Abstract
Astrocytes are important glia cell type in the central nervous system. These cells can undergo transformation to a reactive state upon injury such as focal ischemic stroke (FIS). Reactive astrocytes are distinct from normal or homeostatic astrocytes in morphology, protein profiles and metabolic functions. Glial cell-derived neurotrophic factor (GDNF) was discovered as a potent survival neurotrophic factor for multiple subtypes of neurons and can be released from reactive astrocytes. In our previous study, we found that GDNF expression was upregulated in reactive astrocytes following ischemic stroke. Specific knock out of GDNF in reactive astrocytes exacerbated brain damage and motor deficits after ischemic stroke. Here, using in vitro and in vivo ischemia models, we investigated the effects of GDNF overexpression in astrocytes on neuronal survival and brain recovery after ischemia. We observed that astrocyte specific GDNF overexpression by viral transduction could decrease brain infarction and promote motor function recovery after photothrombosis (PT)-induced FIS. In addition, GDNF overexpression in astrocytes could increase the proliferation of reactive astrocytes and reduce oxidative stress after PT. Using the oxygen-glucose deprivation (OGD) model of cultured astrocytes, we confirmed that this ischemic insult could upregulate GDNF expression and increase its release to extracellular space. Transfection of GDNF DNA plasmid could further increase GDNF release after OGD. To further study the effects of reactive astrocytes-derived extracellular GDNF on neuronal survival after ischemia, cultured neurons subjected to OGD were exposed to astrocyte conditioned medium (ACM). The ACM collected from OGD subjected astrocyte culture could significantly reduce neuronal death, while neutralizing antibodies against GDNF and its receptors including GFRα1, RET and p-RET could suppress this beneficial effect. We also found that reactive astrocytes-derived GDNF could trigger the activation of RET receptors in cultured neurons and suppress neuronal mitochondrial fission and caspase-dependent cell apoptosis after OGD. Overall, our results indicate that reactive astrocytes-derived GDNF could play an important role in neuronal survival and functional recovery and underscore the non-cell autonomy underlying astrocyte-neuron interactions in brain repair after ischemic stroke.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11064-025-04370-6.
Keywords: Reactive astrocytes, GDNF, Oxidative stress, Ischemic stroke
Introduction
Cerebral focal ischemic stroke (FIS) is a clinical condition characterized by neuronal death and motor dysfunction. Currently, the therapeutic strategy against acute FIS is to restore blood flow by thrombolysis or mechanical thrombectomy [1]. However, patients benefiting from these treatments remain limited and survivors commonly experience neurological impairments and psychiatric disorders [2]. Therefore, dedicated research efforts of novel therapeutic avenues are urgently needed to improve outcomes for patients. Following FIS, neurons are unable to sustain their normal activities due to deprivation of oxygen and nutrition, and this leads to complicated pathological processes, including excitotoxicity, oxidative stress, neuroinflammation and immune responses [3]. Among these, oxidative stress, which is elicited by the production of reactive oxygen species (ROS) that overpowers the endogenous antioxidant defense system, is a pivotal event contributing to neuronal apoptosis [4]. Under normal conditions, ROS is produced by the mitochondrial respiratory chain (MRC) as a by-product of ATP generation, mostly in the form of superoxide anions [5]. During ischemic stroke, the depletion of oxygen forces neurons to use glycolysis to produce energy. This metabolic alteration leads to lactate accumulation and acidosis [6]. Acidosis contributes to the elevation of oxidative stress through generating hydroxyl radical, a more reactive form of ROS [6]. During reperfusion, the reversal of complex I in MRC resulting in the overproduction of superoxide anions [7]. Moreover, study has demonstrated that nicotinamide adenine dinucleotide phosphate-oxidases (NOXs), a family of enzymes generating superoxide anions, are also activated during reperfusion [8]. These changes result in ROS accumulation and elevate oxidative stress. ROS can oxidize important cellular component including lipid, DNA and functional proteins, leading to lipid peroxidation, DNA fragmentation, protein denaturation and finally activates caspase dependent cell apoptosis. Therefore, counterbalancing excessive ROS through boosting antioxidative system holds great potential in stroke therapy.
Astrocytes are important glia cell type in the central nervous system (CNS). Under normal conditions, neurons closely interact with astrocytes to avoid oxidative injury [9, 10]. Upon FIS, affected astrocytes undergo a transformation into the reactive state which is characterized by upregulation of glial fibrillary acidic protein (GFAP) and increased proliferation [11, 12]. Reactive astrocytes has been implicated in various pathological conditions, exhibiting either beneficial or detrimental effects [13–15]. A large body of evidence has demonstrated that reactive astrocytes could alleviate neuronal apoptosis and offer brain protection following ischemic stroke [16–18]. However, the underlying mechanism through which reactive astrocytes promote neuronal survival is incompletely understood. Over the past decades, accumulative evidence revealed that reactive astrocytes could protect neurons and brain recovery through two major pathways: release of neurotrophic factors and provision of essential metabolites [19–21].
Glial cell line-derived neurotrophic factor (GDNF) was originally isolated from the supernatant of a rat glioma cell-line and found to be a potent survival neurotrophic factor for dopaminergic neurons [22]. Later, it was also found to have pronounced protective effects for other neuronal subpopulations, including noradrenergic and motor neurons [23]. Thus, GDNF is seen as a therapeutic agent to treat several neurological diseases. In CNS, GDNF is mainly released by microglia and astrocytes, and it can bind to GDNF-family receptor-α1 receptor (GFRα1) located in neuronal cell membrane [24, 25]. This binding triggers the activation of RET tyrosine kinase, driving alterations of neuronal activities [25–27]. Recently, there is strong evidence demonstrating that GDNF-GFRα1-RET signaling pathway contributes to neuronal development, differentiation and survival under both healthy and pathological conditions [28–30]. However, it is not clear whether this mechanism is involved in ischemic brain injury, in particular through astrocyte-neuron interactions.
In our previous study, we found that GDNF expression was upregulated in reactive astrocytes following a mouse model of FIS [15], and specific deletion of GDNF in astrocytes led to increased neuronal death and motor function deficits after photothrombosis (PT)-induced FIS [15]. Additionally, deletion of GDNF in astrocytes was shown to increase oxidative stress and reduced proliferation of reactive astrocytes in the peri-infarct region (PIR) and neurogenesis in the dentate gyrus (DG) [15]. These results strongly indicated that reactive astrocytes-derived GDNF could promote neuronal survival after ischemic stroke. To further study the mechanism of reactive astrocytes-derived GDNF on neuronal survival and brain recovery after ischemic stroke, we overexpressed GDNF in astrocytes and used in vitro and in vivo ischemia models combined with astrocyte conditioned medium (ACM). Our results underscore the non-cell autonomous mechanism in brain repair and suggest that promoting endogenous neurotrophic factor release from reactive astrocytes is a potential therapeutic option for ischemic stroke therapy.
Materials and Methods
Animals
Adult male and female mice with C57BL/6J background aged 8–10 weeks were used in this study. Mice were maintained on a 12 h light:12 h dark cycle (lights on 7 a.m.-7 p.m.) under pathogen-free conditions in the AAALAC-accredited animal facility at the University of Missouri according to institutional guidelines. All experimental procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Missouri Animal Care Quality Assurance Committee (ACQAC). Adult male and female mice were used in the current study.
Cell Transfection and Recombinant Adeno-Associated Virus (rAAV) Injection
DNA plasmids that have an astrocyte-specific gfaABC1D promoter encoding the genes for mRFP (Control) and GDNF, namely pZac2.1-gfaABC1D-mRFP and pZac2.1-gfaABC1D-GDNF plasmids (mRFP and GDNF plasmids for short) were constructed for transfection in cultured cells and for preparation of serotype 5 rAAV vectors, i.e., rAAV5-gfaABC1D-mRFP and rAAV5-gfaABC1D-GDNF (rAAV5-mRFP and rAAV5-GDNF) vectors. These viral vectors were stored in aliquots at -80 oC and thawed on ice before use.
To overexpress GDNF in astrocytes in vitro, primary astrocytes were transfected with the GDNF plasmid using Lipofectamine 2000 reagent (Cat. No.1168019, Thermo Fisher Scientific) [31, 32]. Briefly, before transfection, primary astrocytes were replenished in DMEM/F12 medium without FBS and antibiotic. DNA plasmid and Lipofectamine 2000 reagent were diluted separately in the same medium and incubated at room temperature for 5 min. The diluted DNA and lipofectamine 2000 reagent were then mixed gently and incubated at room temperature for 20 min; the mixtures were added drop by drop to the culture wells. After cells were cultured for 6 h at 37 °C and 5% CO2, the medium was changed back to the complete medium without antibiotic.
To overexpress GDNF in vivo, mice were injected with rAAV5-GDNF vectors [33]. Briefly, mice were anesthetized with ketamine/xylazine under sterile condition and secured in a custom-made stereotaxic frame. A skin incision was made and a small hole with 0.2 mm in diameter was drilled in the skull for injection. Coordinates for injection were (in mm: caudal to bregma, left of midline, ventral to pial surface): cortex (0.5, 3.5, 0.4) and hippocampus (2.1, 2.5, 1.5). Viral vectors (1 µl) were injected with a nanoliter injector (Drummond Scientific Company, PA) through the hole at a rate of 4 nl/s using a glass pipette with a tip of 10–15 μm-diameter. The skin was sutured after injection and mice were sent back to the animal facility after recovery.
In Vitro and in Vivo Ischemia Model
For OGD model [31, 32], astrocyte or neuronal cultures were washed three times with serum- and glucose-free balanced salt solution (BSS) (pH 7.4, mM): NaCl 116, CaCl2 1.8, MgSO4 0.8, KCl 5.4, NaH2PO4 1, NaHCO3 14.7, HEPES 10. Astrocytes or neurons cultured at 37 °C were incubated with BSS in an anaerobic chamber flushed with 99% N2 and 1% air for 6 h–1 h, respectively. After OGD, the BSS was replaced by cell culture medium, and cells were then allowed to recover for 24 h in a culture incubator with humidified atmosphere of 5% CO2 and 95% air. For glutamate excitotoxicity model [34, 35], primary neuronal cultures were exposed to 30 µM glutamate and 3 µM glycine for 24 h at 37 °C in a cultured incubator.
Photothrombosis (PT) was induced as described in our previous studies [36–39]. Briefly, mice were anesthetized by ketamine and xylazine (130 mg and 10 mg/kg body weight). Rose Bengal (30 mg/kg dissolved in saline) (Cat. No. 330000, Sigma) was injected through the tail vein. PT was induced 3 min after injection. An area of 1.5 mm diameter was focally illuminated for 2 min on the intact skull without skin at the center of − 0.8 mm from the bregma and 2.0 mm lateral to the midline (motor cortex) with a green light (540–580 nm) through a×10 objective. The light source was an X-cite 120 PC metal halide lamp (Excelitas Technologies, USA).
Transcardial Perfusion, Infarct Volume Measurements
Transcardial perfusion and infarct volume measurement were described in our previous studies [36–39]. In brief, mice were transcardially perfused with phosphate buffer saline (PBS), followed by ice-cold 4% paraformaldehyde (PFA) in phosphate buffer (pH 7.4). The brain was removed and post-fixed in 4% PFA in PBS at 4 oC overnight. It was then transferred to 30% sucrose for 2–3 days until it sank. Coronal sections of the brain (30 μm) were cut using a cryostat and collected serially on pre-gelatin coated glass slides and stored at -20 oC until use. For Nissl staining, brain sections were stained by 0.25% cresyl violet. The areas of cerebral infarction were delineated and quantified using ImageJ software.
Behavioral Tests
Motor behavioral tests including hanging wire, cylinder and grip force tests were conducted from day 1 to day 21 after PT to assess motor functional recovery as described in our previous studies [11, 38–40]. The pre-training was conducted twice before pre-recording of behavior tests. The pre-recording of behavior tests was conducted one day before PT.
Primary Cortical Astrocyte or Neuronal Cultures
Primary astrocytes were isolated from cortices of P1 C57BL/6J mice. The cortices were digested with 0.25% Trypsin-EDTA (Cat. No. 25300-054, Thermo Fisher Scientific) for 20 min at 37oC. After digestion, the dissociated cells were plated onto 75 cm2 flasks with Dulbecco’s modified Eagle medium (DMEM)/F12 (Cat. No. 11320033, Gibco) supplemented with 10% heated-inactivated fetal bovine serum (FBS) (Cat. No. F2422, Sigma) and 1% antibiotic (Cat. No. 15140122, Gibco). The mixed cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 and 95% air with change of medium every 3 days. Upon reaching confluency, the mixed cultures were mechanically agitated at a rate of 250 rpm for 12 h. The detached cells were discarded, and astrocytes were detached by using 0.25% Trypsin-EDTA and seeded onto cell culture plates or glass coverslips in cell culture plates. Astrocytes were cultured for 8–12 days for experiments with medium changed every 3 days.
Primary cortical neuronal cultures were prepared from embryonic day 15/16 (E15/16) C57BL/6J mice as previously described [31, 34, 35]. Briefly, the cortices of embryos were digested with 0.05% Trypsin-EDTA. The isolated cells were plated onto poly-L-lysine-coated tissue culture plates or glass coverslips in culture plates with Dulbecco’s modified Eagle medium (DMEM)/F12 supplemented with 10% FBS and 0.5% antibiotic overnight. The medium was then changed to Neurobasal medium (Cat. No. 21103049, Gibco) supplemented with 2% B27 serum free supplements (Cat. No. 17504044, Gibco), 0.5 mM L-glutamine (Cat. No. 25030081, Gibco) and 0.5% antibiotic. The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air with 50% medium changed every 3 days. Experiments were conducted between 10 and 12 day in vitro (DIV) as neurons were matured at this age and vulnerable to ischemic injury.
GDNF Assay
The concentration of GDNF in astrocyte medium was determined by GDNF ELISA kit (Cat. No. EK0935, Boster Bio) following the instructions provided by the manufacturer.
Treatment of Primary Neuronal Cultures With Astrocyte Conditioned Medium (ACM)
Primary astrocytes (cultured in 6-well plates) transfected and not transfected with pZac2.1-gfaABC1D-GDNF were subjected to 6 h OGD followed by a 24 h reperfusion. Medium was then harvested as ACM with OGD (ACM (OGD)). ACM (OGD) was used to treat neurons after 1 h OGD with 50% change of neuronal medium. The neurons were allowed to recover for 24 h in a regular cell culture incubator for subsequent assays.
Neuronal Death and Viability Assays
To evaluate neuronal death after ischemia, primary neurons were plated onto glass coverslips in 24 well plates. On the day of assay, cultured cells were rinsed with 1×PBS and incubated with propidium iodide (PI) for 30 min at 37 °C. Cells were then washed with 1×PBS and fixed by 4% PFA for 20 min at room temperature. Neurons were counter stained with Dapi and imaged using a Nikon FN1 epi-fluorescence microscopy equipped with a Cool SNAP-EZ CCD-camera.
To assess neuronal viability following ischemia, neurons were plated on 48 well plates. Thiazolyl blue tetrazolium bromide (MTT) was added to the culture wells and incubated at 37 °C for 4 h in a 5% CO2 atmosphere. The MTT-containing medium was then replaced with dimethyl sulfoxide (DMSO) to dissolve the formed blue formazan. The absorbance was then read at 540 nm.
In Vivo Detection of Reactive Oxygen Species (ROS) by Dihydroethidium (DHE)
To evaluate oxidative stress in vivo, DHE (25 mg/kg) was administered to mice through intraperitoneal (IP) injection two times within 30 min at 6 h after PT. Mice were then sacrificed at 18 h after the second injection. Brain sections were cut, counter stained with Dapi and imaged immediately using fluorescent microscope.
Western Blot Analysis
Western blotting (WB) was used to analyze protein expression in whole cell lysate as described in our previous studies [15, 32, 37, 38, 40, 41]. Briefly, the total protein was extracted from freshly harvested brain tissues or primary cultured cells using a lysis buffer (pH 8.2) plus protease inhibitor (Pierce Biotechnology, IL), and phosphatase inhibitor cocktails (Sigma, MO). The protein concentration of cell lysate was determined with a BCA protein assay kit (Cat. 23227, Thermo Fisher Scientific). Equivalent amounts of protein from each sample were diluted with Laemmli buffer, boiled for 5 min, subjected to electrophoresis in 10% SDS-polyacrylamide gels and subsequently transferred to PVDF membranes. Membranes were blocked for 1 h with 5% (w/v) BSA in Tris-buffered saline containing 0.1% Tween 20 (TBST) and were incubated overnight at 4oC in 1% (w/v) BSA with primary antibodies. The membranes were then incubated with rabbit HRP (Horseradish peroxidase)-conjugated anti-mouse IgG (1:1000; Millipore) or goat HRP-conjugated anti-rabbit IgG (1:1000; Millipore) diluted in 1% (w/v) BSA in TBS-T for 1 h at room temperature. The membranes were then exposed to Clarity Western ECL substrate (Bio-Rad, CA) and signals were visualized by Gel Documentation Imaging System. The primary antibodies include a mouse anti-GDNF monoclonal antibody (1:1000, Cat. No. SC-13147, Santa Cruz Biotechnology), a mouse anti-GFR α1 monoclonal antibody (1:1000, Cat. No. SC-271546, Santa Cruz Biotechnology), a rabbit anti-RET monoclonal antibody (1:500, Cat. No. 3223, Cell Signaling), a rabbit anti-P-RET polyclonal antibody (1:500, Cat. No. SAB4504530, Sigma), a rabbit anti-DRP 1 polyclonal antibody (1:1000, Cat. No. ABT155, Sigma), a rabbit anti-P-DRP 1 polyclonal antibody (1:500, Cat. No. 3455, Cell Signaling), a rabbit anti-Caspase 9 polyclonal antibody (1:1000, Cat. No. 9504 S, Cell Signaling), a rabbit anti-Cleaved caspase 3 polyclonal antibody (1:1000, Cat. No. 9661, Cell Signaling), a mouse anti-Synaptophysin monoclonal antibody (1:1000, Cat. No. S5768, Sigma), a mouse anti-vGluT1 monoclonal antibody (1:1000, Cat. No. NBP2-59329, Novus), a mouse anti-GluR1 monoclonal antibody (1:1000, Cat. No. NBP2-22399, Novus), a rabbit anti-GluR2 monoclonal antibody (1:1000, Cat. No. NBP2-75510, Novus), a rabbit anti-NMDAR2A polyclonal antibody (1:500, NB300-105, Novus), a rabbit anti-NMDAR2B polyclonal antibody (1:500, NB300-106, Novus), a mouse anti-β-actin monoclonal antibody (1:5000, Cat. No. SC-47778, Santa Cruz Biotechnology).
Immunochemical Analyses
The procedures of immunostaining were described in our previous studies [39–41]. PFA-fixed brain sections were blocked by 10% donkey serum-0.03% Triton-1×PBS for 1 h at room temperature and washed with 1×PBS for three times. Brain sections were subsequently incubated overnight at 4 °C with primary antibodies diluted in 1% donkey serum-0.03% tripon-1×PBS, and then incubated with an Alexa 488-conjugated donkey-anti-mouse IgG (1:300; Cat. No. R37114, Invitrogen), an Alexa 488-conjugated donkey-anti-rabbit IgG (1:300; Cat. No. A21206, Invitrogen) or an Alexa 568-conjugated donkey-anti-rabbit IgG (1:300; Cat. No. A10042, Invitrogen) for 4 h in the dark at room temperature. Fluorescent images were acquired using a Nikon FN1 epi-fluorescence microscopy equipped with a CoolSNAP-EZ CCD-camera or an Olympus Fluoview 1000 confocal microscope. The primary antibodies include a mouse anti-GFAP monoclonal antibody (1:300; Cat. No. MAB360, Sigma), a rabbit anti-GDNF polyclonal antibody (1:300, Cat. No. SC-328, Santa Cruz Biotechnology), a rabbit anti-Iba 1 polyclonal antibody (1:300, Cat. No. 019-19741, Fujifilm).
Statistical Analysis
Quantitative data were expressed as mean ± s.e.m. Statistical assessments were made by a student’s t-test for two groups or a one-way ANOVA (Bonferroni post hoc test) for multiple groups. p < 0.05 was considered statistically significant.
Results
Overexpression of GDNF in Astrocytes Decreased Brain Lesion and Promoted Motor Function Recovery After PT
To realize specific GDNF overexpression in astrocytes, we constructed a GDNF expressing DNA plasmid that contains an astrocyte-specific gfaABC1D promoter for transfection and rAAV preparation [33]. Our data demonstrated that overexpression of GDNF in astrocytes can be achieved in vitro and in vivo by DNA transfection and viral transduction (Online Resource 1).
To assess whether overexpression of GDNF in astrocytes could improve stroke outcomes in mouse model, we injected rAAV5-GDNF and rAAV5-mRFP vectors into the cortex and induced photothrombosis ischemic stroke 14 days later (Fig. 1a, b). Initially, we conducted immunostaining and confirmed GDNF or mRFP expressions in cortical reactive astrocytes in the PIR 7 days after PT (Fig. 1c, d). We also conducted WB analysis of synaptic markers in the PIR and found that GDNF overexpression in astrocytes did not affect the protein levels of synaptophysin, GluRs (GluR1 and GluR2) and NMDARs (NMDAR 2A and NMDAR 2B) but reduced vGluT1 expression compared with the control mice (Fig. 1e-l), suggesting alterations of glutamatergic neurotransmission after ischemic stroke by astrocytic GDNF overexpression. To determine whether astrocytic GDNF could offer brain protection after stroke, we evaluated brain lesion using Nissl staining. Mice injected with rAAV5-GDNF vectors to overexpress GDNF in astrocytes (group 1) showed reduced infarct volumes at 2 and 7 days after PT as compared to the control mice injected with rAAV5-mRFP vectors (Ctrl or group 2) (Fig. 2a-c). Next, we further evaluated the impact of GDNF in astrocytes on functional recovery through a battery of motor behavioral tests. From all tests including hanging wire, grip force, and cylinder tests, the group 2 mice exhibited more severe functional deficits than the group 1 mice (Fig. 2d-g). Overall, these results demonstrated that overexpression of GDNF in astrocytes can reduce brain lesion and facilitate motor function recovery following ischemic stroke.
Fig. 1.
The effects of GDNF overexpression in astrocytes on synaptic proteins following PT. (a) An illustration of the viral injection. IC: ischemic core; PIR: peri-infarct region. (b) Experimental timeline. (c, d) Fluorescent images showing the expressions of GDNF and mRFP in cortical astrocytes 7 days after PT. (e-l) WB images (e) and summary data (f-l) of protein expressions in cortical tissues 7 days after PT. In (c-l) rAAV5-GDNF and rAAV5-mRFP virus were injected 2 weeks before PT. N = 4 mice for each group. *p < 0.05, **p < 0.01, ***p < 0.001; (Student’s t-test)
Fig. 2.
Overexpression of GDNF in astrocytes reduced brain lesion and promoted motor function recovery after PT. (a) Representative images of Nissl staining showing the brain infarct areas of viral injected mice 2 days after PT. The white dash lines outline the damage area. (b, c) Quantification of infarct volumes at 2 (b) and 7 (c) days after PT. N = 4 mice for each group 2 days after PT and 7 mice for each group 7 days after PT. (d-g) Evaluation of motor behavior function by cylinder (d, e), hanging wire (f), and grip force (g) tests at different timepoints before and after PT. N = 8 and 7 mice injected with rAAV5-mRFP and rAAV5-GDNF vectors. *p < 0.05, **p < 0.01, ***<0.001; (Student’s t-test)
GDNF Overexpression in Astrocytes Promoted Astrocytic Proliferation and Reduced Oxidative Stress After PT
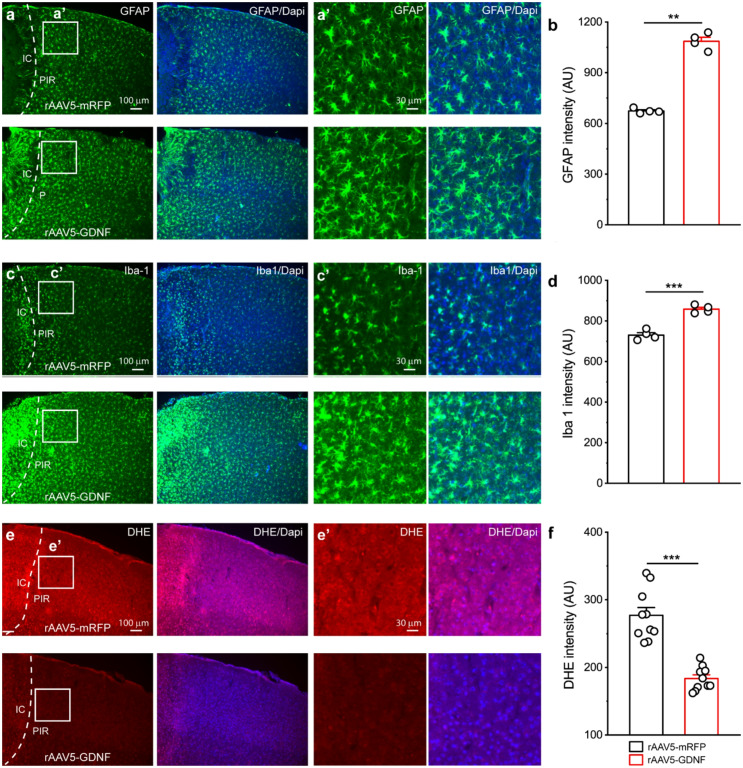
Reactive astrogliosis is a common feature following CNS injury [42–44]. It has been demonstrated that reactive astrocytes can promote neuronal survival following ischemic stroke [44–46]. Our previous study showed that deletion of GDNF could decrease astrocytic proliferation after ischemic stroke [15]. Therefore, we asked whether GDNF overexpression could promote astrocytic proliferation following ischemic stroke. Accordingly, we conducted GFAP staining in motor cortex 4 days after PT, a timepoint when proliferation of reactive astrocytes reaches its maximal rate. Our results showed that group 1 mice showed upregulation of GFAP in the PIR as compared to group 2 control mice, indicating promotion of astrocyte activation due to GDNF overexpression (Fig. 3a, b). Interestingly, group 1 mice also showed an increase in Iba expression, suggesting that microglia activation was also facilitated by astrocyte-derived GDNF (Fig. 3c, d).
Fig. 3.
GDNF overexpression promoted proliferation of reactive astrocytes and reduced oxidative stress in PIR following PI. (a-d) Representative fluorescent images and signal analysis of GFAP (a, b) and Iba 1 (c, d) expressions in the PIR from rAAV5-mRFP and rAAV5-GDNF viruses injected mice at day 4 post stroke. The right panels were the high-resolution images of the boxed region in the left panels. N = 4 mice for each group. (e-f) Fluorescent images of DHE and Dapi and analysis of DHE signal in the PIR of mRFP and GDNF viruses transduced mice at 4 days after stroke. The right panels were the high-resolution images of the boxed region in the left panels. Notice the decreases in DHE signal in GDNF overexpression mice. Data were averaged from 10 images for each group. *p < 0.05, **p < 0.01, ***p < 0.001; (Student’s t-test)
Neurons possess high efficiency of mitochondrial oxidative phosphorylation and produce low levels of reactive oxygen species (ROS) under normal conditions. However, following ischemic stroke, mitochondrial dysfunction could contribute to accumulation of ROS, thus elevating oxidative stress [47]. To investigate whether GDNF overexpression in astrocytes could lower oxidative stress in the PIR after ischemic stroke, DHE, a fluorescent probe for the detection of ROS was injected to label ROS. Results showed that DHE fluorescent intensity was significantly reduced in the PIR in group 1 mice at day 4 post stroke as compared to group 2 mice (Fig. 3e, f).
Reactive Astrocytes Promoted Neuronal Survival After OGD Through Release of GDNF
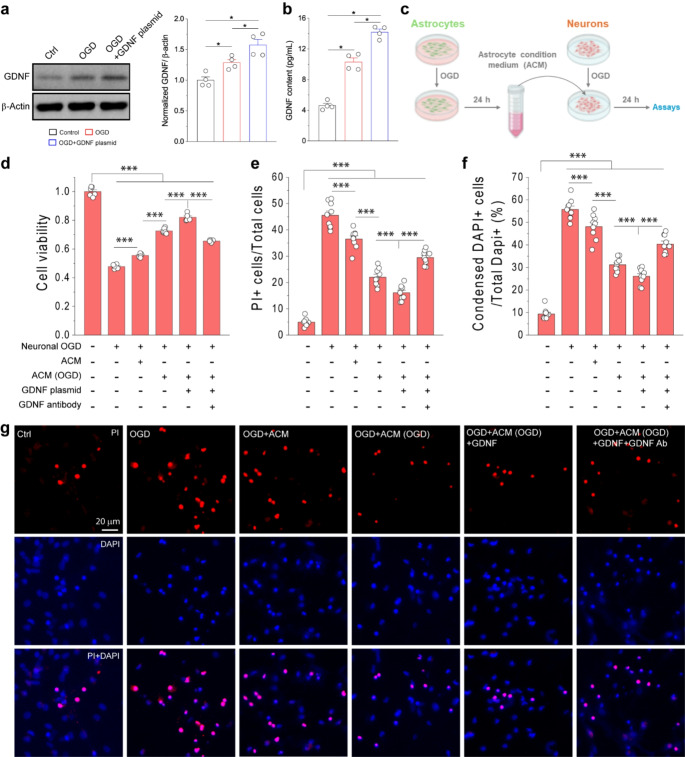
To test whether overexpression of GDNF in astrocytes could promote neuronal survival and reduce neuronal death after ischemia, we used the OGD model of primary astrocytic and neuronal cultures. Initially, Western blot analysis showed the protein levels of GDNF were increased following OGD (Fig. 4a), consistent with the observation in the mouse PT model. The GDNF levels were further increased by transfection with the GDNF plasmid (Fig. 4a). Importantly, GDNF levels in astrocyte medium were also elevated after OGD and further increased upon DNA plasmid transfection (Fig. 4b). Thus, this result showed that OGD could upregulate GDNF expression in reactive astrocytes and increase its release to extracellular space. Next, we collected ACM from astrocyte cultures with different conditions and used these ACMs to treat neuronal cultures during the 24 h-reoxygenation period after 1 h OGD (Fig. 4c). Neuronal viability was assessed by MTT assay (Fig. 4d). Our data showed that treatment of neurons with ACM from astrocyte culture subjected to 6 h OGD followed by 24 h reperfusion, i.e., ACM (OGD), significantly increased cell viability after OGD as compared to neurons without ACM (OGD) treatment. Notably, ACM from normal astrocyte culture without OGD also showed beneficial effects on neuronal viability albeit less effective compared to ACM (OGD). ACM collected from OGD-subjected astrocyte cultures transfected GDNF DNA plasmid, i.e., ACM (OGD + GDNF), further increased neuronal viability. To exclusively determine whether the protective effect of ACM (OGD + GDNF) is GDNF dependent, we treated OGD-subjected neurons with ACM (OGD) containing GDNF neutralizing agent during reoxygenation period. The addition of GDNF antibody in ACM (OGD) abrogated the beneficial impact of ACM (OGD) on neuronal viability.
Fig. 4.
Astrocyte-derived GDNF reduced neuronal death after OGD. (a, b) WB images and analysis of GDNF expression in primary cultured astrocytes (a) and GDNF content in the medium (b) at different conditions. N = 4 independent experiments. For OGD condition, primary cultured astrocytes were subjected to 6 h OGD and followed by 24 h reperfusion for western blot and ELISA analysis. (c) Schematic diagram showing experimental design. Primary astrocytes with or without transfection of GDNF plasmid were subjected to 6 h OGD. Astrocyte conditioned medium from astrocyte cultures not subjected or subjected to OGD, namely ACM and ACM(OGD) of different conditions were collected 24 h later and added to primary neuronal cultures after subjected to 1 h OGD followed by 24 h reoxygenation for neuronal viability and death assays. (d-f) Neuronal viability, percentages of PI + neurons and neurons with condensed nuclei after 1 h OGD and followed by 24 h reoxygenation at different conditions. The percentage of PI + neurons was calculated based on the total number of neurons based on DAPI staining. (g) Fluorescent images of neurons stained with PI and Dapi at different conditions. Data in (d) were averaged values from 6 replicates of representative experiment; data in (e, f) were averaged from cell counting of 9 images in each condition. *p < 0.05, **p < 0.01, ***p < 0.001; (one-way ANOVA test)
To further assess the effects of ACM (OGD) on neuronal death and apoptosis after OGD, we used propidium iodine (PI) and Dapi to stain primary neuronal cultures after OGD [31, 48] (Fig. 4e-g). Consistent with MTT assay, both ACM and ACM (OGD) reduced OGD-induced neuronal death and apoptosis. ACM (OGD + GDNF) further reduced OGD-induced neuronal death and apoptosis while inclusion of GDNF antibody the conditioned medium to neutralize GDNF attenuated the protective effect of ACM (OGD). In addition, the effects of ACM(OGD) and GDNF antibody on neuronal viability and death after OGD were also confirmed in a glutamate excitotoxicity model (Online Resource 1). Collectively, our results demonstrated that reactive astrocytes could promote neuronal survival and suppressed neuronal death after ischemic injury through released GDNF.
Reactive Astrocytes-Derived GDNF Triggered the Activation of Neuronal RET Receptors To Promote Neuronal Survival After OGD
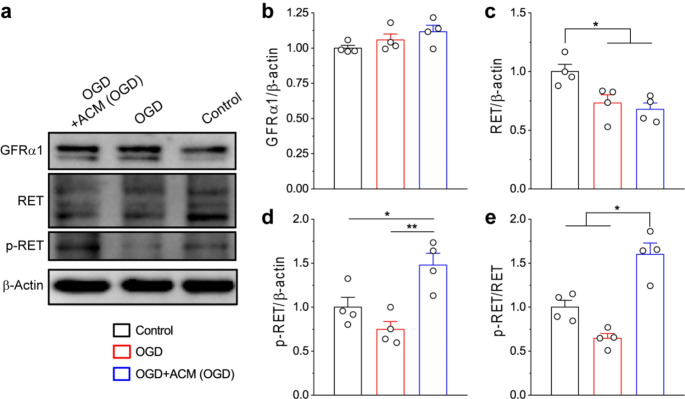
In the CNS, GDNF released by glia cells could mediate neuronal functions and survival mainly through the RET-dependent signaling pathways [11, 25–30]. Activation of RET receptor is known to undergo a two-step process. In the first step, GDNF binds to GFRα1 to form a complex; in the second step, the GDNF-GFRα1 complex triggers the phosphorylation of RET receptor, which initiates downstream intracellular signaling pathways. To investigate whether reactive astrocytes-released GDNF could lead to activation of RET receptors in neurons after ischemia, we carried out Western blot analysis of OGD neurons treated with and without ACM (OGD) (Fig. 5). Our results demonstrated that OGD did not affect the protein levels of GFRα1 and p-RET receptors in neurons, but reduced RET levels (Fig. 5a-d); however, the treatment by ACM(OGD) significantly increased the phosphorylation of RET receptor and elevated p-RET/RET ratio after OGD, suggesting that reactive astrocytes-derived GDNF could drive the activation of neuronal RET receptor (Fig. 5a, d, e).
Fig. 5.
Reactive astrocytes-derived GDNF triggered the activation of RET receptors in primary neurons after OGD. (a-e) WB images of protein expressions (a) and analysis (b-e) in primary cultured neurons after OGD. Summary data in (b-e) were averaged from 4 replicates. *p < 0.05, **p < 0.01; (one-way ANOVA test)
To determine whether reactive astrocytes-released GDNF could protect neuron against OGD through interacting with GFRα1 and RET receptors in neurons, we added GFRα1, RET and p-RET antibodies to neuronal cultures during reperfusion time after OGD and analyzed neuronal viability and death (Fig. 6). As demonstrated in Fig. 4, treatment of ACM (OGD) increased neuronal viability and reduced neuronal death following OGD (Fig. 6a-d). However, addition of GFRα1 antibody to block the formation of GDNF-GFRα1 complex significantly reduced neuronal viability and increased neuronal death after OGD. Similar results were also observed after addition of RET and p-RET antibodies which could block the phosphorylation of RET (p-RET) receptor and activation of downstream signaling pathways, respectively.
Fig. 6.
Astrocyte-derived GDNF protected neurons against OGD through the activation of neuronal GDNF receptors. (a-c) Neuronal viability and death based on PI staining and condensed nuclei after 1 h OGD and followed by 24 h reoxygenation at different conditions. (d) Representative fluorescent images of neurons stained with PI and Dapi at different conditions. Data in (a) were averaged values for 6 replicates of representative experiment; data in (b, c) were averaged from cell counting in 9 images in each condition. *p < 0.05, **p < 0.01, ***p < 0.001; (one-way ANOVA test)
Taken together, these results demonstrated that reactive astrocytes-derived GDNF could reduce neuronal death after OGD by activating GFRα1-RET signaling pathway in neurons, highlighting the non-cell autonomous effect of reactive astrocytes-derived GDNF on neuronal survival after OGD.
Reactive Astrocytes-Derived GDNF Could Inhibit Neuronal Mitochondrial Fission and Apoptosis After OGD
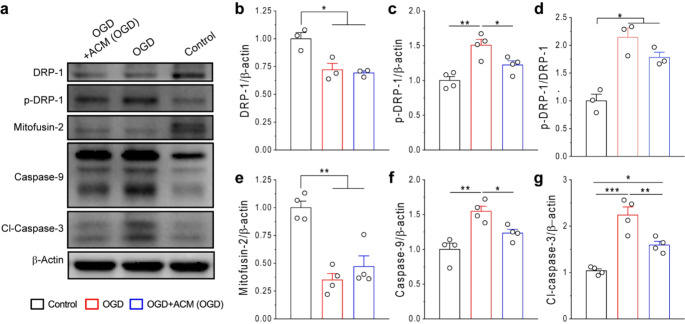
Mitochondrial dysfunction is an important factor responsible for neuronal death following ischemic injury. A previous study showed that the GDNF/RET signaling pathway could prevent degeneration of dopaminergic neurons through restoring mitochondria functions [49]. In this study, we assessed the effect of reactive astrocytes-derived GDNF on neuronal mitochondrial quality after OGD by examining the expressions of DRP1, p-DRP1 and mitofusin 2, three proteins known to regulate mitochondrial fusion and fission (Fig. 7a-e). Our results showed that expressions of DRP 1 and mitofusin 2 were downregulated while the protein levels of p-DRP-1 was increased in primary cortical neurons following OGD. These results suggest that mitochondria fission is promoted. Interestingly, ACM (OGD) treatment reduced the protein levels of p-DRP1, with no significant effect on DRP1 and mitofusin 2. These data suggest that reactive astrocytes-derived GDNF alleviating mitochondrial fission.
Fig. 7.
Reactive astrocyte-derived GDNF inhibited mitochondrial fission and apoptosis of primary neurons after OGD. (a-g) WB images (a) and analysis (b-g) of protein expressions in primary neurons after OGD. Data were from 3–4 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; (one-way ANOVA test)
Lastly, we investigated the effects of GDNF/RET signaling on neuronal apoptosis by examining the protein levels of caspase-9 and cleaved caspase 3, two factors mediating apoptotic cell death. As expected, OGD upregulated caspase-9 and cleaved caspase 3 levels in primary neuronal cultures, whereas ACM (OGD) treatment significantly suppressed their upregulations after OGD (Fig. 7a, f, g). These results are consistent with neuronal death assay by PI and Dapi stainings (Figs. 4 and 6). Taking together, our data suggest that GDNF released from reactive astrocytes drives the activation of GFRα-RET-dependent signaling pathways, thereby suppressing mitochondrial fission and cell apoptosis in primary neurons after OGD.
Discussion
In the current study, we explored whether and how reactive astrocytes-derived GDNF could promote neuronal survival and brain recovery after ischemic stroke using both in vitro and in vivo models. In our in vivo study, we observed that astrocyte specific GDNF overexpression by rAAV transduction could significantly decrease brain infarction and promote motor function recovery after PT. Meanwhile, we also observed that GDNF overexpression facilitated astrocytic proliferation and reduced oxidative stress in the PIR. In in vitro study, we found that treatment of OGD subjected neurons with ACM(OGD) containing increased GDNF released from reactive astrocytes could promote neuron survival and reduce neuronal death, trigger activation of the neuronal GFRα1-RET signaling pathway, and inhibit neuronal mitochondrial fission and apoptosis (Fig. 8).
Fig. 8.
Schematic diagram illustrating the role of astrocytic GDNF in neuronal survival and brain recovery after ischemic stroke. Astrocytes are activated following ischemic stroke and become reactive astrocytes. Reactive astrocytes release GDNF into extracellular space and binds to GFRα1 protein anchor on neuronal cell membrane. The GDNF-GFRα1 complexes trigger the phosphorylation of RET receptors in neurons and initiate intracellular signaling pathways, which suppress mitochondrial fission and reduce oxidative stress. These changes inhibit caspase regulated cell apoptosis and promote neuronal survival and brain recovery after ischemic stroke
Reactive astrogliosis is known to accompany many central nervous system (CNS) pathological conditions such as ischemia, neuroinflammation and neurodegeneration [50]. During this process, affected astrocytes undergo transformation to a reactive state and become reactive astrocytes. Reactive astrocytes typically exhibit upregulation of GFAP expression, an intermediate filament protein vital for astroglia cyto-architecture and functions [12]. Studies using GFAP null mice suggest the important role of GFAP to retain the function of reactive astrocytes and reduce brain damage following ischemia [51, 52]. Reactive astrocytes could release neurotrophic factors to modulate neuronal activities; however, the interaction between GFAP and neurotrophins release is not well established. An investigation revealed a null mutation of GFAP in mice conferred protection for striatal neurons from metabolic and excitotoxic insults through promoting GDNF release from astrocytes, indicating GFAP could regulate GDNF production [53]. Here we observed that GDNF overexpression in cortical astrocytes could upregulate GFAP expression. This evidence, combined with our previous study showing that astrocyte specific knockout of GDNF could inhibit GFAP expression and cell proliferation, demonstrating that GDNF could also mediate GFAP expression and astroglia functions [15].
In the CNS, GDNF can modulate neuronal activities and functions mainly through the GFRα1-RET dependent signaling pathway [25–30]. Under this event, GDNF can bind to RET receptors and subsequently trigger the phosphorylation of RET at four different tyrosine residues. Evidence further showed that phosphorylation of these residues could stimulate a similar profile of downstream signaling pathways responsible for cell survival, proliferation, differentiation, and neurite growth [54]. RET receptor is expressed in multiple neuronal populations including sympathetic, sensory, motor and dopaminergic neurons [55]. It is also detected in different brain regions such as cortex, hippocampus and striatum [56]. In the present study, we tested whether reactive astrocytes-derived GDNF could promote neuronal survival and rescue neuronal death after ischemia through activation of RET receptors in neurons. We employed ACM from control and OGD-subjected astrocyte cultures to treat OGD cortical neurons during reperfusion period. Assays of neuronal viability and neuronal death demonstrated that supplementation of ACM (OGD) could reduce neuronal death following ischemia, and addition of GDNF antibody in ACM (OGD) blocked this beneficial effect. These results are in agreement with the notion of neuronal protective role of astrocytes-derived GDNF on ischemic injury. To exclusively determine whether GDNF acts with neuronal GDNF receptor pathway, GFRα1 antibody, RET antibody or p-RET antibody were added to neuronal cultures after OGD, and results showed that these treatments significantly attenuated the protective effect of ACM (OGD). Although RET expression was not significantly altered, the protein levels of p-RET in cortical neurons was dramatically elevated after OGD when treated with ACM(OGD). Altogether, these results demonstrated that reactive astrocytes-released GDNF could prevent ischemia-induced neuronal loss through the RET dependent pathway.
Moreover, the decreased expression of p-DRP-1 (Ser616) after ACM (OGD) treatment suggested suppressing neuronal mitochondrial fission after OGD. Following ischemia, mitochondrial fission mediated by p-DRP-1 (Ser 616) can be detected in both primary cultured neurons and brain cortical tissues [57, 58]. Mitochondrial fission leads to fragmentated mitochondria, thus reduces efficiency of oxidative phosphorylation and contributes to neuronal death after ischemic injury [59–61]. Inhibition of mitochondrial fission can attenuate neuronal death after ischemic stroke [61]. Our data indicate that reactive astrocytes-derived GDNF suppressed mitochondrial fission in neurons via the RET dependent signaling pathway. We also found that ACM (OGD) treatment reduced expressions of cleaved caspase-3 and caspase 9 in neurons subjected to OGD, suggesting inhibition of the cell apoptotic pathway. Collectively, our results confirm a novel mechanism for reactive astrocytes-derived GDNF to inhibit mitochondrial fission and suppress cell apoptosis after ischemia.
To investigate whether reactive astrocytes-derived GDNF could alleviate neurodegeneration after stroke in vivo, we conducted WB analysis of cortical tissues from both GDNF and mRFP viruses injected mice. Our data displayed that GDNF overexpression in reactive astrocytes did not affect the expression levels of synaptophysin, GluRs and NMDARs, but reduced vGluT1 protein levels in the PIR after stroke. In the CNS, vGluT1 is specifically expressed in glutamatergic neurons and mediates the loading of presynaptic vesicles with glutamate, the first step of glutamatergic neurotransmission [62–64]. It has been reported that ischemia upregulated vGluT1 expression in the brain and leading to excitotoxicity, which contributes to the elevation of oxidative stress and neuronal death [64, 65]. Recently, several studies indicated vGluT1 as a therapeutic target for ischemic stroke [64, 66, 67]. For instance, reduced expression of vGluT1 was shown to attenuate brain infarction and neurological deficits after ischemic stroke [67] and inhibition of vGluT1 function offered neuroprotection in ischemic brain [64]. Our results suggest that GDNF overexpression in astrocytes may modulate glutamatergic neurotransmission and suppress excitotoxicity through decreasing vGluT1-mediated glutamate reloading, thereby exerting a brain protection effect after ischemic stroke. However, efforts are needed to validate the signaling pathways by which GDNF regulates vGluT1 expression.
Oxidative stress, induced by the accumulation of reactive oxygen species (ROS), is the primary factor that leads to brain damage after ischemic stroke. During ischemic stroke, the depletions of glucose and oxygen disrupt mitochondria oxidative phosphorylation and alter the cellular metabolic profiles, thereby promoting ROS productions and elevating oxidative stress [8, 67]. In addition, the excessive release of glutamate results in intracellular accumulation of Ca2+, which triggers the activation of signaling pathways responsible for overproduction of free radicals and aggravates oxidative stress induced injury [68, 69]. During reperfusion, the resupplies of glucose and oxygen reactivates mitochondrial respiratory chain, which further facilitating ROS accumulation. Unfortunately, the disruption of glucose metabolism reduces the cellular capacity to synthesize antioxidants rapidly, which exacerbates the escalation of oxidative stress. Among all cell types, neurons are especially vulnerable to oxidative stress due to the high rate of oxidative phosphorylation and limited ability to synthesize ROS scavengers, thus in CNS, neurons are highly dependent on astrocytes to defend against oxidative stress. According to previous investigations, astrocytes can efficiently supply neurons with glutathione (GSH), the primary antioxidant [9, 70, 71]. This metabolic interaction restores neuronal functions under a range of pathological situations [72–74]. However, this point may not be comprehensive, as astrocytes could protect neurons from ROS triggered injury via other routes [75–77]. For example, astrocyte secreted GDNF was observed to protect neurons against 6-OHDA cytotoxicity via upregulation of GSH synthesis [75]. Overexpression of GDNF in hippocampal astrocytes in 3×Tg-AD mice could preserve learning and memory deficits due to the abolishment of oxidative stress by GDNF [76]. In our previous study, we found that specific knockout of GDNF in astrocytes could elevate oxidative stress and increase neuronal death in PIR following stroke [15]. Consistent with these findings, we demonstrated that overexpression of GDNF in astrocytes could downregulate ROS production in PIR following ischemic stroke, corroborating our in vitro results that ACM (OGD) alleviated mitochondria fission in neurons, which contribute to the downregulation of oxidative stress.
In summary, our study demonstrated the beneficial effects of GDNF derived from reactive astrocytes to offer neuronal survival and functional recovery after ischemic stroke. Reactive astrocytes-derived GDNF could drive the activation of GFRα1-RET signaling pathways in neurons, which could inhibit mitochondrial fission and lower oxidative stress. Our study thus highlights the non-cell autonomous effect of reactive astrocytes-derived GDNF on neuronal survival through astrocyte-neuron interactions and suggests that astrocytic GDNF may have implications in stroke therapy.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author Contributions
Zhe Zhang: Writing-original draft, Writing-review & editing, Investigation, Methodology, Data curation, Formal analysis. Nannan Zhang: Investigation, Methodology, Data curation, Formal analysis. Shinghua Ding: Writing-review & editing, Supervision, Funding acquisition, Conceptualization, Project administration.
Funding
This work was supported by the National Institute of Health [R01NS069726 (NINDS), R01NS123023 (NINDS)] and American Heart Association (AHA) [16GRNT31280014] to SD.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saver JL, Goyal M, Van der Lugt AA, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, Cardona P (2016) Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 316:1279–1289 [DOI] [PubMed] [Google Scholar]
- 2.Lan XY, Liang XS, Cao MX, Qin HM, Chu CY, Boltze J, Li S (2024) NCAM mimetic peptide P2 synergizes with bone marrow mesenchymal stem cells in promoting functional recovery after stroke. J Cereb Blood Flow Metab 44:1128–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38:1167–1186 [DOI] [PubMed] [Google Scholar]
- 4.Beckman KB, Ames BN (1998) Mitochondrial aging: open questions a. Ann N Y Acad Sci 1:118–127 [DOI] [PubMed] [Google Scholar]
- 5.Rice ME (2011) H2O2: a dynamic neuromodulator. Neuroscientist 17:389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying W, Han SK, Miller JW, Swanson RA (1999) Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem 73:1549–1556 [DOI] [PubMed] [Google Scholar]
- 7.Shirley R, Ord EN, Work LM (2014) Oxidative stress and the use of antioxidants in stroke. Antioxidants 3:472–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, SongY S, Chan PH (2009) Inhibition of NADPH oxidase is neuroprotective after ischemia—reperfusion. J Cereb Blood Flow Metab 29:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic Cooperation. Cell Metab 14:724–738 [DOI] [PubMed] [Google Scholar]
- 10.Bonvento G, Bolaños JP (2021) Astrocyte-neuron metabolic Cooperation shapes brain activity. Cell Metab 33:1546–1564 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Sun GY, Ding S (2021) Glial cell line-derived neurotrophic factor and focal ischemic stroke. Neurochem Res 46:2638–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhury GR, Ding S (2016) Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis 85:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967 [DOI] [PubMed] [Google Scholar]
- 14.Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H (2013) Reactive astrocytes secrete lcn2 to promote neuron death. PNAS 110:4069–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang N, Zhang Z, He R, Li H, Ding S (2020) LAST-CreERT2 mediated deletion of GDNF increases brain damage and exacerbates long‐term stroke outcomes after focal ischemic stroke in mouse model. Glia 68:2395–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becerra-Calixto A, Cardona-Gómez GP (2017) The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci 10:88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson MR, Fuertes CJ, Dunn AK, Drew MR, Jones TA (2021) Reactive astrocytes facilitate vascular repair and remodeling after stroke. Cell Rep 35:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Y, Duan R, Ding W, Gu Q, Liu M, Zhou J, Sun J, Zhu J (2022) Astrocyte-derived Exosomal nicotinamide phosphoribosyltransferase (Nampt) ameliorates ischemic stroke injury by targeting AMPK/mTOR signaling to induce autophagy. Cell Death Dis 13:1057–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Yuan Z, Yang S, Zhu Y, Xue M, Zhang J, Leng L (2023) Brain energy metabolism: astrocytes in neurodegenerative diseases. CNS Neurosci Ther 29:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Duan X, Li S, Zhang R, Dai X, Lu M (2024) Unveiling the role of astrocytes in postoperative cognitive dysfunction. Ageing Res Rev 95:102223 [DOI] [PubMed] [Google Scholar]
- 21.Alhadidi QM, Bahader GA, Arvola O, Kitchen P, Shah ZA, Salman MM (2023) Astrocytes in functional recovery following central nervous system injuries. J Physiol 602:1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Ouaamari Y, Van den Bos J, Willekens B, Cools N, Wens I (2023) Neurotrophic factors as regenerative therapy for neurodegenerative diseases: current status, challenges and future perspectives. Int J Mol Sci 24:3866–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed RA, Hayat M, Qaiser H, Uzair M, Al-Regaiey K, Khallaf R, Kaleem I, Bashir S (2024) Aging-Related protein alterations in the brain. J Alzheimer’s Dis 99:1–18 [DOI] [PubMed] [Google Scholar]
- 24.Singh G, Sikder A, Phatale V, Srivastava S, Singh SB, Khatri DK (2023) Therapeutic potential of GDNF in neuroinflammation: targeted delivery approaches for precision treatment in neurological diseases. J Drug Deliv 87:104876 [Google Scholar]
- 25.Houghton FM, Adams SE, Ríos AS, Masino L, Purkiss AG, Briggs DC, Ledda F, McDonald NQ (2023) Architecture and regulation of a GDNF-GFRα1 synaptic adhesion assembly. Nat Commun 14:7551–7565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte Azevedo M, Sander S, Tenenbaum L (2020) GDNF, a neuron-derived factor upregulated in glial cells during disease. J Clin Med 9:456–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394 [DOI] [PubMed] [Google Scholar]
- 28.Drinkut A, Tillack K, Meka DP, Schulz JB, Kügler S, Kramer ER (2016) RET is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis 7:e2359–e2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong Z, Zhang QY, Liu J, Wang ZQ, Zhang Y, Xiao Q, Lu J, Zhou HY, Chen SD (2009) Phosphoproteome study reveals Hsp27 as a novel signaling molecule involved in GDNF-induced neurite outgrowth. J Proteome Res 8:2768–2787 [DOI] [PubMed] [Google Scholar]
- 30.Bespalov MM, Saarma M (2007) GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci 28:68–74 [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Li H, Ding S (2016) Pre-B-cell colony-enhancing factor protects against apoptotic neuronal death and mitochondrial damage in ischemia. Sci Rep 6:324160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Zhang Z, Zhang N, Li H, Zhang L, Baines CP, Ding S (2019) Subcellular NAMPT-mediated NAD+ salvage pathways and their roles in bioenergetics and neuronal protection after ischemic injury. J Neurochem 151:732–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Wang T, Sun GY, Ding S (2010) Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience 170:992–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Li H, Ding S (2014) The effects of NAD+ on apoptotic neuronal death and mitochondrial biogenesis and function after glutamate excitotoxicity. Int J Mol Sci 15:20449–20468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi J, Li H, Ye SQ, Ding S (2012) Pre-B‐cell colony‐enhancing factor exerts a neuronal protection through its enzymatic activity and the reduction of mitochondrial dysfunction in in vitro ischemic models. J Neurochem 120:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding S, Wang T, Cui W (2009) Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia 57:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S (2010) Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab 20:1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Xie Y, Zhang N, Yu Y, Zhang Q, Ding S (2015) Disruption of IP3R2-mediated Ca2+ signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke. Cell Calcium 58:565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Zhang N, Lin HY, Yu Y, Cai QY, Ma L, Ding S (2014) Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neurosci 15:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, Swerdlow RH, Wang X, Ding S (2017) Deletion of Nampt in projection neurons of adult mice leads to motor dysfunction, neurodegeneration, and death. Cell Rep 9:2184–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Zhang N, Sun G, Ding S (2013) Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN Neuro 5:195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pekny M, Pekna M (2016) Reactive gliosis in the pathogenesis of CNS diseases. BBA-Mol Basis Dis 1862:483–491 [DOI] [PubMed] [Google Scholar]
- 43.Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248 [DOI] [PMC free article] [PubMed]
- 44.Sims NR, Yew WP (2017) Reactive astrogliosis in stroke: contributions of astrocytes to recovery of neurological function. Neurochem Int 107:88–103 [DOI] [PubMed] [Google Scholar]
- 45.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K, Larsson K, Yabe T (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481 [DOI] [PubMed] [Google Scholar]
- 46.Bhatti MS, Frostig RD (2023) Astrocyte-neuron lactate shuttle plays a pivotal role in sensory-based neuroprotection in a rat model of permanent middle cerebral artery occlusion. Sci Rep 13:12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orellana-Urzúa S, Rojas I, Líbano L, Rodrigo R (2020) Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des 26:4246–4260 [DOI] [PubMed] [Google Scholar]
- 48.Crowley LC, Scott AP, Marfell BJ, Boughaba JA, Chojnowski G, Waterhouse NJ (2016) Measuring cell death by Propidium iodide uptake and flow cytometry. Cold Spring Harb Protoc 7:647–651 [DOI] [PubMed] [Google Scholar]
- 49.Meka DP, Müller-Rischart AK, Nidadavolu P, Mohammadi B, Motori E, Ponna SK, Aboutalebi H, Bassal M, Annamneedi A, Finckh B, Miesbauer M (2015) Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. J Clin Invest 125:1873–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94:1077–1098 [DOI] [PubMed] [Google Scholar]
- 51.Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM (2000) High susceptibility to cerebral ischemia in GFAP-null mice. J Cereb Blood Flow Metab 20:1040–1044 [DOI] [PubMed] [Google Scholar]
- 52.Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML (2004) Loss of glial fibrillary acidic protein results in decreased glutamate transport and Inhibition of PKA-induced EAAT2 cell surface trafficking. Mol Brain Res 124:114–123 [DOI] [PubMed] [Google Scholar]
- 53.Hanbury R, Ling ZD, Wuu J, Kordower JH (2003) GFAP knockout mice have increased levels of GDNF that protect striatal neurons from metabolic and excitotoxic insults. J Comp Neurol 461:307–316 [DOI] [PubMed] [Google Scholar]
- 54.Coulpier M, Anders J, Ibáñez CF (2002) Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem 277:1991–1999 [DOI] [PubMed] [Google Scholar]
- 55.Runeberg-Roos P, Saarma M (2007) Neurotrophic factor receptor RET: structure, cell biology, and inherited diseases. Ann Med 39:572–580 [DOI] [PubMed] [Google Scholar]
- 56.Tohda C, Joyashiki E (2009) Sominone enhances neurite outgrowth and Spatial memory mediated by the neurotrophic factor receptor. RET Br J Pharmacol 157:1427–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flippo KH, Gnanasekaran A, Perkins GA, Ajmal A, Merrill RA, Dickey AS, Taylor SS, McKnight GS, Chauhan AK, Usachev YM, Strack S (2018) AKAP1 protects from cerebral ischemic stroke by inhibiting Drp1-dependent mitochondrial fission. J Neurosci 38:8233–8242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SD, Lin TK, Yang DI, Lee SY, Shaw FZ, Liou CW, Chuang YC (2015) Roles of PTEN-induced putative kinase 1 and dynamin-related protein 1 in transient global ischemia-induced hippocampal neuronal injury. Biochem Biophys Res Commun 460:397–403 [DOI] [PubMed] [Google Scholar]
- 59.Jia J, Jin H, Nan D, Yu W, Huang Y (2021) New insights into targeting mitochondria in ischemic injury Apoptosis 26: 163–183 [DOI] [PubMed]
- 60.Zhou X, Chen H, Wang L, Lenahan C, Lian L, Ou Y, He Y (2021) Mitochondrial dynamics: a potential therapeutic target for ischemic stroke. Front Aging Neurosci 13:721428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Wang HY, Wu B, Cheng CY, Xiao W, Wang ZZ, Yang YY, Li P, Yang H (2017) Ginkgolide K attenuates neuronal injury after ischemic stroke by inhibiting mitochondrial fission and GSK-3β-dependent increases in mitochondrial membrane permeability. Oncotarget 8:44682–44693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martineau M, Guzman RE, Fahlke C, Klingauf J (2017) VGLUT1 functions as a glutamate/proton exchanger with chloride channel activity in hippocampal glutamatergic synapses. Nat Commun 8:2279–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniels RW, Miller BR, DiAntonio A (2011) Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis 41:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pomierny B, Krzyżanowska W, Skórkowska A, Jurczyk J, Bystrowska B, Budziszewska B, Pera J (2023) Inhibition of vesicular glutamate transporters (VGLUTs) with Chicago Sky blue 6B before focal cerebral ischemia offers neuroprotection. Mol Neurobiol 6:3130–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu Y, Zhan Q, Zhang H, Liu X, Huang L, Li H, Yuan Q (2017) Increased susceptibility to ischemic brain injury in neuroplastin 65-deficient mice likely via glutamate excitotoxicity. Front Cell Neurosci 11:110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haque MN, Hannan MA, Dash R, Choi SM, Moon IS (2021) The potential LXRβ agonist stigmasterol protects against hypoxia/reoxygenation injury by modulating mitophagy in primary hippocampal neurons. PYTOEY 81:153415 [DOI] [PubMed] [Google Scholar]
- 67.Skórkowska A, Krzyżanowska W, Bystrowska B, Torregrossa R, Whiteman M, Pomierny B, Budziszewska B (2024) The hydrogen sulfide donor AP39 reduces glutamate-mediated excitotoxicity in a rat model of brain ischemia. Neuroscience 539:86–102 [DOI] [PubMed] [Google Scholar]
- 68.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294:C460–C466 [DOI] [PubMed] [Google Scholar]
- 69.Dohare P, Hyzinski-Garcia MC, Vipani A, Bowens NH, Nalwalk JW, Feustel PJ, Keller RW Jr, Jourd’heuil D, Mongin AA (2014) The neuroprotective properties of the superoxide dismutase mimetic tempol correlate with its ability to reduce pathological glutamate release in a rodent model of stroke. Free Radic Biol Med 77:168–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patiño P, Parada E, Farré-Alins V, Molz S, Cacabelos R, Marco-Contelles J, López MG, Tasca CI, Ramos E, Romero A, Egea J (2016) Melatonin protects against oxygen and glucose deprivation by decreasing extracellular glutamate and Nox-derived ROS in rat hippocampal slices. Neurotoxicology 57:61–68 [DOI] [PubMed] [Google Scholar]
- 71.Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH (2003) Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23:3394–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y, Zhang H, Zhang X, Wang W, Chen Y, Cai Z, Wang Q, Wang J, Shi Y (2023) Promotion of astrocyte-neuron glutamate-glutamine shuttle by SCFA contributes to the alleviation of Alzheimer’s disease. Redox Biol 62:102690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miao Y, Qiu Y, Lin Y, Miao Z, Zhang J, Lu X (2011) Protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism. Mol Biol Rep 38:3235–3242 [DOI] [PubMed] [Google Scholar]
- 75.Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M (2009) Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Dis 33:405–411 [DOI] [PubMed] [Google Scholar]
- 76.Revilla S, Ursulet S, Álvarez-López MJ, Castro‐Freire M, Perpiñá U, García‐Mesa Y, Bortolozzi A, Giménez‐Llort L, Kaliman P, Cristòfol R, Sarkis C (2014) Lenti‐GDNF gene therapy protects against Alzheimer’s disease‐like neuropathology in 3xTg‐AD mice and MC65 cells. CNS Neurosci Ther 20:961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabezas R, Baez-Jurado E, Hidalgo-Lanussa O, Echeverria V, Ashrad GM, Sahebkar A, Barreto GE (2019) Growth factors and neuroglobin in astrocyte protection against neurodegeneration and oxidative stress. Mol Neurobiol 56:2339–2351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.