. 10.1099/00207713-32-1-140 [Google Scholar]
- 69.Boscaro V, Fokin SI, Schrallhammer M et al (2013) Revised systematics of Holospora-like bacteria and characterization of “Candidatus Gortzia infectiva”, a novel macronuclear symbiont of Paramecium jenningsi. Microb Ecol 65:255–267. 10.1007/s00248-012-0110-2 [DOI] [PubMed] [Google Scholar]
- 70.Fraune S, Bosch TCG (2007) Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra. Proc Natl Acad Sci U S A 104:13146–13151. 10.1073/pnas.0703375104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eschbach E, Pfannkuchen M, Schweikert M et al (2009) “Candidatus Paraholospora nucleivisitans”, an intracellular bacterium in Paramecium sexaurelia shuttles between the cytoplasm and the nucleus of its host. Syst Appl Microbiol 32:490–500. 10.1016/j.syapm.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 72.Kim E, Park JS, Simpson AGB et al (2010) Complex array of endobionts in Petalomonas sphagnophila, a large heterotrophic euglenid protist from Sphagnum-dominated peatlands. ISME J 4:1108–1120. 10.1038/ismej.2010.40 [DOI] [PubMed] [Google Scholar]
- 73.Du X, Li X, Wang Y et al (2012) Phylogenetic diversity of nitrogen fixation genes in the intestinal tract of Reticulitermes chinensis Snyder. Curr Microbiol 65:547–551. 10.1007/s00284-012-0185-5 [DOI] [PubMed] [Google Scholar]
- 74.Schulz F, Lagkouvardos I, Wascher F et al (2014) Life in an unusual intracellular niche: a bacterial symbiont infecting the nucleus of amoebae. ISME J 8:1634–1644. 10.1038/ismej.2014.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hess S, Suthaus A, Melkonian M (2016) “Candidatus Finniella” (Rickettsiales, Alphaproteobacteria), novel endosymbionts of viridiraptorid amoeboflagellates (Cercozoa, Rhizaria). Appl Environ Microbiol 82:659–670. 10.1128/AEM.02680-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Georgiades K, Madoui M-A, Le P et al (2011) Phylogenomic analysis of Odyssella thessalonicensis fortifies the common origin of Rickettsiales, Pelagibacter ubique and Reclimonas americana mitochondrion. PLoS ONE 6:e24857. 10.1371/journal.pone.0024857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferla MP, Thrash JC, Giovannoni SJ, Patrick WM (2013) New rRNA gene-based phylogenies of the Alphaproteobacteria provide perspective on major groups, mitochondrial ancestry and phylogenetic instability. PLoS ONE 8:e83383. 10.1371/journal.pone.0083383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, Wu M (2015) An integrated phylogenomic approach toward pinpointing the origin of mitochondria. Sci Rep 5:7949. 10.1038/srep07949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kroer P, Kjeldsen KU, Nyengaard JR et al (2016) A novel extracellular gut symbiont in the marine worm Priapulus caudatus (Priapulida) reveals an alphaproteobacterial symbiont clade of the Ecdysozoa. Front Microbiol 7:539. 10.3389/fmicb.2016.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dittmer J, Bredon M, Moumen B et al (2023) The terrestrial isopod symbiont “Candidatus Hepatincola porcellionum” is a potential nutrient scavenger related to Holosporales symbionts of protists. ISME Commun 3:18. 10.1038/s43705-023-00224-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castelli M, Gammuto L, Podushkina D et al (2025) Hepatincolaceae (Alphaproteobacteria) are distinct from Holosporales and independently evolved to associate with Ecdysozoa. Environ Microbiol 27:e70028. 10.1111/1462-2920.70028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.George EE, Tashyreva D, Kwong WK et al (2022) Gene transfer agents in bacterial endosymbionts of microbial eukaryotes. Genome Biol Evol 14:evac099. 10.1093/gbe/evac099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chuvochina M, Mussig AJ, Chaumeil P-A et al (2023) Proposal of names for 329 higher rank taxa defined in the Genome Taxonomy Database under two prokaryotic codes. FEMS Microbiol Lett 370:071. 10.1093/femsle/fnad071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hördt A, López MG, Meier-Kolthoff JP et al (2020) Analysis of 1,000+ type-strain genomes substantially improves taxonomic classification of Alphaproteobacteria. Front Microbiol 11:468. 10.3389/fmicb.2020.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Z, Petersen JM, Martijn J et al (2020) A novel alphaproteobacterium with a small genome identified from the digestive gland of multiple species of abalone. Environ Microbiol Rep 12:387–395. 10.1111/1758-2229.12845 [DOI] [PubMed] [Google Scholar]
- 86.Lundgren JG, Lehman RM, Chee-sanford J (2007) Bacterial communities within digestive tracts of ground beetles (Coleoptera: Carabidae). Ann Entomol Soc Am 100:275–282. 10.1603/0013-8746(2007)100[275:BCWDTO]2.0.CO;2 [Google Scholar]
- 87.Ley RE, Hamady M, Lozupone C et al (2008) Evolution of mammals and their gut microbes. Science 320:1647–1651. 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roeselers G, Mittge EK, Stephens WZ et al (2011) Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. 10.1038/ismej.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sudakaran S, Salem H, Kost C, Kaltenpoth M (2012) Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae). Mol Ecol 21:6134–6151. 10.1111/mec.12027 [DOI] [PubMed] [Google Scholar]
- 90.Wu S, Tian J, Wang G et al (2012) Characterization of bacterial community in the stomach of yellow catfish (Pelteobagrus fulvidraco). World J Microbiol Biotechnol 28:2165–2174. 10.1007/s11274-012-1022-5 [DOI] [PubMed] [Google Scholar]
- 91.Grice EA, Kong HH, Conlan S et al (2009) Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192. 10.1126/science.1171700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson WR, Torralba M, Fair PA et al (2009) Novel diversity of bacterial communities associated with bottlenose dolphin upper respiratory tracts. Environ Microbiol Rep 1:555–562. 10.1111/j.1758-2229.2009.00080.x [DOI] [PubMed] [Google Scholar]
- 93.Kong HH, Oh J, Deming C et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22:850–859. 10.1101/gr.131029.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Rensburg JJ, Lin H, Gao X et al (2015) The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio 6:e01315-01315. 10.1128/mBio.01315-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lanzoni O, Szokoli F, Schrallhammer M et al (2023) “Candidatus Intestinibacterium parameciiphilum”-member of the “Candidatus Paracaedibacteraceae” family (Alphaproteobacteria, Holosporales) inhabiting the ciliated protist Paramecium. Int Microbiol 27:659–671. 10.1007/s10123-023-00414-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Potekhin A, Nekrasova I, Flemming F (2021) In shadow of Holospora – the continuous quest for new Holosporaceae members. Protistology 15:127–141. 10.21685/1680-0826-2021-15-3-3 [Google Scholar]
- 97.Potekhin A, Schweikert M, Nekrasova I et al (2018) Complex life cycle, broad host range and adaptation strategy of the intranuclear Paramecium symbiont Preeria caryophila comb. nov. FEMS Microbiol Ecol 94. 10.1093/femsec/fiy076 [DOI] [PubMed]
- 98.Fokin SI, Serra V, Ferrantini F et al (2019) “Candidatus Hafkinia simulans” gen. nov., sp. nov., a novel Holospora-like bacterium from the macronucleus of the rare brackish water ciliate Frontonia salmastra (Oligohymenophorea, Ciliophora): multidisciplinary characterization of the new endosymbiont and its host. Microb Ecol 77:1092–1106. 10.1007/s00248-018-1311-0 [DOI] [PubMed] [Google Scholar]
- 99.Vakkerov-Kouzova ND, Rautian MS (2011) Obtaining and characterization of “Holospora curviuscula” and Holospora obtusa, bacterial symbionts of the macronuclei of Paramecium bursaria and Paramecium caudatum. Microbiology 80:728–732. 10.1134/S0026261711050171 [PubMed] [Google Scholar]
- 100.Lanzoni O, Fokin SI, Lebedeva N et al (2016) Rare freshwater ciliate Paramecium chlorelligerum Kahl, 1935 and its macronuclear symbiotic bacterium “Candidatus Holospora parva”. PLoS ONE 11:e0167928. 10.1371/journal.pone.0167928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garushyants SK, Beliavskaia AY, Malko DB et al (2018) Comparative genomic analysis of Holospora spp., intranuclear symbionts of paramecia. Front Microbiol 9:738. 10.3389/fmicb.2018.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wackerow-Kouzova ND, Myagkov DV (2021) Clarification of the taxonomic position of Paramecium caudatum micronucleus symbionts. Curr Microbiol 78:4098–4102. 10.1007/s00284-021-02667-7 [DOI] [PubMed] [Google Scholar]
- 103.Serra V, Fokin SI, Castelli M et al (2016) “Candidatus Gortzia shahrazadis”, a novel endosymbiont of Paramecium multimicronucleatum and a revision of the biogeographical distribution of Holospora-Like Bacteria. Front Microbiol 7. 10.3389/fmicb.2016.01704 [DOI] [PMC free article] [PubMed]
- 104.Beliavskaia AY, Predeus AV, Garushyants SK et al (2020) New intranuclear symbiotic bacteria from macronucleus of Paramecium putrinum—“Candidatus Gortzia Yakutica”. Diversity 12:198. 10.3390/d12050198 [Google Scholar]
- 105.McDonald R, Schreier HJ, Watts JEM (2012) Phylogenetic analysis of microbial communities in different regions of the gastrointestinal tract in Panaque nigrolineatus, a wood-eating fish. PLoS ONE 7:e48018. 10.1371/journal.pone.0048018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.West PT, Probst AJ, Grigoriev IV et al (2018) Genome-reconstruction for eukaryotes from complex natural microbial communities. Genome Res 28:569–580. 10.1101/gr.228429.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fokin SI, Serra V (2022) Bacterial symbiosis in ciliates (Alveolata, Ciliophora): roads traveled and those still to be taken. J Eukaryot Microbiol 69:e12886. 10.1111/jeu.12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tashyreva D, Votýpka J, Yabuki A et al (2025) Description of new diplonemids (Diplonemea, Euglenozoa) and their endosymbionts: charting the morphological diversity of these poorly known heterotrophic flagellates. Protist 177:126090. 10.1016/j.protis.2025.126090 [DOI] [PubMed] [Google Scholar]
- 109.Mujakić I, Andrei A-Ş, Shabarova T et al (2021) Common presence of phototrophic Gemmatimonadota in temperate freshwater lakes. mSystems 6:e01241-20. 10.1128/mSystems.01241-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sánchez-Navarro R, Nuhamunada M, Mohite OS et al (2022) Long-read metagenome-assembled genomes improve identification of novel complete biosynthetic gene clusters in a complex microbial activated sludge ecosystem. mSystems 7:e0063222. 10.1128/msystems.00632-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Allers E, Wright JJ, Konwar KM et al (2013) Diversity and population structure of Marine Group A bacteria in the Northeast subarctic Pacific Ocean. ISME J 7:256–268. 10.1038/ismej.2012.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lesaulnier C, Papamichail D, McCorkle S et al (2008) Elevated atmospheric CO2 affects soil microbial diversity associated with trembling aspen. Environ Microbiol 10:926–941. 10.1111/j.1462-2920.2007.01512.x [DOI] [PubMed] [Google Scholar]
- 113.Yoon HY, Lee SY (2019) An assessment of the bacterial diversity found in dental unit waterlines using the Illumina MiSeq. Biocontrol Sci 24:201–212. 10.4265/bio.24.201 [DOI] [PubMed] [Google Scholar]
- 114.Leyva JM, Martínez-Porchas M, Hernández-López J et al (2018) Identifying the causal agent of necrotizing hepatopancreatitis in shrimp: multilocus sequence analysis approach. Aquac Res 49:1795–1802. 10.1111/are.13633 [Google Scholar]
- 115.Hook SE, Twine NA, Simpson SL et al (2014) 454 pyrosequencing-based analysis of gene expression profiles in the amphipod Melita plumulosa: transcriptome assembly and toxicant induced changes. Aquat Toxicol 153:73–88. 10.1016/j.aquatox.2013.11.022 [DOI] [PubMed] [Google Scholar]
- 116.Fortin SG, Song B, Anderson IC, Reece KS (2022) Blooms of the harmful algae Margalefidinium polykrikoides and Alexandrium monilatum alter the York River Estuary microbiome. Harmful Algae 114:102216. 10.1016/j.hal.2022.102216 [DOI] [PubMed] [Google Scholar]
- 117.Nakai R, Abe T, Baba T et al (2012) Microflorae of aquatic moss pillars in a freshwater lake, East Antarctica, based on fatty acid and 16S rRNA gene analyses. Polar Biol 35:425–433. 10.1007/s00300-011-1090-2 [Google Scholar]
- 118.Brodie EL, Desantis TZ, Joyner DC et al (2006) Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl Environ Microbiol 72:6288–6298. 10.1128/AEM.00246-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L-M, Liu F, Tan W-F et al (2008) Microbial DNA extraction and analyses of soil iron–manganese nodules. Soil Biol Biochem 40:1364–1369. 10.1016/j.soilbio.2007.01.004 [Google Scholar]
- 120.Zhang W, Huang Z, He L, Sheng X (2012) Assessment of bacterial communities and characterization of lead-resistant bacteria in the rhizosphere soils of metal-tolerant Chenopodium ambrosioides grown on lead-zinc mine tailings. Chemosphere 87:1171–1178. 10.1016/j.chemosphere.2012.02.036 [DOI] [PubMed] [Google Scholar]
- 121.Liu R, Zhang Y, Ding R et al (2009) Comparison of archaeal and bacterial community structures in heavily oil-contaminated and pristine soils. J Biosci Bioeng 108:400–407. 10.1016/j.jbiosc.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 122.KurtE W, Kan J, Polson SW, Williamson SJ (2011) Optimizing the indirect extraction of prokaryotic DNA from soils. Soil Biol Biochem 43:736–748. 10.1016/j.soilbio.2010.04.017 [Google Scholar]
- 123.Heinzel E, Hedrich S, Janneck E et al (2009) Bacterial diversity in a mine water treatment plant. Appl Environ Microbiol 75:858–861. 10.1128/AEM.01045-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schauer R, Bienhold C, Ramette A, Harder J (2010) Bacterial diversity and biogeography in deep-sea surface sediments of the South Atlantic Ocean. ISME J 4:159–170. 10.1038/ismej.2009.106 [DOI] [PubMed] [Google Scholar]
- 125.Anantharaman K, Brown CT, Hug LA et al (2016) Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat Commun 7:13219. 10.1038/ncomms13219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Watanabe T, Kojima H, Fukui M (2016) Identity of major sulfur-cycle prokaryotes in freshwater lake ecosystems revealed by a comprehensive phylogenetic study of the dissimilatory adenylylsulfate reductase. Sci Rep 6:36262. 10.1038/srep36262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weber CF, King GM (2010) Distribution and diversity of carbon monoxide-oxidizing bacteria and bulk bacterial communities across a succession gradient on a Hawaiian volcanic deposit. Environ Microbiol 12:1855–1867. 10.1111/j.1462-2920.2010.02190.x [DOI] [PubMed] [Google Scholar]
- 128.Slama HB, Chenari Bouket A, Alenezi FN et al (2021) Olive mill and olive pomace evaporation pond’s by-products: toxic level determination and role of indigenous microbiota in toxicity alleviation. Appl Sci 11:5131. 10.3390/app11115131 [Google Scholar]
- 129.Schweikert M, Meyer B (2001) Characterization of intracellular bacteria in the freshwater dinoflagellate Peridinium cinctum. Protoplasma 217:177–184. 10.1007/BF01283398 [DOI] [PubMed] [Google Scholar]
- 130.Schmidt HJ, Görtz H-D, Quackenbush RL (1987) Caedibacter caryophila sp. nov., a killer symbiont inhabiting the macronucleus of Paramecium caudatum. Int J Syst Evol Microbiol 37:459–462. 10.1099/00207713-37-4-459 [Google Scholar]
- 131.Schrallhammer M, Fokin SI, Schleifer K-H, Petroni G (2006) Molecular characterization of the obligate endosymbiont “Caedibacter macronucleorum” Fokin and Görtz, 1993 and of its host Paramecium duboscqui strain Ku4-8. J Eukaryot Microbiol 53:499–506. 10.1111/j.1550-7408.2006.00133.x [DOI] [PubMed] [Google Scholar]
- 132.Kusch J, Stremmel M, Breiner H-W et al (2000) The toxic symbiont Caedibacter caryophila in the cytoplasm of Paramecium novaurelia. Microb Ecol 40:330–335. 10.1007/s002480000034 [DOI] [PubMed] [Google Scholar]
- 133.Flemming FE, Grosser K, Schrallhammer M (2021) Natural shifts in endosymbionts’ occurrence and relative frequency in their ciliate host population. Front Microbiol 12:791615. 10.3389/fmicb.2021.791615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fokin SI (2004) Bacterial endocytobionts of ciliophora and their interactions with the host cell. Int Rev Cytol 236:181–249. 10.1016/S0074-7696(04)36005-5 [DOI] [PubMed] [Google Scholar]
- 135.Brodie EL, DeSantis TZ, Parker JPM et al (2007) Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci 104:299–304. 10.1073/pnas.0608255104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin Y, Wang L, Xu K et al (2021) Revealing taxon-specific heavy metal-resistance mechanisms in denitrifying phosphorus removal sludge using genome-centric metaproteomics. Microbiome 9:67. 10.1186/s40168-021-01016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matsuo J, Kawaguchi K, Nakamura S et al (2010) Survival and transfer ability of phylogenetically diverse bacterial endosymbionts in environmental Acanthamoeba isolates. Environ Microbiol Rep 2:524–533. 10.1111/j.1758-2229.2009.00094.x [DOI] [PubMed] [Google Scholar]
- 138.Chan LL, Mak JW, Ambu S, Chong PY (2018) Identification and ultrastructural characterization of Acanthamoeba bacterial endocytobionts belonging to the Alphaproteobacteria class. PLoS ONE 13:e0204732. 10.1371/journal.pone.0204732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guazzaroni M-E, Herbst F-A, Lores I et al (2013) Metaproteogenomic insights beyond bacterial response to naphthalene exposure and bio-stimulation. ISME J 7:122–136. 10.1038/ismej.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huddy RJ, Sachdeva R, Kadzinga F et al (2021) Thiocyanate and organic carbon inputs drive convergent selection for specific autotrophic Afipia and Thiobacillus strains within complex microbiomes. Front Microbiol 12:643368. 10.3389/fmicb.2021.643368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Youssef N, Sheik CS, Krumholz LR et al (2009) Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16S rRNA gene-based environmental surveys. Appl Environ Microbiol 75:5227–5236. 10.1128/AEM.00592-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Casar CP, Momper LM, Kruger BR, Osburn MR (2021) Iron-fueled life in the continental subsurface: deep mine microbial observatory, South Dakota, USA. Appl Environ Microbiol 87:e0083221. 10.1128/AEM.00832-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaw AK, Halpern AL, Beeson K et al (2008) It’s all relative: ranking the diversity of aquatic bacterial communities. Environ Microbiol 10:2200–2210. 10.1111/j.1462-2920.2008.01626.x [DOI] [PubMed] [Google Scholar]
- 144.Parks DH, Rinke C, Chuvochina M et al (2017) Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2:1533–1542. 10.1038/s41564-017-0012-7 [DOI] [PubMed] [Google Scholar]
- 145.Acosta-González A, Rosselló-Móra R, Marqués S (2013) Characterization of the anaerobic microbial community in oil-polluted subtidal sediments: aromatic biodegradation potential after the Prestige oil spill. Environ Microbiol 15:77–92. 10.1111/j.1462-2920.2012.02782.x [DOI] [PubMed] [Google Scholar]
- 146.Wu Y-H, Liao L, Wang C-S et al (2013) A comparison of microbial communities in deep-sea polymetallic nodules and the surrounding sediments in the Pacific Ocean. Deep Sea Res Part I 79:40–49. 10.1016/j.dsr.2013.05.004 [Google Scholar]
- 147.Zhang Y, Kitajima M, Whittle AJ, Liu W-T (2017) Benefits of genomic insights and crispr-cas signatures to monitor potential pathogens across drinking water production and distribution systems. Front Microbiol 8:2036. 10.3389/fmicb.2017.02036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dirren S, Posch T (2016) Promiscuous and specific bacterial symbiont acquisition in the amoeboid genus Nuclearia (Opisthokonta). FEMS Microbiol Ecol 92:fiw105. 10.1093/femsec/fiw105 [DOI] [PubMed] [Google Scholar]
- 149.Choi SH, Cho MK, Ahn SC et al (2009) Endosymbionts of Acanthamoeba isolated from domestic tap water in Korea. Korean J Parasitol 47:337–344. 10.3347/kjp.2009.47.4.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Müller A, Walochnik J, Wagner M, Schmitz-Esser S (2016) A clinical Acanthamoeba isolate harboring two distinct bacterial endosymbionts. Eur J Protistol 56:21–25. 10.1016/j.ejop.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 151.dos Santos DL, Virginio VG, Berté FK et al (2022) Clinical and molecular diagnosis of Acanthamoeba keratitis in contact lens wearers in southern Brazil reveals the presence of an endosymbiont. Parasitol Res 121:1447–1454. 10.1007/s00436-022-07474-y [DOI] [PubMed] [Google Scholar]
- 152.Scharf SA, Friedrichs L, Bock R et al (2024) Oxford Nanopore Technology-based identification of an Acanthamoeba castellanii endosymbiosis in microbial keratitis. Microorganisms 12:2292. 10.3390/microorganisms12112292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yarza P, Yilmaz P, Pruesse E et al (2014) Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol 12:635–645. 10.1038/nrmicro3330 [DOI] [PubMed] [Google Scholar]
- 154.Li X, Zhang Z, Luo J et al (2022) Arbuscular mycorrhizal fungi and nitrilotriacetic acid regulated Suaeda salsa growth in Cd-contaminated saline soil by driving rhizosphere bacterial assemblages. Environ Exp Bot 193:104669. 10.1016/j.envexpbot.2021.104669 [Google Scholar]
- 155.Seitz VA, McGivern BB, Daly RA et al (2022) Variation in root exudate composition influences soil microbiome membership and function. Appl Environ Microbiol 88:e0022622. 10.1128/aem.00226-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Probst AJ, Ladd B, Jarett JK et al (2018) Differential depth distribution of microbial function and putative symbionts through sediment-hosted aquifers in the deep terrestrial subsurface. Nat Microbiol 3:328–336. 10.1038/s41564-017-0098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bornemann TLV, Adam PS, Turzynski V et al (2022) Genetic diversity in terrestrial subsurface ecosystems impacted by geological degassing. Nat Commun 13:284. 10.1038/s41467-021-27783-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huo L, Zhao S, Shi B et al (2021) Bacterial community change and antibiotic resistance promotion after exposure to sulfadiazine and the role of UV/H2O2-GAC treatment. Chemosphere 283:131214. 10.1016/j.chemosphere.2021.131214 [DOI] [PubMed] [Google Scholar]
- 159.Rivière D, Desvignes V, Pelletier E et al (2009) Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J 3:700–714. 10.1038/ismej.2009.2 [DOI] [PubMed] [Google Scholar]
- 160.Huang Y, Deng Y, Law JC-F et al (2021) Acesulfame aerobic biodegradation by enriched consortia and Chelatococcus spp.: kinetics, transformation products, and genomic characterization. Water Res 202:117454. 10.1016/j.watres.2021.117454 [DOI] [PubMed] [Google Scholar]
- 161.Schneider D, Aßmann N, Wicke D et al (2020) Metagenomes of wastewater at different treatment stages in central Germany. Microbiol Resour Announc 9:e00201-e220. 10.1128/MRA.00201-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yao N, Li W, Hu L, Fang N (2024) Do mould inhibitors alter the microbial community structure and antibiotic resistance gene profiles on textiles? Sci Total Environ 911:168808. 10.1016/j.scitotenv.2023.168808 [DOI] [PubMed] [Google Scholar]
- 163.Rodríguez-Díaz C, Martín-Reyes F, Taminiau B et al (2023) The metagenomic composition and effects of fecal-microbe-derived extracellular vesicles on intestinal permeability depend on the patient’s disease. Int J Mol Sci 24:4971. 10.3390/ijms24054971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parks DH, Chuvochina M, Waite DW et al (2018) A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. 10.1038/nbt.4229 [DOI] [PubMed] [Google Scholar]
- 165.Goh KM, Shahar S, Chan K-G et al (2019) Current status and potential applications of underexplored prokaryotes. Microorganisms 7:468. 10.3390/microorganisms7100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Williams MM, Domingo JWS, Meckes MC et al (2004) Phylogenetic diversity of drinking water bacteria in a distribution system simulator. J Appl Microbiol 96:954–964. 10.1111/j.1365-2672.2004.02229.x [DOI] [PubMed] [Google Scholar]
- 167.Pan Y, Ren Q, Chen P et al (2021) Insight into microbial community aerosols associated with electronic waste handling facilities by culture-dependent and culture-independent methods. Front Public Health 9. 10.3389/fpubh.2021.657784 [DOI] [PMC free article] [PubMed]
- 168.Yuhana M, Horath T, Hanselmann K (2006) Bacterial community shifts of a high mountain lake in response to variable simulated conditions: availability of nutrients, light and oxygen. Biotropia - The Southeast Asian Journal of Tropical Biology 13. 10.11598/btb.2006.13.2.219
- 169.Santofimia E, González-Toril E, López-Pamo E et al (2013) Microbial diversity and its relationship to physicochemical characteristics of the water in two extreme acidic pit lakes from the Iberian Pyrite Belt (SW Spain). PLoS ONE 8:e66746. 10.1371/journal.pone.0066746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.García-Moyano A, González-Toril E, Aguilera Á, Amils R (2012) Comparative microbial ecology study of the sediments and the water column of the Río Tinto, an extreme acidic environment. FEMS Microbiol Ecol 81:303–314. 10.1111/j.1574-6941.2012.01346.x [DOI] [PubMed] [Google Scholar]
- 171.Headd B, Engel AS (2014) Biogeographic congruency among bacterial communities from terrestrial sulfidic springs. Front Microbiol 5:473. 10.3389/fmicb.2014.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gihring TM, Moser DP, Lin L-H et al (2006) The distribution of microbial taxa in the subsurface water of the Kalahari Shield, South Africa. Geomicrobiol J 23:415–430. 10.1080/01490450600875696 [Google Scholar]
- 173.Gomez-Alvarez V, Humrighouse BW, Revetta RP, Santo Domingo JW (2015) Bacterial composition in a metropolitan drinking water distribution system utilizing different source waters. J Water Health 13:140–151. 10.2166/wh.2014.057 [DOI] [PubMed] [Google Scholar]
- 174.Kim L, Pagaling E, Zuo YY, Yan T (2014) Impact of substratum surface on microbial community structure and treatment performance in biological aerated filters. Appl Environ Microbiol 80:177–183. 10.1128/AEM.03001-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marshall Hathaway JJ, Garcia MG, Balasch MM et al (2014) Comparison of bacterial diversity in Azorean and Hawai’ian lava Ccave microbial mats. Geomicrobiol J 31:205–220. 10.1080/01490451.2013.777491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Y, Yang Z, Ni J et al (2022) Toxicity and modulation of silver nanoparticles synthesized using abalone viscera hydrolysates on bacterial community in aquatic environment. Front Microbiol 13. 10.3389/fmicb.2022.968650 [DOI] [PMC free article] [PubMed]
- 177.Elshahed MS, Youssef NH, Spain AM et al (2008) Novelty and uniqueness patterns of rare members of the soil biosphere. Appl Environ Microbiol 74:5422–5428. 10.1128/AEM.00410-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cruz-Martínez K, Suttle KB, Brodie EL et al (2009) Despite strong seasonal responses, soil microbial consortia are more resilient to long-term changes in rainfall than overlying grassland. ISME J 3:738–744. 10.1038/ismej.2009.16 [DOI] [PubMed] [Google Scholar]
- 179.Qi X, Wang E, Xing M et al (2012) Rhizosphere and non-rhizosphere bacterial community composition of the wild medicinal plant Rumex patientia. World J Microbiol Biotechnol 28:2257–2265. 10.1007/s11274-012-1033-2 [DOI] [PubMed] [Google Scholar]
- 180.Lin X, Kennedy D, Fredrickson J et al (2012) Vertical stratification of subsurface microbial community composition across geological formations at the Hanford Site. Environ Microbiol 14:414–425. 10.1111/j.1462-2920.2011.02659.x [DOI] [PubMed] [Google Scholar]
- 181.Yin H, Cao L, Qiu G et al (2008) Molecular diversity of 16S rRNA and gyrB genes in copper mines. Arch Microbiol 189:101–110. 10.1007/s00203-007-0298-6 [DOI] [PubMed] [Google Scholar]
- 182.Vannini C, Ferrantini F, Ristori A et al (2012) Betaproteobacterial symbionts of the ciliate Euplotes: origin and tangled evolutionary path of an obligate microbial association. Environ Microbiol 14:2553–2563. 10.1111/j.1462-2920.2012.02760.x [DOI] [PubMed] [Google Scholar]
- 183.Hongoh Y, Ohkuma M, Kudo T (2003) Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae). FEMS Microbiol Ecol 44:231–242. 10.1016/S0168-6496(03)00026-6 [DOI] [PubMed] [Google Scholar]
- 184.Nakajima H, Hongoh Y, Usami R et al (2005) Spatial distribution of bacterial phylotypes in the gut of the termite Reticulitermes speratus and the bacterial community colonizing the gut epithelium. FEMS Microbiol Ecol 54:247–255. 10.1016/j.femsec.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 185.Yang H, Schmitt-Wagner D, Stingl U, Brune A (2005) Niche heterogeneity determines bacterial community structure in the termite gut (Reticulitermes santonensis). Environ Microbiol 7:916–932. 10.1111/j.1462-2920.2005.00760.x [DOI] [PubMed] [Google Scholar]
- 186.Ikeda-Ohtsubo W, Faivre N, Brune A (2010) Putatively free-living ’Endomicrobia’- ancestors of the intracellular symbionts of termite gut flagellates? Environ Microbiol Rep 2:554–559. 10.1111/j.1758-2229.2009.00124.x [DOI] [PubMed] [Google Scholar]
- 187.Strassert JFH, Köhler T, Wienemann THG et al (2012) ‘Candidatus Ancillula trichonymphae’, a novel lineage of endosymbiotic Actinobacteria in termite gut flagellates of the genus Trichonympha. Environ Microbiol 14:3259–3270. 10.1111/1462-2920.12012 [DOI] [PubMed] [Google Scholar]
- 188.Mies US, Hervé V, Kropp T et al (2024) Genome reduction and horizontal gene transfer in the evolution of Endomicrobia-rise and fall of an intracellular symbiosis with termite gut flagellates. mBio 15(6):e0082624. 10.1128/mbio.00826-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miyake S, Ngugi DK, Stingl U (2016) Phylogenetic diversity, distribution, and cophylogeny of giant bacteria (Epulopiscium) with their surgeonfish hosts in the Red Sea. Front Microbiol 7. 10.3389/fmicb.2016.00285 [DOI] [PMC free article] [PubMed]
- 190.Mehrshad M, Salcher MM, Okazaki Y et al (2018) Hidden in plain sight-highly abundant and diverse planktonic freshwater Chloroflexi. Microbiome 6:176. 10.1186/s40168-018-0563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Crump BC, Hobbie JE (2005) Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr 50:1718–1729. 10.4319/lo.2005.50.6.1718 [Google Scholar]
- 192.Hamilton TL, Jones DS, Schaperdoth I, Macalady JL (2014) Metagenomic insights into S(0) precipitation in a terrestrial subsurface lithoautotrophic ecosystem. Front Microbiol 5:756. 10.3389/fmicb.2014.00756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang L, Gao G, Tang X, Shao K (2014) Impacts of different salinities on bacterial biofilm communities in fresh water. Can J Microbiol 60:319–326. 10.1139/cjm-2013-0808 [DOI] [PubMed] [Google Scholar]
- 194.Li D, Li Z, Yu J et al (2010) Characterization of bacterial community structure in a drinking water distribution system during an occurrence of red water. Appl Environ Microbiol 76:7171–7180. 10.1128/AEM.00832-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ruff SE, Humez P, de Angelis IH et al (2023) Hydrogen and dark oxygen drive microbial productivity in diverse groundwater ecosystems. Nat Commun 14:3194. 10.1038/s41467-023-38523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu M, Zhang Y, Yang M et al (2012) Abundance and distribution of tetracycline resistance genes and mobile elements in an oxytetracycline production wastewater treatment system. Environ Sci Technol 46:7551–7557. 10.1021/es301145m [DOI] [PubMed] [Google Scholar]
- 197.Blunt SM, Sackett JD, Rosen MR et al (2018) Association between degradation of pharmaceuticals and endocrine-disrupting compounds and microbial communities along a treated wastewater effluent gradient in Lake Mead. Sci Total Environ 622–623:1640–1648. 10.1016/j.scitotenv.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 198.Gibson I, Bedingfield G, Horne RW, Bird B (1986) Electron microscope study of the structure of Holospora caryophila. Micron and Microscopica Acta 17:247–257. 10.1016/0739-6260(86)90006-7 [Google Scholar]
- 199.Görtz H-D, Ahlers N, Robenek H (1989) Ultrastructure of the infectious and reproductive forms of Holospora obtusa, a bacterium infecting the macronucleus of Paramecium caudatum. Microbiology 135:3079–3085. 10.1099/00221287-135-11-3079 [Google Scholar]
- 200.Fujishima M, Sawabe H, Iwatsuki K (1990) Scanning electron microscopic observation of differentiation from the reproductive short form to the infectious long form of Holospora obtusa. J Protozool 37:123–128. 10.1111/j.1550-7408.1990.tb05881.x [Google Scholar]
- 201.Fokin SI, Lebedeva NA, Potekhin A et al (2023) Holospora-like bacteria “Candidatus Gortzia yakutica” and Preeria caryophila: ultrastructure, promiscuity, and biogeography of the symbionts. Eur J Protistol 90:125998. 10.1016/j.ejop.2023.125998 [DOI] [PubMed] [Google Scholar]
- 202.Preer LB (1969) Alpha, an infectious macronuclear symbiont of Paramecium aurelia. J Protozool 16:570–578. 10.1111/j.1550-7408.1969.tb02315.x [DOI] [PubMed] [Google Scholar]
- 203.Wiemann M (1989) The release of Holospora obtusa from Paramecium caudatum observed with a new device for extended in vivo microscopy. J Protozool 36:176–179. 10.1111/j.1550-7408.1989.tb01069.x [Google Scholar]
- 204.Wiemann M, Görtz H-D (1989) Release of the endonucleobiotic bacterium Holospora elegans from its host cell Paramecium caudatum. Eur J Protistol 25:100–108. 10.1016/S0932-4739(89)80021-5 [DOI] [PubMed] [Google Scholar]
- 205.Fokin S, Sabaneyeva E (1997) Release of endonucleobiotic bacteria Holospora bacillata and Holospora curvata from the macronucleus of their host cells Paramecium woodruffi and Paramecium calkinsi. Endocytobiosis and Cell Research 12:49–55 [Google Scholar]
- 206.Fokin S (1998) Strategies of the macronuclear dndocytobionts of Paramecium during the sexual process of the host. Symbiosis 25:323–342 [Google Scholar]
- 207.Fokin SI, Brigge T, Brenner J, Görtz H-D (1996) Holospora species infecting the nuclei of Paramecium appear to belong into two groups of bacteria. Eur J Protistol 32:19–24. 10.1016/S0932-4739(96)80072-1 [Google Scholar]
- 208.Fujishima M, Nagahara K, Kojima Y, Sayama Y (1991) Sensitivity of the infectious long form of the macronuclear endosymbiont Holospora obtusa of the ciliate Paramecium caudatum against chemical and physical factors. Eur J Protistol 27:119–126. 10.1016/S0932-4739(11)80333-0 [DOI] [PubMed] [Google Scholar]
- 209.Barhey K, Gibson I (1984) A study on the conditions for infection of Holospora caryophila, a macronuclear symbiont of Paramecium biaurelia. Micron Microscopica Acta 15:261–268. 10.1016/0739-6260(84)90040-6 [Google Scholar]
- 210.Görtz H-D, Wiemann M (1989) Route of infection of the bacteria Holospora elegans and Holospora obtusa into the nuclei of Paramecium caudatum. Eur J Protistol 24:101–109. 10.1016/S0932-4739(89)80037-9 [DOI] [PubMed] [Google Scholar]
- 211.Fokin SI, Skovorodkin IN, Sabaneyeva EV (2003) Initial steps of infection of the ciliate Paramecium with bacteria Holospora sp. Tsitologiia 45:1227–1233 [PubMed] [Google Scholar]
- 212.Iwatani K, Dohra H, Lang BF et al (2005) Translocation of an 89-kDa periplasmic protein is associated with Holospora infection. Biochem Biophys Res Commun 337:1198–1205. 10.1016/j.bbrc.2005.09.175 [DOI] [PubMed] [Google Scholar]
- 213.Sabaneyeva EV, Fokin SI, Gavrilova EV, Kornilova ES (2005) Nocodazole inhibits macronuclear infection with Holospora obtusa in Paramecium caudatum. Protoplasma 226:147–153. 10.1007/s00709-005-0121-7 [DOI] [PubMed] [Google Scholar]
- 214.Sabaneyeva EV, Derkacheva ME, Benken KA et al (2009) Actin-based mechanism of Holospora obtusa trafficking in Paramecium caudatum. Protist 160:205–219. 10.1016/j.protis.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 215.Fujishima M, Fujita M (1985) Infection and maintenance of Holospora obtusa, a macronucleus-specific bacterium of the ciliate Paramecium caudatum. J Cell Sci 76:179–187. 10.1242/jcs.76.1.179 [DOI] [PubMed] [Google Scholar]
- 216.Kawai M, Fujishima M (2000) Invasion of the macronucleus of Paramecium caudatum by the bacterium Holospora obtusa: fates of the bacteria and timings of invasion steps. Eur J Protistol 36:46–52. 10.1016/S0932-4739(00)80022-X [Google Scholar]
- 217.Görtz HD, Lellig S, Miosga O, Wiemann M (1990) Changes in fine structure and polypeptide pattern during development of Holospora obtusa, a bacterium infecting the macronucleus of Paramecium caudatum. J Bacteriol 172:5664–5669. 10.1128/jb.172.10.5664-5669.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wiemann M, Görtz HD (1991) Identification and localization of major stage-specific polypeptides of infectious Holospora obtusa with monoclonal antibodies. J Bacteriol 173:4842–4850. 10.1128/jb.173.15.4842-4850.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dohra H, Fujishima M, Hoshide K (1994) Monoclonal antibodies specific for periplasmic materials of the macronuclear specific bacterium Holospora obtusa of the ciliate Paramecium caudatum. Eur J Protistol 30:288–294. 10.1016/S0932-4739(11)80075-1 [Google Scholar]
- 220.Dohra H, Yamamoto K, Fujishima M, Ishikawa H (1997) Cloning and sequencing of gene coding for a periplasmic 5.4 kDa peptide of the macronucleus-specific symbiont Holospora obtusa of the ciliate Paramecium caudatum. Zoolog Sci 14:69–75. 10.2108/zsj.14.69 [DOI] [PubMed] [Google Scholar]
- 221.Fujishima M, Dohra H, Kawai M (1997) Quantitative changes in periplasmic proteins of the macronucleus-specific bacterium Holospora obtusa in the infection process of the ciliate Paramecium caudatum. J Eukaryot Microbiol 44:636–642. 10.1111/j.1550-7408.1997.tb05971.x [DOI] [PubMed] [Google Scholar]
- 222.Fujishima M, Kawano H, Miyakawa I (2023) A 63-kDa periplasmic protein of the endonuclear symbiotic bacterium Holospora obtusa secreted to the outside of the bacterium during the early infection process binds weakly to the macronuclear dna of the host Paramecium caudatum. Microorganisms 11:155. 10.3390/microorganisms11010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ehrsam E, Görtz H-D (1999) Surface proteins of the gram-negative bacterium Holospora obtusa bind to macronuclear proteins of its host Paramecium caudatum. Eur J Protistol 35:304–308. 10.1016/S0932-4739(99)80008-X [Google Scholar]
- 224.Abamo F, Dohra H, Fujishima M (2008) Fate of the 63-kDa periplasmic protein of the infectious form of the endonuclear symbiotic bacterium Holospora obtusa during the infection process. FEMS Microbiol Lett 280:21–27. 10.1111/j.1574-6968.2007.01023.x [DOI] [PubMed] [Google Scholar]
- 225.Görtz HD, Fujishima M (1983) Conjugation and meiosis of Paramecium caudatum infected with the micronucleus-specific bacterium Holospora elegans. Eur J Cell Biol 32:86–91 [PubMed] [Google Scholar]
- 226.Dohra H, Tanaka K, Suzuki T et al (2014) Draft genome sequences of three Holospora species (Holospora obtusa, Holospora undulata, and Holospora elegans), endonuclear symbiotic bacteria of the ciliate Paramecium caudatum. FEMS Microbiol Lett 359:16–18. 10.1111/1574-6968.12577 [DOI] [PubMed] [Google Scholar]
- 227.Skoblo II, Borchsenius ON, Lebedeva NA, Osipov DV (1990) Symbiogenic lysis of bacteria Holospora acuminata in the generative nucleus of the ciliate Paramecium bursaria. Tsitologiya 32:515–519 [Google Scholar]
- 228.Skoblo II, Borkhsenius ON, Lebedeva NA, Osipov DV (1990) Selective lysis of the symbiotic bacterium Holospora acuminata in the genetive nucleus of the ciliate Paramecium bursaria. Cytology 32:515–519 [Google Scholar]
- 229.Ossipov DV, Skoblo II, Borchsenius ON, Lebedeva NA (1993) Interactions between Paramecium bursaria (Protozoa, Ciliophora, Hymenostomatida) and their nuclear symbionts. Eur J Protistol 29:61–71. 10.1016/S0932-4739(11)80298-1 [DOI] [PubMed] [Google Scholar]
- 230.Fokin SI, Skovorodkin IN (1997) Experimental analysis of the resistance of Paramecium caudatum (ciliophora) against infection by bacterium Holospora undulata. Eur J Protistol 33:214–218. 10.1016/S0932-4739(97)80039-9 [Google Scholar]
- 231.Lohse K, Gutierrez A, Kaltz O (2006) Experimental evolution of resistance in Paramecium caudatum against the bacterial parasite Holospora undulata. Evolution 60:1177–1186. 10.1554/05-656.1 [PubMed] [Google Scholar]
- 232.Kaltz O, Koella JC (2003) Host growth conditions regulate the plasticity of horizontal and vertical transmission in Holospora undulata, a bacterial parasite of the protozoan Paramecium caudatum. Evolution 57:1535–1542. 10.1111/j.0014-3820.2003.tb00361.x [DOI] [PubMed] [Google Scholar]
- 233.Restif O, Kaltz O (2006) Condition-dependent virulence in a horizontally and vertically transmitted bacterial parasite. Oikos 114:148–158. 10.1111/j.2006.0030-1299.14611.x [Google Scholar]
- 234.Magalon H, Nidelet T, Martin G, Kaltz O (2010) Host growth conditions influence experimental evolution of life history and virulence of a parasite with vertical and horizontal transmission. Evolution 64:2126–2138. 10.1111/j.1558-5646.2010.00974.x [DOI] [PubMed] [Google Scholar]
- 235.Dusi E, Gougat-Barbera C, Berendonk TU, Kaltz O (2015) Long-term selection experiment produces breakdown of horizontal transmissibility in parasite with mixed transmission mode. Evolution 69:1069–1076. 10.1111/evo.12638 [DOI] [PubMed] [Google Scholar]
- 236.Duncan AB, Fellous S, Kaltz O (2011) Reverse evolution: selection against costly resistance in disease-free microcosm populations of Paramecium caudatum. Evolution 65:3462–3474. 10.1111/j.1558-5646.2011.01388.x [DOI] [PubMed] [Google Scholar]
- 237.Nidelet T, Kaltz O (2007) Direct and correlated responses to selection in a host-parasite system: testing for the emergence of genotype specificity. Evolution 61:1803–1811. 10.1111/j.1558-5646.2007.00162.x [DOI] [PubMed] [Google Scholar]
- 238.Adiba S, Huet M, Kaltz O (2010) Experimental evolution of local parasite maladaptation. J Evol Biol 23:1195–1205. 10.1111/j.1420-9101.2010.01985.x [DOI] [PubMed] [Google Scholar]
- 239.Weiler J, Zilio G, Zeballos N et al (2020) Among-strain variation in resistance of Paramecium caudatum to the endonuclear parasite Holospora undulata: geographic and lineage-specific patterns. Front Microbiol 11. 10.3389/fmicb.2020.603046 [DOI] [PMC free article] [PubMed]
- 240.Fels D, Kaltz O (2006) Temperature-dependent transmission and latency of Holospora undulata, a micronucleus-specific parasite of the ciliate Paramecium caudatum. Proc Royal Soc B: Biol Sci 273:1031–1038. 10.1098/rspb.2005.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Duncan AB, Fellous S, Kaltz O (2011) Temporal variation in temperature determines disease spread and maintenance in Paramecium microcosm populations. Proc Royal Soc B: Biol Sci 278:3412–3420. 10.1098/rspb.2011.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Duncan AB, Gonzalez A, Kaltz O (2013) Stochastic environmental fluctuations drive epidemiology in experimental host–parasite metapopulations. Proc Royal Soc B: Biol Sci 280:20131747. 10.1098/rspb.2013.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bella C, Koehler L, Grosser K et al (2016) Fitness impact of obligate intranuclear bacterial symbionts depends on host growth phase. Front Microbiol 7. 10.3389/fmicb.2016.02084 [DOI] [PMC free article] [PubMed]
- 244.Nidelet T, Koella JC, Kaltz O (2009) Effects of shortened host life span on the evolution of parasite life history and virulence in a microbial host-parasite system. BMC Evol Biol 9:65. 10.1186/1471-2148-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Castelli M, Lanzoni O, Fokin SI et al (2015) Response of the bacterial symbiont Holospora caryophila to different growth conditions of its host. Eur J Protistol 51:98–108. 10.1016/j.ejop.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 246.Fellous S, Quillery E, Duncan AB, Kaltz O (2010) Parasitic infection reduces dispersal of ciliate host. Biol Let 7:327–329. 10.1098/rsbl.2010.0862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nørgaard LS, Zilio G, Saade C et al (2021) An evolutionary trade-off between parasite virulence and dispersal at experimental invasion fronts. Ecol Lett 24:739–750. 10.1111/ele.13692 [DOI] [PubMed] [Google Scholar]
- 248.Zilio G, Nørgaard LS, Petrucci G et al (2021) Parasitism and host dispersal plasticity in an aquatic model system. J Evol Biol 34:1316–1325. 10.1111/jeb.13893 [DOI] [PubMed] [Google Scholar]
- 249.Zilio G, Nørgaard LS, Gougat-Barbera C et al (2023) Travelling with a parasite: the evolution of resistance and dispersal syndromes during experimental range expansion. Proc Royal Soc B: Biol Sci 290:20221966. 10.1098/rspb.2022.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Duncan AB, Dusi E, Schrallhammer M et al (2018) Population-level dynamics in experimental mixed infections: evidence for competitive exclusion among bacterial parasites of Paramecium caudatum. Oikos 127:1380–1389. 10.1111/oik.05280 [Google Scholar]
- 251.Smurov AO, Fokin SI (1998) Resistance of Paramecium caudatum infected with endonuclear bacteria Holospora against salinity impact. Proc Zool Inst RAS 276:175–178 [Google Scholar]
- 252.Fujishima M, Kawai M, Yamamoto R (2005) Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol Lett 243:101–105. 10.1016/j.femsle.2004.11.053 [DOI] [PubMed] [Google Scholar]
- 253.Duncan AB, Fellous S, Accot R et al (2010) Parasite-mediated protection against osmotic stress for Paramecium caudatum infected by Holospora undulata is host genotype specific. FEMS Microbiol Ecol 74:353–360. 10.1111/j.1574-6941.2010.00952.x [DOI] [PubMed] [Google Scholar]
- 254.Dohra H, Fujishima M, Ishikawa H (1998) Structure and expression of a GroE-homologous operon of a macronucleus-specific symbiont Holospora obtusa of the ciliate Paramecium caudatum. J Eukaryot Microbiol 45:71–79. 10.1111/j.1550-7408.1998.tb05072.x [DOI] [PubMed] [Google Scholar]
- 255.Hori M, Fujishima M (2003) The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J Eukaryot Microbiol 50:293–298. 10.1111/j.1550-7408.2003.tb00137.x [DOI] [PubMed] [Google Scholar]
- 256.Hori M, Fujii K, Fujishima M (2008) Micronucleus-specific bacterium Holospora elegans irreversibly enhances stress gene expression of the host Paramecium caudatum. J Eukaryot Microbiol 55:515–521. 10.1111/j.1550-7408.2008.00352.x [DOI] [PubMed] [Google Scholar]
- 257.Schulz F, Horn M (2015) Intranuclear bacteria: inside the cellular control center of eukaryotes. Trends Cell Biol 25:339–346. 10.1016/j.tcb.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 258.Pond FR, Gibson I, Lalucat J, Quackenbush RL (1989) R-body-producing bacteria. Microbiol Rev 53:25–67. 10.1128/mr.53.1.25-67.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kusch J, Görtz H-D (2006) Towards an understanding of the killer trait: Caedibacter endocytobionts in Paramecium. In: Overmann J (ed) Molecular Basis of Symbiosis. Springer, Berlin, Heidelberg, pp 61–76 [DOI] [PubMed] [Google Scholar]
- 260.Preer JRJ, Jurand A (1968) The relation between virus-like particles and R bodies of Paramecium aurelia. Genetics Research 12:331–340. 10.1017/S0016672300011915 [DOI] [PubMed] [Google Scholar]
- 261.Jurand A, Preer JR, Rudman BM (1978) Further investigations on the prelethal effects of the killing action of kappa containing killer stocks of Paramecium aurelia. J Exp Zool 206:25–47. 10.1002/jez.1402060105 [Google Scholar]
- 262.Preer LB, Rudman BM, Preer JR, Jurand A (1974) Induction of R nodies by ultraviolet light in killer paramecia. Microbiology 80:209–215. 10.1099/00221287-80-1-209 [Google Scholar]
- 263.Dilts JA, Quackenbush RL (1986) A mutation in the R body-coding sequence destroys expression of the killer trait in P. tetraurelia. Science 232:641–643. 10.1126/science.3008334 [DOI] [PubMed] [Google Scholar]
- 264.Preer JR, Siegel RW, Stark PS (1953) The relationship between Kappa and Paramecin in Paramecium aurelia. Proc Natl Acad Sci U S A 39:1228–1233. 10.1073/pnas.39.12.1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mueller JA (1963) Separation of kappa particles with infective activity from those with killing activity and identification of the infective particles in Paramecium aurelia. Exp Cell Res 30:492–508. 10.1016/0014-4827(63)90326-4 [Google Scholar]
- 266.Schrallhammer M, Galati S, Altenbuchner J et al (2012) Tracing the role of R-bodies in the killer trait: absence of toxicity of R-body producing recombinant E. coli on paramecia. Eur J Protistol 48:290–296. 10.1016/j.ejop.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 267.Koehler L, Flemming FE, Schrallhammer M (2019) Towards an ecological understanding of the killer trait – a reproducible protocol for testing its impact on freshwater ciliates. Eur J Protistol 68:108–120. 10.1016/j.ejop.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 268.Grimes GW, Preer JRJ (1971) Further observations on the correlation between kappa and phage-like particles in Paramecium. Genetics Research 18:115–116. 10.1017/S0016672300012477 [Google Scholar]
- 269.Quackenbush RL, Burbach JA (1983) Cloning and expression of DNA sequences associated with the killer trait of Paramecium tetraurelia stock 47. Proc Natl Acad Sci 80:250–254. 10.1073/pnas.80.1.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kanabrocki JA, Lalucat J, Cox BJ, Quackenbush RL (1986) Comparative study of refractile (R) bodies and their genetic determinants: relationship of type 51 R bodies to R bodies produced by Pseudomonas taeniospiralis. J Bacteriol 168:1019–1022. 10.1128/jb.168.2.1019-1022.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Heruth DP, Pond FR, Dilts JA, Quackenbush RL (1994) Characterization of genetic determinants for R body synthesis and assembly in Caedibacter taeniospiralis 47 and 116. J Bacteriol 176:3559–3567. 10.1128/jb.176.12.3559-3567.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jeblick J, Kusch J (2005) Sequence, transcription activity, and evolutionary origin of the R-body coding plasmid pKAP298 from the intracellular parasitic bacterium Caedibacter taeniospiralis. J Mol Evol 60:164–173. 10.1007/s00239-004-0002-2 [DOI] [PubMed] [Google Scholar]
- 273.Quackenbush RL, Cox BJ, Kanabrocki JA (1986) Extrachromosomal elements of extrachromosomal elements of Paramecium and their extrachromosomal elements. In: Wickner RB, Hinnebusch A, Lambowitz AM et al (eds) Extrachromosomal elements in lower eukaryotes. Springer, US, Boston, MA, pp 265–278 [DOI] [PubMed] [Google Scholar]
- 274.Kusch J, Czubatinski L, Nachname S et al (2002) Competitive advantages of Caedibacter-infected paramecia. Protist 153:47–58. 10.1078/1434-4610-00082 [DOI] [PubMed] [Google Scholar]
- 275.Daugherty RM, Linka N, Audia JP et al (2004) The nucleotide transporter of Caedibacter caryophilus exhibits an extended substrate spectrum compared to the analogous ATP/ADP translocase of Rickettsia prowazekii. J Bacteriol 186:3262–3265. 10.1128/JB.186.10.3262-3265.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Shropshire JD, Leigh B, Bordenstein SR (2020) Symbiont-mediated cytoplasmic incompatibility: what have we learned in 50 years? eLife 9:e61989. 10.7554/eLife.61989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Raymann K, Bobay L-M, Doak TG et al (2013) A genomic survey of Reb homologs suggests widespread occurrence of R-bodies in proteobacteria. G3 (Bethesda) 3:505–516. 10.1534/g3.112.005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Akiba N, Aono T, Toyazaki H et al (2010) phrR-Like gene praR of Azorhizobium caulinodans ORS571 is essential for symbiosis with Sesbania rostrata and is involved in expression of reb genes. Appl Environ Microbiol 76:3475–3485. 10.1128/AEM.00238-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Matsuoka J, Ishizuna F, Kurumisawa K et al (2017) Stringent expression control of pathogenic R-body production in legume symbiont Azorhizobium caulinodans. mBio 8. 10.1128/mbio.00715-17. 10.1128/mbio.00715-17 [DOI] [PMC free article] [PubMed]
- 280.Hirakawa T, Matsuoka J-I, Morohashi K et al (2021) LysR-type transcriptional regulator of high temperature inducible class A β-lactamase gene controls pathogenic R-body production genes in Azorhizobium caulinodans. Soil Science and Plant Nutrition 67:57–70. 10.1080/00380768.2020.1840263 [Google Scholar]
- 281.Wang B, Lin Y-C, Vasquez-Rifo A et al (2021) Pseudomonas aeruginosa PA14 produces R-bodies, extendable protein polymers with roles in host colonization and virulence. Nat Commun 12:4613. 10.1038/s41467-021-24796-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ramírez B, Guevara M, Alfaro R et al (2021) A cross-sectional study of shrimp pathogens in wild shrimp, Penaeus vannamei and Penaeus stylirostris in Tumbes, Peru. Aquac Res 52:1118–1126. 10.1111/are.14969 [Google Scholar]
- 283.Lightner DV (1996) A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp. World Aquaculture Society Publishing
- 284.Frelier PF, Loy JK, Kruppenbach B (1993) Transmission of necrotizing hepatopancreatitis in Penaeus vannamei. J Invertebr Pathol 61:44–48. 10.1006/jipa.1993.1008 [Google Scholar]
- 285.Frelier PF, Sis RF, Bell TA, Lewis DH (1992) Microscopic and ultrastructural studies of necrotizing hepatopancreatitis in Pacific white shrimp (Penaeus vannamei) cultured in Texas. Vet Pathol 29:269–277. 10.1177/030098589202900401 [DOI] [PubMed] [Google Scholar]
- 286.Lightner DV, Redman RM, Bonami JR (1992) Morphological evidence for a single bacterial etiology in Texas necrotizing hepatopancreatitis in Penaeus vannamei (Crustacea: Decapoda). Dis Aquat Org 13:235 [Google Scholar]
- 287.Leyva JM, Martínez-Porchas M, Vargas-Albores F et al (2017) Análisis bioinformático del sistema flagelar de la alphaproteobacteria tipo rickettsia Candidatus Hepatobacter penaei. Rev Biol Mar Oceanogr 52:121–130. 10.4067/S0718-19572017000100010 [Google Scholar]
- 288.Briñez B, Aranguren F, Salazar M (2003) Fecal samples as DNA source for the diagnosis of necrotizing hepatopancreatitis (NHP) in Penaeus vannamei broodstock. Dis Aquat Organ 55:69–72. 10.3354/dao055069 [DOI] [PubMed] [Google Scholar]
- 289.Bradley-Dunlop DJ, Pantoja C, Lightner DV (2004) Development of monoclonal antibodies fordetection of necrotizing hepatopancreatitisin penaeid shrimp. Dis Aquat Org 60:233–240. 10.3354/dao060233 [DOI] [PubMed] [Google Scholar]
- 290.Aranguren LF, Dhar AK (2018) Detection and quantification of Hepatobacter penaei bacteria (NHPB) by new PCR and quantitative PCR assays. Dis Aquat Org 131:49–57. 10.3354/dao03285 [DOI] [PubMed] [Google Scholar]
- 291.Lightner DV, Redman RM, Pantoja CR et al (2012) Historic emergence, impact and current status of shrimp pathogens in the Americas. J Invertebr Pathol 110:174–183. 10.1016/j.jip.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 292.Morales-Covarrubias MS, Tlahuel-Vargas L, Martínez-Rodríguez I et al (2012) Necrotising hepatobacterium (NHPB) infection in Penaeus vannamei with florfenicol and oxytetracycline: a comparative experimental study. Revista Científica de la Facultad de Ciencias Veterinarias de la Universidad del Zulia 23:72–80 [Google Scholar]
- 293.Avila-Villa LA, Martínez-Porchas M, Gollas-Galván T et al (2011) Evaluation of different microalgae species and Artemia (Artemia franciscana) as possible vectors of necrotizing hepatopancreatitis bacteria. Aquaculture 318:273–276. 10.1016/j.aquaculture.2011.05.043 [Google Scholar]
- 294.Mendoza-Cano F, Sánchez-Paz A, Encinas-García T et al (2013) First record of necrotizing hepatopancreatitis bacterium (NHP-B) associated with the zooplankton samples from the Gulf of California. Mexico Mar Biodivers Rec 6:e85. 10.1017/S1755267213000535 [Google Scholar]
- 295.Morales-Covarrubias MS, García-Aguila N, Bolan-Mejía del MC, García OGZ (2020) Prevalence of the major diseases in Penaeus vannamei farmed of Sinaloa Mexico. Revista Científica de la Facultad de Ciencias Veterinarias 29:198–206. https://produccioncientificaluz.org/index.php/cientifica/article/view/32298
- 296.Betts-Hampikian HJ, Fields KA (2010) The chlamydial type III secretion mechanism: revealing cracks in a tough nut. Front Microbiol 1:114. 10.3389/fmicb.2010.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gillespie JJ, Kaur SJ, Rahman MS et al (2015) Secretome of obligate intracellular Rickettsia. FEMS Microbiol Rev 39:47–80. 10.1111/1574-6976.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Meir A, Macé K, Lukoyanova N et al (2020) Mechanism of effector capture and delivery by the type IV secretion system from Legionella pneumophila. Nat Commun 11:2864. 10.1038/s41467-020-16681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Schön ME, Martijn J, Vosseberg J et al (2022) The evolutionary origin of host association in the Rickettsiales. Nat Microbiol 7:1189–1199. 10.1038/s41564-022-01169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sassera D, Lo N, Epis S et al (2011) Phylogenomic evidence for the presence of a flagellum and cbb(3) oxidase in the free-living mitochondrial ancestor. Mol Biol Evol 28:3285–3296. 10.1093/molbev/msr159 [DOI] [PubMed] [Google Scholar]
- 301.Schulz F, Martijn J, Wascher F et al (2016) A Rickettsiales symbiont of amoebae with ancient features. Environ Microbiol 18:2326–2342. 10.1111/1462-2920.12881 [DOI] [PubMed] [Google Scholar]
- 302.Collingro A, Köstlbacher S, Mussmann M et al (2017) Unexpected genomic features in widespread intracellular bacteria: evidence for motility of marine Chlamydiae. ISME J 11:2334–2344. 10.1038/ismej.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Floriano AM, Biffignandi GB, Castelli M et al (2023) The evolution of intramitochondriality in Midichloria bacteria. Environ Microbiol 25:2102–2117. 10.1111/1462-2920.16446 [DOI] [PubMed] [Google Scholar]
- 304.Burki F, Sandin MM, Jamy M (2021) Diversity and ecology of protists revealed by metabarcoding. Curr Biol 31:R1267–R1280. 10.1016/j.cub.2021.07.066 [DOI] [PubMed] [Google Scholar]
- 305.Taylor MJ (2003) Wolbachia in the inflammatory pathogenesis of human filariasis. Ann N Y Acad Sci 990:444–449. 10.1111/j.1749-6632.2003.tb07409.x [DOI] [PubMed] [Google Scholar]
- 306.Schrallhammer M (2010) The killer trait of Paramecium and its causative agents. Palaeodiversity 3:79–88 [Google Scholar]
- 307.Borchsenius ON, Skoblo II, Ossipov DV (1983) Holospora curviuscula a new species of macronuclear symbiotic bacteria of Paramecium bursaria. Cytologia 25:91–97 [Google Scholar]
- 308.Ossipov DV, Skoblo II, Rautian MS, Podlipaev SA (1980) Holospora acuminata a new species of symbiotic bacterium from the micronucleus of the ciliate Paramecium bursaria Focke. Cytologia 22:922–929 [Google Scholar]
- 309.Dohra H, Suzuki H, Suzuki T et al (2013) Draft genome sequence of Holospora undulata strain HU1, a micronucleus-specific symbiont of the ciliate Paramecium caudatum. Genome Announcements 1: e00664-13 10.1128/genomea.00664-13. 10.1128/genomea.00664-13 [DOI] [PMC free article] [PubMed]
- 310.Shiohama Y, Takeshita K, Hirakata Y et al (2022) Complete genome sequence of “Candidatus Hydrogenosomobacter endosymbioticus”, an intracellular bacterial symbiont of the anaerobic ciliate Scuticociliate GW7. Microbiol Resour Announc 11:e0115021. 10.1128/mra.01150-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Suzuki H, Dapper AL, Jackson CE et al (2015) Draft genome sequence of Caedibacter varicaedens, a Kappa killer endosymbiont bacterium of the ciliate Paramecium biaurelia. Genome Announc 3:e01310-e1315. 10.1128/genomeA.01310-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.