Abstract
Introduction
A systematic literature review and network meta-analysis (NMA) were conducted to compare the short-term efficacy of lebrikizumab to other biologic and Janus kinase (JAK) inhibitor monotherapies approved for moderate-to-severe atopic dermatitis in adults and adolescents.
Methods
The NMA included randomized, double-blind, placebo-controlled monotherapy phase 2 and 3 trials of biologics (lebrikizumab 250 mg every 2 weeks [Q2W], dupilumab 300 mg Q2W, and tralokinumab 300 mg Q2W) and JAK inhibitors (abrocitinib 100/200 mg daily, baricitinib 2/4 mg daily, and upadacitinib 15/30 mg daily) at approved doses. Efficacy outcomes included the proportions of patients achieving Eczema Area and Severity Index (EASI) improvement, an Investigator Global Assessment of 0 or 1 (IGA 0/1), and a ≥ 4-point improvement in pruritus/itch numeric rating scale score at 12 weeks (abrocitinib) or 16 weeks (other treatments). Itch was also assessed at week 4. A Bayesian NMA employing baseline risk-adjusted random effects models was used to estimate treatment differences.
Results
Twenty-two monotherapy studies involving 8531 patients were included in the NMA. By week 12/16, lebrikizumab had superior odds of achieving IGA 0/1 and itch improvement compared to baricitinib and tralokinumab; similar odds to dupilumab, abrocitinib, and upadacitinib 15 mg; and inferior odds to upadacitinib 30 mg. Additionally, lebrikizumab had a higher probability of improving EASI than baricitinib 2 mg; similar probability to baricitinib 4 mg, tralokinumab, dupilumab, abrocitinib, and upadacitinib 15 mg; and lower probability than upadacitinib 30 mg daily. At week 4, lebrikizumab had superior odds of improving itch compared to tralokinumab; similar odds to baricitinib, dupilumab, and abrocitinib 100 mg; and inferior odds to abrocitinib 200 mg and upadacitinib.
Conclusion
Among biologics, lebrikizumab was comparable to dupilumab and superior to tralokinumab in improving response rates at week 16. Upadacitinib 30 mg was the only JAK inhibitor with superior response rates compared to lebrikizumab.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-025-01357-7.
Keywords: Atopic dermatitis, Eczema Area and Severity Index, Investigator Global Assessment, Lebrikizumab, Network meta-analysis, Pruritus/itch Numeric Rating Scale
Key Summary Points
| Advanced systemic monotherapies are available for moderate-to-severe atopic dermatitis (AD), but there is limited evidence comparing these monotherapies in head-to-head clinical trials. |
| The present network meta-analysis (NMA) aimed to evaluate the short-term efficacy of approved interleukin (IL)-13/IL-4 biologics (lebrikizumab, dupilumab, tralokinumab) and Janus kinase (JAK) inhibitors (abrocitinib, baricitinib, and upadacitinib) as monotherapies for moderate-to-severe AD. |
| Among biologics, lebrikizumab was comparable to dupilumab and was superior to tralokinumab in improving response rates at week 16. |
| Upadacitinib 30 mg daily had superior efficacy as compared to all biologics at week 16. |
| This NMA suggests that lebrikizumab is a highly promising first-line biologic for moderate-to-severe AD, offering patients meaningful improvements in the signs and symptoms of AD. |
Introduction
Atopic dermatitis (AD) is a chronic, burdensome inflammatory skin disease characterized by skin lesions, pruritus, and sleep disturbance [1–3]. Pruritus is the universal symptom in AD [4] and is particularly troublesome at night, disrupting sleep and leading to daytime sleepiness, ultimately impairing health-related quality-of-life and increasing the economic burden placed on patients [1, 2, 5, 6]. Patients with moderate-to-severe AD are often initially treated with topical corticosteroids; however, many patients require long-term systemic management to control symptoms. Advanced systemic treatments can be given as monotherapy or combined with topical corticosteroids and include biologics (e.g., dupilumab, tralokinumab, and lebrikizumab), and Janus kinase (JAK) inhibitors (e.g., abrocitinib, baricitinib, and upadacitinib) [7].
Although JAK inhibitors have shown efficacy in treating AD and have a fast onset of action, their use requires careful monitoring to minimize the risk of side effects [8–12]. The JAK inhibitors have US Food and Drug Administration (FDA)-issued boxed warnings and other labeling advisories that alert healthcare providers to the risk of serious infections, mortality, cancer, cardiovascular events, and thrombosis [13–15]. In contrast, biologics such as dupilumab, tralokinumab, and lebrikizumab have more favorable safety profiles in AD [16]. Dupilumab and tralokinumab are approved for moderate-to-severe AD in adults and adolescents [17–20]. Recent clinical trials demonstrated that lebrikizumab monotherapy has a robust efficacy and safety profile in adults and adolescents with moderate-to-severe AD [21–25], and lebrikizumab is now approved for the treatment of moderate-to-severe AD in adults and adolescents 12 years and older with a body weight of at least 40 kg who are candidates for systemic therapy [26, 27].
Evidence from head-to-head clinical trials comparing AD monotherapies is limited. The Heads Up phase 3 trial compared the short-term efficacy of upadacitinib (30 mg orally once daily [QD]) with dupilumab (300 mg subcutaneously every 2 weeks [Q2W]) without a placebo arm [28]. This trial, the first to directly compare a JAK inhibitor with a biologic, found that approximately 10% more patients treated with upadacitinib achieved a clinical response than patients treated with dupilumab at week 16. However, the efficacy of other AD monotherapies, including lebrikizumab, has not been compared in head-to-head clinical trials. A network meta-analysis (NMA) is a widely accepted and robust method to compare multiple treatments that have not been directly compared in randomized clinical trials [29, 30]. Previous NMAs have examined the short-term efficacy of treatments for moderate-to-severe AD in adults [31–36]. These NMAs, however, did not include adolescents, well-conducted phase 2 clinical trials, and the most recent phase 3 clinical trials [31–35], or they included non-approved doses, including doses lower than those approved for treatment of AD, which could affect efficacy outcomes [31, 32]. A recent NMA also combined data from both monotherapy and combination therapy trials in their analysis [36]. The objective of this NMA was to evaluate the short-term efficacy of lebrikizumab monotherapy as compared with other advanced systemic monotherapies used to treat adults or adolescents with moderate-to-severe AD.
Methods
Ethical Approval
This study is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Sources and Study Selection
A systematic literature review was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [37]. Phase 2 and 3 randomized, double-blind, placebo-controlled clinical trials evaluating advanced systemic monotherapies in adults (≥ 18 years) and/or adolescents (≥ 12 to < 18 years) with moderate-to-severe AD were eligible for inclusion (Table S1). Advanced systemic monotherapies included lebrikizumab, abrocitinib, baricitinib, dupilumab, tralokinumab, and upadacitinib. Only approved dosing regimens were included to ensure relevance of the results to clinical practice and policy decision-making [13, 14, 17–20, 38]. Different doses of the same monotherapy were considered independent treatments. The systematic literature search was performed on records up to April 2023, with no additional trials of advanced systemic monotherapies published as of August 2024. Data sources included electronic databases, conference abstracts, clinical trial registries, and reference lists of published literature. A detailed description of the search strategies for each database is provided in Tables S2–4. A feasibility assessment was conducted to determine which studies identified in the systematic literature review could be included in the NMA. Risk of bias was assessed for each study using the Cochrane Risk of Bias assessment tool [39]. Additional information on the search strategy used to identify eligible studies and the subsequent feasibility assessment is described in the Supplementary Methods.
Efficacy Outcomes
Efficacy outcomes included the proportions of patients achieving Eczema Area and Severity Index (EASI) improvement ≥ 90% from baseline (EASI 90), ≥ 75% from baseline (EASI 75), and ≥ 50% from baseline (EASI 50) [40]; the proportion of patients achieving an Investigator Global Assessment of 0 (clear) or 1 (almost clear) (IGA 0/1) [41] with a 2-point improvement; and the proportion of patients achieving a ≥ 4-point improvement in pruritus/itch numeric rating scale (NRS) score from baseline [42]. Included studies either did not define the IGA scale (n = 17) or used the validated IGA for AD (vIGA-AD) scale (n = 5) [43]. Timepoints for efficacy endpoints were selected to assess responses during the initial phase of treatment (4–16 weeks). EASI, IGA, and pruritus/itch NRS responses were evaluated at week 16 for lebrikizumab, baricitinib, dupilumab, tralokinumab, and upadacitinib and at week 12 for abrocitinib. Early response to treatment was also assessed at week 4 using the pruritus/itch NRS. Non-responder imputation (NRI) was used for all analyses to address missing outcome data. This approach was explicitly reported in most studies. In cases where the imputation method was not explicitly reported, it was assumed that NRI was used.
Statistical Analysis
To estimate differences in efficacy between lebrikizumab and each comparator, Bayesian NMAs were conducted in accordance with National Institute for Health and Care Excellence (NICE) guidelines [44–46] using OpenBUGS (version 3.2.3) and R (version 4.2.2) through the R package R2OpenBUGS [47–49]. Fixed effects (FE) and random effects (RE) models with or without adjustment for baseline risk were independently fitted for each outcome [44, 45]. Baseline risk-adjusted models were preferred if the baseline risk coefficient had credible intervals (CrIs) that did not contain zero. Based on model fit statistics, the appropriate RE or FE model (with or without baseline risk adjustment) was selected. RE models were preferred over FE models unless the deviance information criterion (DIC) of the FE model was meaningfully lower (i.e., ≤ 5 points) than that of the RE model [50, 51]. A difference in DIC of 3–7 points is often considered meaningful when comparing different models [52]. Additional information on model specification and assessment of fit is described in the Supplementary Methods.
Absolute probabilities, numbers needed to treat (NNT), odd ratios (ORs), and surface under the cumulative ranking curve (SUCRA) scores were estimated. Comparative efficacy was determined by examining the 95% CrIs for ORs. Lebrikizumab was considered superior or inferior to another treatment if that treatment’s 95% CrIs excluded 1. Sensitivity analyses were conducted in networks restricted to only phase 3 trials. Sensitivity analyses were also conducted to adjust for baseline disease severity through network meta-regressions for EASI and IGA 0/1 response networks. The network meta-regression models for the respective networks adjusted for mean EASI at baseline and the proportion of patients with an IGA of 4 at baseline.
Results
Systematic Literature Review
Of 8392 unique records identified in the systematic literature review, 2318 records were assessed for eligibility, and 53 records of monotherapy studies were identified that reported unique results. Twenty-two clinical trials of advanced systemic monotherapies were considered eligible for the NMA assessment (Fig. 1). These 22 trials included 8531 patients who received either placebo or one of the following monotherapies: lebrikizumab 250 mg Q2W, abrocitinib 100 mg QD and 200 mg QD, baricitinib 2 mg QD and 4 mg QD, dupilumab 300 mg Q2W, tralokinumab 300 mg Q2W, and upadacitinib 15 mg QD and 30 mg QD. All 22 included studies were double-blind, randomized, placebo-controlled clinical trials and were published between 2016 and 2023. All except two studies were multinational trials: NCT03912259 [53] was a trial of dupilumab conducted solely in China, and NCT03443024 [54] was a trial of lebrikizumab conducted solely in the USA. Six of the included studies were phase 2 or 2b trials, and 16 were phase 3 trials. All studies were at least 12 weeks in duration. Two studies had a crossover design (ECZTRA 3 and Rising Up); however, crossover was allowed only at week 16 and did not affect the endpoints of this NMA.
Fig. 1.
PRISMA diagram for the SLR. Fifty-three unique studies were identified by the SLR and assessed for their eligibility to be included in NMAs. Studies that did not report on a lebrikizumab dose of interest or an intervention or dose not approved by the European Medicines Agency or US Food and Drug Administration for AD were excluded. To ensure appropriate study design, extension studies or studies with a non-randomized initial treatment period were excluded. To remove bias arising from absence of a placebo control arm, studies that were not placebo controlled were excluded. Studies that did not report data on outcomes of interest were excluded. Twenty-two monotherapy studies were eligible for inclusion in NMAs. AD, atopic dermatitis; NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses; SLR, systematic literature review
Feasibility Assessment and Model Fit
Overall, NMAs were deemed to be feasible for all efficacy outcomes. Networks were defined by the availability of outcomes reported in the included trials (Fig. 2). For most studies, data were available for all outcomes of interest. All 22 included studies had data available for EASI and IGA endpoints. Twenty studies had data available for reduction in pruritus/itch NRS from baseline to week 16, and 17 studies had data available for reduction in pruritus/itch NRS from baseline to week 4. The risk of bias assessment showed that the overall risk of bias among studies included in this NMA was limited (Fig. S1). All studies reported all outcome data and had adequate methods of randomization, allocation concealment, and blinding. One study was assessed as having unclear reporting of outcome data because two patients did not receive study treatment and were excluded from the efficacy analysis. Another study was considered to potentially have other sources of bias due to a small sample size.
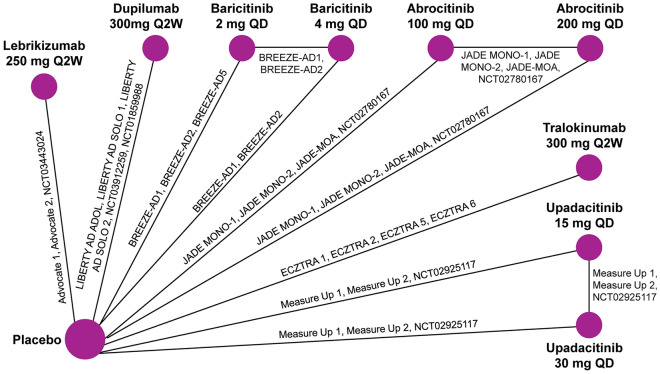
Fig. 2.
Network meta-analysis diagram. This NMA diagram shows the network for EASI response and IGA 0/1 response. The network for ≥ 4-point reduction in pruritus/itch NRS at week 16 is identical, except without the ECZTRA 5 and JADE MOA trials. The network for ≥ 4-point reduction in pruritus/itch NRS at week 4 is similar except without the ECZTRA 5, JADE MOA, NCT03912259, NCT01859988, and NCT02925117 trials. EASI, eczema area and severity index; IGA, Investigator Global Assessment; NMA, network meta-analysis; NRS, numeric rating scale; QD, daily; Q2W, every 2 weeks
Variation across studies in baseline characteristics, including potential treatment effect modifiers, was considered minimal (Tables S5–6, Fig. S2–13). Heterogeneity between studies in baseline mean age and time since AD diagnosis was identified for two trials in adolescents (ECZTRA 6 and AD ADOL). Reported race was mostly consistent across studies; however, one study conducted in China (EFC15116) included only Asian patients. Body mass index (BMI) was comparable among the few studies that reported this baseline characteristic. Heterogeneity in baseline EASI, proportion of patients with a baseline IGA of 4, and placebo EASI response was observed. Goodness-of-fit statistics supported the use of RE models adjusted for baseline risk for all outcomes (Tables S7–10).
Efficacy Endpoints
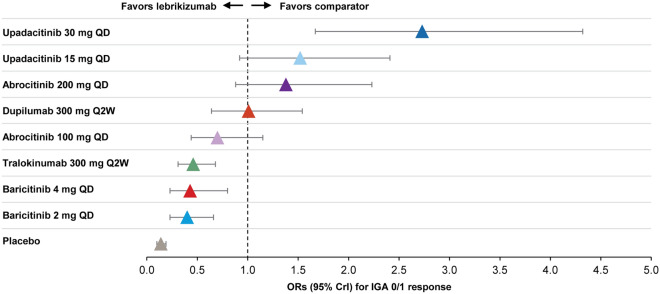
Lebrikizumab 250 mg Q2W had superior odds of achieving an IGA 0/1 by week 16 as compared to baricitinib 2 mg QD (OR 0.40, 95% CrI 0.23–0.66), baricitinib 4 mg QD (OR 0.43, 95% CrI 0.23–0.80), and tralokinumab 300 mg Q2W (OR 0.46, 95% CrI 0.31–0.68) (Fig. 3). Lebrikizumab 250 mg Q2W had comparable odds of achieving an IGA 0/1 by week 12 or 16 relative to abrocitinib 100 mg QD (OR 0.70, 95% CrI 0.44–1.15), dupilumab 300 mg Q2W (OR 1.01, 95% CrI 0.64–1.54), abrocitinib 200 mg QD (OR 1.38, 95% CrI 0.88–2.23), and upadacitinib 15 mg QD (OR 1.52, 95%CrI 0.92, 2.41). Lebrikizumab 250 mg Q2W had inferior odds of achieving IGA 0/1 as compared to upadacitinib 30 mg QD (OR 2.73, 95% CrI 1.67–4.32).
Fig. 3.
IGA 0/1 response relative to lebrikizumab at week 12/16. The forest plot was derived from Bayesian NMA using a baseline-risk adjusted RE model. Results are given as ORs and 95% CrIs for each comparator treatment versus lebrikizumab. For each trial, endpoints were measured at the primary endpoint timepoint (i.e., week 12 for abrocitinib and week 16 for all other treatments). CrI, credible interval; IGA, investigator global assessment; NMA, network meta-analysis; OR, odds ratio; QD, daily; Q2W, every 2 weeks; RE, random effects
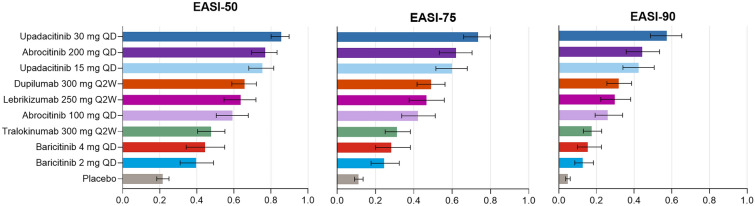
Patients receiving upadacitinib 30 mg QD had the highest probability of achieving an EASI 75 response followed by patients receiving abrocitinib 200 mg QD, upadacitinib 15 mg QD, dupilumab 300 mg Q2W, and lebrikizumab 250 mg Q2W (Fig. 4). Patients receiving lebrikizumab 250 mg Q2W had a higher probability of achieving an EASI 75 response than patients receiving tralokinumab 300 mg Q2W, baricitinib 4 mg QD, and baricitinib 2 mg QD. Lebrikizumab 250 mg Q2W had a similar probability of achieving an EASI 75 response as dupilumab 300 mg Q2W. Treatment rankings for EASI 50 and 90 response were similar to those for EASI 75 response.
Fig. 4.
Probability (95% CrI) of achieving EASI response at week 12/16. The bar graph shows the absolute probability of achieving EASI 50, 75, and 90, along with the corresponding 95% CrIs, using a baseline-risk adjusted RE model. For each trial, endpoints were measured at the primary endpoint timepoint (i.e., week 12 for abrocitinib and week 16 for all other treatments). CrI, credible interval; EASI, Eczema Area and Severity Index; QD, daily; Q2W, every 2 weeks; RE, random effects
Pruritus/itch NRS response for all treatments was assessed at week 4 and week 12 or 16. At week 4, patients receiving lebrikizumab 250 mg Q2W had superior odds of achieving a ≥ 4-point reduction in pruritus/itch NRS as compared to patients receiving tralokinumab 300 mg Q2W (OR 0.37, 95% CrI 0.19–0.70) (Fig. 5a). Lebrikizumab 250 mg Q2W had comparable odds of achieving a ≥ 4-point reduction in pruritus/itch NRS at week 4 relative to baricitinib 2 mg QD (OR 0.52, 95% CrI 0.27–1.01), dupilumab 300 mg Q2W (OR 0.67, 95% CrI 0.38–1.18), baricitinib 4 mg QD (OR 0.93, 95% CrI 0.46–1.94), and abrocitinib 100 mg QD (OR 1.33, 95% CrI 0.68–2.50). Lebrikizumab 250 mg Q2W had inferior odds of achieving a ≥ 4-point reduction in pruritus/itch NRS as compared to upadacitinib 15 mg QD (OR 2.90, 95% CrI 1.59–5.22), abrocitinib 200 mg QD (OR 3.16, 95% CrI 1.63–5.86), and upadacitinib 30 mg QD (OR 4.87, 95% CrI 2.68–8.79).
Fig. 5.
≥4 point reduction in pruritus/itch NRS response relative to lebrikizumab. The forest plot was derived from Bayesian NMA using a baseline-risk adjusted RE model. Results are given as ORs and 95% CrIs for each comparator treatment versus lebrikizumab. a Pruritus/itch NRS response at week 4. b Pruritus/itch NRS response at week 12/16. For each trial, endpoints were measured at the primary endpoint timepoint (i.e., week 12 for abrocitinib and week 16 for all other treatments). CrI, credible interval; NMA, network metaanalysis; NRS, numeric rating scale; OR, odds ratio; QD, daily; Q2W, every 2 weeks; RE, random effects
At week 12 or 16, however, the ranking of other monotherapies changed because of differences in the degree of improvement in itch reduction between weeks 4 and 12/16 (Fig. 5b). Patients receiving lebrikizumab 250 mg Q2W had better odds of achieving a ≥ 4-point improvement in pruritus/itch NRS at week 16 than patients receiving baricitinib 2 mg QD (OR 0.28, 95% CrI 0.15–0.51), baricitinib 4 mg QD (OR 0.35, 95% CrI 0.18–0.68), and tralokinumab 300 mg Q2W (OR 0.39, 95% CrI 0.23–0.63). Lebrikizumab 250 mg Q2W had comparable odds of pruritus/itch NRS improvement relative to abrocitinib 100 mg QD (OR 0.78, 95% CrI 0.47–1.24), dupilumab 300 mg Q2W (OR 0.79, 95% CrI 0.48–1.24), upadacitinib 15 mg QD (OR 1.19, 95% CrI 0.72–1.93), and abrocitinib 200 mg QD (OR 1.43, 95% CrI 0.86–2.28). Lebrikizumab 250 mg Q2W had inferior odds of achieving a ≥ 4-point reduction in pruritus/itch NRS as compared to upadacitinib 30 mg QD at week 16 relative (OR 1.84, 95% CrI 1.08–2.93). SUCRA-based treatment rankings indicated substantial overlap in CrIs (Tables S12–13).
NNT
Overall, NNT rankings for achieving IGA 0/1, EASI 75, or ≥ 4-point reduction in pruritus/itch NRS were generally consistent across treatments (Table S11). Lebrikizumab 250 mg Q2W had an NNT of 3.93 (95% CrI 3.04–5.33) for IGA 0/1, which was more favorable than tralokinumab 300 mg Q2W (8.82; 95% CrI 6.36–13.03) and baricitinib 2 mg QD (10.54; 95% CrI 6.49–21.47). Lebrikizumab’s NNT for IGA 0/1 was comparable to abrocitinib 200 mg QD (3.04; 95% CrI 2.41–4.05), upadacitinib 15 mg QD (2.84; 95% CrI 2.30–3.95), dupilumab 300 mg Q2W (3.89; 95% CrI 3.11–5.46), abrocitinib 100 mg QD (5.42; 95% CrI 3.90–8.12), and baricitinib 4 mg QD (9.35; 95% CrI 5.28–21.09). Only upadacitinib 30 mg QD (2.01; 95% CrI 1.74–2.57) had a more favorable NNT than lebrikizumab for IGA 0/1.
Lebrikizumab 250 mg Q2W had an NNT of 2.82 (95% CrI 2.28–3.66) for EASI 75, which was more favorable than tralokinumab (5.00; 95% CrI 3.85–6.76), baricitinib 4 mg QD (5.82; 95% CrI 3.80–10.43), and baricitinib 2 mg QD (7.55; 95% CrI 4.91–13.86). Lebrikizumab’s NNT for EASI 75 was comparable to abrocitinib 200 mg QD (1.97; 95% CrI 1.71–2.33), upadacitinib 15 mg QD (2.04; 95% CrI 1.79–2.43), dupilumab 300 mg Q2W (2.64; 95% CrI 2.27–3.16), and abrocitinib 100 mg QD (3.22; 95% CrI 2.56–4.27). Only upadacitinib 30 mg QD (1.60; 95% CrI 1.47–1.80) had a more favorable NNT than lebrikizumab for EASI 75.
For a ≥ 4-point reduction in pruritus/itch NRS at week 4, lebrikizumab 250 mg Q2W had an NNT of 4.95 (95% CrI 3.43–7.74), which was more favorable than tralokinumab 300 mg Q2W (16.67; 95% CrI 8.35–47.44) but less favorable than upadacitinib 30 mg QD (1.75; 95% CrI 1.50–2.22), abrocitinib 200 mg QD (2.15; 95% CrI 1.77–2.81), and upadacitinib 15 mg QD (2.25; 95% CrI 1.80–3.08). Lebrikizumab’s NNT for week-4 itch response was comparable to dupilumab 300 mg Q2W (7.43; 95% CrI 5.03–12.20), abrocitinib 100 mg QD (3.87; 95% CrI 2.81–5.79), baricitinib 4 mg QD (5.27; 95% CrI 3.15–10.71), and baricitinib 2 mg QD (10.04; 95% CrI 5.62–23.10). For a ≥ 4-point reduction in pruritus/itch NRS at week 12/16, lebrikizumab 250 mg Q2W had an NNT of 2.90 (95% CrI 2.26–3.80), which was more favorable than tralokinumab 300 mg Q2W (7.03; 95% CrI 4.81–11.17), baricitinib 4 mg QD (8.10; 95% CrI 4.42–19.64), and baricitinib 2 mg QD (11.12; 95% CrI 6.11–29.30). Lebrikizumab’s NNT for week-12/16 itch response was comparable to dupilumab 300 mg Q2W (3.47; 95% CrI 2.78–4.58), abrocitinib 100 mg QD (3.50; 95% CrI 2.67–4.91), upadacitinib 15 mg QD (2.57; 95% CrI 2.09–3.29), abrocitinib 200 mg QD (2.31; 95% CrI 1.90–2.93), and upadacitinib 30 mg QD (2.02; 95% CrI 1.74–2.46).
Sensitivity Analyses
Secondary analyses including only phase 3 trials and network meta-regressions adjusting for baseline EASI and IGA yielded findings generally consistent with the primary analyses. In an analysis of phase 3 trials only, findings for EASI response, IGA 0/1 response, and pruritus/itch NRS response were comparable to the primary analysis (Table S14). When adjusting for baseline mean EASI and the proportion of patients with an IGA score ≥ 4 at baseline, results were broadly comparable to the primary analysis. The CrIs of the estimated baseline severity coefficient included zero, and model fit was not improved in these adjusted models as compared to the corresponding primary analysis model (Tables S15–16). It was therefore concluded that the primary analyses were not biased by the limited variation seen in baseline severity across studies.
Discussion
This NMA assessed the short-term efficacy of lebrikizumab and other advanced systemic monotherapies approved for moderate-to-severe AD. Lebrikizumab was superior to tralokinumab on all measured outcomes at week 16. Although lebrikizumab was comparable to dupilumab on all measured outcomes at week 16, it showed a trend toward better odds for itch improvement than dupilumab at weeks 4 and 16. This may be due to lebrikizumab’s potent and selective inhibition of interleukin (IL)-13 [55]. As compared to JAK inhibitors, lebrikizumab was superior to baricitinib for all outcomes at week 16 and comparable to abrocitinib 100 mg QD. However, abrocitinib 200 mg QD and upadacitinib 15 mg QD were superior to lebrikizumab for EASI response, though lebrikizumab was comparable to these JAK inhibitors for IGA 0/1 and itch reduction at week 12/16. Only upadacitinib 30 mg QD was superior to lebrikizumab in comparative analyses. This finding is consistent with a recent NMA comparing the efficacy of targeted systemic therapies in alleviating pruritus at week 16 in patients with moderate-to-severe AD [56]. When considering the clinically relevant measure of NNT, our study showed that lebrikizumab was superior to tralokinumab and baricitinib and comparable to abrocitinib, dupilumab, and upadacitinib 15 mg QD at week 12/16. Although lebrikizumab had worse NNT outcomes than upadacitinib 30 mg for IGA 0/1 and EASI 75 at week 16, it had comparable NNT outcomes for itch reduction. Taken together, these findings highlight lebrikizumab 250 mg Q2W as a promising first-line treatment option for moderate-to-severe AD, with short-term efficacy comparable to or better than most other advanced systemic treatments.
Although this NMA and others [31–36] assessed short-term efficacy, AD is a chronic disease that requires long-term disease control and treatment. The efficacy of biologics to maintain response between weeks 16 and 52 has been evaluated in maintenance clinical trials [57–59]. In these trials, patients who achieved a clinical response at week 16 were re-randomized to receive active treatment or placebo (treatment withdrawal) for an additional 36 weeks of maintenance therapy. Data from these trials cannot be connected in an NMA because the placebo arms in these trials cannot be used as a common comparator. Responses in the placebo arms may reflect a pharmacodynamic effect of the drug that carries forward from induction treatment into the maintenance period of these studies. Indeed, in the ADvocate 1 and 2 trials, approximately half of patients who achieved a response to lebrikizumab at week 16 and were randomized to the withdrawal arm maintained their IGA 0/1 and EASI 75 responses at week 52 [57]. In contrast, the majority of patients who achieved a response to dupilumab or tralokinumab at week 16 and were randomized to the withdrawal arm relapsed at week 52 [58, 59]. These findings indicate that lebrikizumab may have a durable effect during treatment pauses.
Maintenance trials of biologics also included active treatment arms with drugs at varying dosing intervals. Lebrikizumab Q4W had better efficacy than lebrikizumab Q2W [57]. At week 52, IGA 0/1 and EASI 75 were maintained by 76.9% and 81.7% of patients treated with lebrikizumab Q4W. Patients receiving dupilumab also maintained response at week 52 when dupilumab was administered weekly (QW) or Q2W; however, longer dosage intervals resulted in a worsening of clinical response [58]. After 52 weeks, IGA 0/1 and EASI 75 were maintained by 54.0% and 71.6% of patients treated with dupilumab 300 mg QW/Q2W. Additionally, more frequent dosing with tralokinumab Q2W proved to be superior to less frequent Q4W dosing. At week 52, 55.9% of patients maintained IGA 0/1, and 57.3% maintained EASI 75 with tralokinumab Q2W [59]. In a recent indirect comparative analysis, lebrikizumab Q4W provided superior long-term maintenance of IGA 0/1 and equal long-term maintenance of EASI 75 as compared to dupilumab QW/Q2W in adult patients with moderate-to-severe AD who had responded to 16 weeks of induction treatment [60]. Taken together, these findings indicate that lebrikizumab may be preferable to dupilumab and tralokinumab in maintaining skin clearance and itch reduction among patients who achieve a clinical response at week 16. Additionally, lebrikizumab requires less frequent dosing than dupilumab or tralokinumab; however, tralokinumab Q4W may be considered for patients with lower body weight. These factors are important considerations for the long-term management of AD and may allow treatment plans to be tailored to individual patient needs and preferences.
Long-term trials of JAK inhibitors have used different study designs that preclude indirect comparison with biologics [61–63]. For example, in a phase 3 trial, patients receiving upadacitinib continued their assigned treatment from week 16 to 52 regardless of their response status at week 16 [63]. At week 52, approximately 84% of patients continuing upadacitinib 30 mg achieved EASI 75 and 65% achieved IGA 0/1. Although the Heads Up phase 3 trial compared the short-term efficacy of upadacitinib 30 mg QD to dupilumab 300 mg Q2W, head-to-head trials with longer durations are needed to assess treatment efficacy.
In addition to efficacy, several other factors must be considered when comparing biologics and JAK inhibitors, including their safety profiles. Current evidence for the safety of dupilumab, tralokinumab, and lebrikizumab monotherapy indicates that these drugs are well tolerated in patients with AD [64]. In an integrated safety analysis of eight lebrikizumab clinical trials, most adverse events were nonserious, mild or moderate in severity, and did not lead to treatment discontinuation [65]. Moreover, a recent indirect comparison demonstrated that lebrikizumab Q4W and dupilumab QW/Q2W had similar overall adverse event rates in adult patients with moderate-to-severe AD [60]. Conjunctivitis and injection site reactions were some of the most common adverse events reported for all three biologics [17, 20, 27]. Lebrikizumab had fewer injection site reactions than dupilumab or tralokinumab [17, 20, 27]. Although the incidence of conjunctivitis and other ophthalmologic adverse events was low [65–68], additional long-term safety data are needed to further differentiate the safety profile of these drugs.
Despite the promising responses observed in trials of upadacitinib 30 mg QD, the safety of JAK inhibitors has raised concerns. In the phase 3 Heads Up trial, upadacitinib was associated with increased risks of infection, eczema herpeticum, herpes zoster, and laboratory-related adverse events as compared to dupilumab, which may limit its use in certain patient populations [28]. Additionally, recent meta-analyses of JAK inhibitors used to treat moderate-to-severe AD showed that abrocitinib and upadacitinib increased the incidence of treatment-related adverse events [11] and that JAK inhibitors increased the incidence of herpes zoster, acne, headache, blood creatinine phosphokinase elevation, and nausea [12]. Other serious adverse events reported for JAK inhibitors, though rare, were malignancy, venous thromboembolism, and major adverse cardiovascular events; however, adverse events were only monitored during short follow-up periods mostly limited to 16 weeks or less [12]. The FDA-issued boxed warnings and other labeling advisories for upadacitinib and abrocitinib alert healthcare providers to the risk of serious infections, mortality, cancer, cardiovascular events, and thrombosis [13, 14]. Practical guides for the management of patients with AD treated with JAK inhibitors emphasize the importance of individualized patient assessment and laboratory and clinical monitoring to mitigate risks [69, 70]. These guides recommend considering JAK inhibitors for patients 65 years or older with specific clinical phenotypes, such as high itch-NRS scores or major involvement of sensitive areas, such as the face, neck, hands, or genitalia. They also advise evaluating baseline risk factors, including advanced age, history of venous thromboembolisms or malignancy, heart/kidney/liver conditions, and pregnancy/lactation, as well as regular monitoring during treatment for hematologic/metabolic anomalies, pregnancy, and infection. Healthcare providers must assess whether the benefits of JAK inhibitor therapy outweigh the potential risks and evaluate the need for baseline and/or ongoing safety monitoring. For these reasons, upadacitinib and abrocitinib are recommended in patients with moderate-to-severe AD with inadequate response to systemic therapy, including biologics, or when such therapies are inadvisable [71].
A strength of this NMA is that it is based on a robust systematic literature review and comprehensive feasibility assessment, leading to an analysis of high-quality evidence with limited potential for bias and secondary analyses demonstrating consistent findings. Bayesian analyses were conducted using robust statistical methodologies [45, 72], which were used to fit multiple models to identify the most appropriate for each outcome. In particular, baseline risk-adjusted RE models were selected to accommodate heterogeneity and adjust for placebo response rate differences across trials. Additionally, this NMA is the first to incorporate available phase 2 and 3 monotherapy data for lebrikizumab alongside dupilumab, tralokinumab, baricitinib, abrocitinib, and upadacitinib. Previous NMAs evaluating the efficacy of JAK inhibitors and biologics as monotherapies for AD have not included lebrikizumab [11, 34, 73], only included results from an early phase clinical trial of lebrikizumab [74], or included combination treatments with topical anti-inflammatory medications [36]. A recent NMA that compared lebrikizumab to other monotherapies included 13 trials [33], whereas the present NMA included 9 additional trials: six were phase 2 or 2b trials in adults (NCT03443024 [54], JADE MOA [75], NCT02780167 [76], NCT01859988 [77], ECZTRA 5 [78], and NCT02925117 [79]), and three were phase 3 trials, two in adolescents (AD ADOL [80] and ECZTRA 6 [81]), and one in adults (NCT03912259 [53]). In addition, this NMA included comparisons of 4-week itch response, whereas other NMAs have only included comparisons of 16-week itch response. Only approved doses were included in networks to ensure relevance of the results to clinical practice and to inform policy and decision-making surrounding the use of lebrikizumab. Most studies were multinational, with representation from Europe, North America, South America, Asia, and Oceania, supporting the generalizability of the NMA findings across multiple geographies. Taken together, these strengths support the internal validity of this NMA and generalizability of its findings to multiple settings and geographies.
Another strength of this NMA is its focus on monotherapy trials to accurately assess the single-agent efficacy of each drug; however, this may not fully reflect real-world practice where combination therapy is commonly used among patients. A limitation of this NMA is that abrocitinib trials used an endpoint of 12 weeks for initial treatment, whereas other trials used an endpoint of 16 weeks. Although a 12-week treatment period may underestimate abrocitinib’s efficacy relative to other agents assessed at 16 weeks, this limitation was considered acceptable to ensure a comprehensive analysis of available treatments for moderate-to-severe AD. Moreover, this NMA does not include 2-week efficacy data. Future studies should focus on assessing the early-onset efficacy of these therapies to better understand their rapid treatment effects. Another limitation of this NMA is that it did not compare safety outcomes because not all included trials reported safety data at 16 weeks. In addition, the exact IGA and itch scales used in the clinical trials varied; however, the concepts of itch and skin clearance measured by each of these scales were similar across studies. To ensure validity and comparability of results, future clinical trials should employ standardized scales, such as the vIGA-AD. Finally, one limitation of this NMA is the differing baseline characteristics among the included trials. Although our methodology included adjustments for baseline risk and used meta-regression analyses to account for key treatment effect modifiers, randomized head-to-head trials are still needed to definitively control for confounding factors.
Conclusion
This NMA shows that lebrikizumab has similar efficacy to dupilumab in achieving itch improvement and skin clearance after 16 weeks of treatment in patients with moderate-to-severe AD. This finding suggests that selective inhibition of IL-13 alone is an effective treatment strategy. Additionally, this NMA shows that lebrikizumab has superior clinical efficacy compared to tralokinumab, which may be explained by lebrikizumab’s distinct mechanism of action and pharmacokinetics profile. While both biologics target IL-13, lebrikizumab has a higher binding affinity and slower dissociation rate than tralokinumab [55]. Taken together with the known safety profiles of these treatments, these findings support the use of lebrikizumab as a first-line systemic treatment option for patients with moderate-to-severe AD. Future research should investigate the long-term safety and efficacy of systemic monotherapies and explore drug performance that accounts for potential withdrawals during the long-term management of this chronic disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing
Medical writing was provided by Michael Franklin, MS, of PPD (clinical research business of Thermo Fisher Scientific) in accordance with Good Publication Practice guidelines and was paid for by Eli Lilly and Company.
Author Contributions
Erin Johansson, Martin Dossenbach, Gaia Gallo, Marta Casillas, Andrei Karlsson, Tristan Curteis, and Thomas Bieber contributed to the study conception and design. Analysis and interpretation of the data was performed by Raj Chovatiya, Lisa Beck, Alan D. Irvine, James Del Rosso, Martin Dossenbach, Andrei Karlsson, Buelent Akmaz, Kahled Ezzedine, Peter Foley, Kamata Masahiro, Amy S. Paller, Luis Puig, and Marni Wiseman. All authors participated in the development and review of the manuscript and approved the manuscript submitted to this publication.
Funding
This study was funded by Eli Lilly and Company.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
Declarations
Conflict of Interest
Jonathan I. Silverberg has served as advisor, speaker, or consultant for AbbVie, Asana Biosciences, Dermavant Sciences, Galderma, GlaxoSmithKline, Glenmark, Kiniksa, LEO Pharma, Lilly, Menlo Therapeutics, Novartis, Pfizer, Realm Pharma, and Regeneron-Sanofi and is a researcher for GlaxoSmithKline. Thomas Bieber has served as a speaker, consultant, and investigator for AbbVie, Affibody, Almirall, AnaptysBio, Arena, Asana Biosciences, ASLAN Pharmaceuticals, Bayer Health, BioVerSys, Boehringer-Ingelheim, Bristol Myers Squibb, Connect Pharma, Dermavant Sciences, Domain Therapeutics, EQRx, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO Pharma, LG Chem, Lilly, L’Oréal, MSD, Novartis, Numab, OM Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron and UCB; and is the founder and chairman of the board of Davos Biosciences. Amy S. Paller has received honoraria for consulting for AbbVie, Abeona, Apogee, Arcutis, Aslan, BioCryst, Boehringer-Ingelheim, Bristol-Myers-Squibb, Dermavant, Galderma, Incyte, Johnson and Johnson, Krystal Biotech, LEO, Mitsubishi Tanabe, Nektar, Primus, Procter and Gamble, Regeneron, Sanofi, Seanergy, TWI Biotech, and UCB. She has served as an investigator without honoraria for AbbVie, Applied Pharma Research, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, Regeneron, Timber, and UCB. Lisa Beck has received grants from AbbVie, AstraZeneca, DermTech, Kiniksa, Pfizer, Regeneron, Ribon Therapeutics, and Sanofi; has received speaker’s fees or honoraria from Sanofi, Genzyme, Maruho/Galderma; has served as consultant and advisory board member for Abbvie, Allakos, Amgen, Arena Pharmaceuticals, Astra-Zeneca, Cara Therapeutics, DermTech, Escient Pharmaceuticals, Evelo Biosciences, Galderma, Genzyme, Glaxo-Smith Kline, Incyte, Invea Therapeutics, Janssen, LEO Pharma, Merck, Nektar Therapeutics, Novartis, Numab Therapeutics, Pfizer, Rapt Therapeutics, Regeneron, Ribon Therapeutics, Sanofi/Genzyme, Sanofi-Aventis, Simpson Healthcare, Stealth BioTherapeutics, Trevi Therapeutics, UCB, Union Therapeutics, and Xencor; is a member of the data monitoring committee for Novartis; and owns stocks in Gilead, Medtronic, and Moderna. Masahiro Kamata has received honoraria for lectures from AbbVie and Eli Lilly. Luis Puig has received consultancy/speaker’s honoraria from and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius-Kabi, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, STADA, Sun-Pharma, and UCB. Luis Puig, an Editorial Board member of Dermatology and Therapy, was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Marni Wiseman has served as consultant, speaker, advisory board member, and clinical trial investigator for AbbVie, Amgen, Arcutis, Asana BioSciences, AstraZeneca, Bausch Health, Bristol Myers Squibb, Celgene, Dermira, Eli Lilly, Galderma, Glenmark, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Principia, PRCL Research, Regeneron, Sanofi, UCB, and La Roche-Posay. Khaled Ezzedine has received consulting fees from AbbVie, Incyte, La Roche-Posay, Pfizer, Pierre Fabre, Sanofi, MSD, Bristol Myers Squibb, and Almirall. Alan D. Irvine is a consultant and/or advisory board member and/or is on the Data Safety Monitoring Board for: AbbVie, Almirall, Arena Pharmaceuticals, BenevolentAI, Eli Lilly and Company, LEO Pharma, Novartis, Pfizer, Regeneron, and Sanofi; has received research grants from: AbbVie and Pfizer; is on the board of directors of: the International Eczema Council; provides research support to: Regeneron; and is on the speaker’s bureau for: AbbVie, Eli Lilly and Company, Regeneron, and Sanofi Genzyme. Peter Foley has received grants from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sanofi, Sun Pharma, and UCB Pharma; has served as an investigator for AbbVie, Amgen, Argenx, Arcutis, Aslan, AstraZeneca, Boehringer Ingelheim, Botanix, Bristol Myers Squibb, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Eli Lilly, Evelo, Galderma, Genentech, Geneseq, GlaxoSmithKline, Hexima, Incyte, Janssen, Kymab, LEO Pharma, Merck, MedImmune, Novartis, Pfizer, Regeneron Pharmaceuticals, Reistone, Roche, Sanofi, Sun Pharma, Teva, UCB, and Valeant; has served as advisory board member for AbbVie, Amgen, Aslan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, LEO Pharma, Mayne Pharma, Merck, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and Valeant; has served as a consultant for Aslan, Bristol Myers Squibb, Eli Lilly, Galderma, GenesisCare, Hexima, Janssen, LEO Pharma, MedImmune, Mayne Pharma, Novartis, Pfizer, Roche, and UCB; has received travel grants from AbbVie, Eli Lilly, Galderma, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Roche, Sun Pharma, and Sanofi; and has received speaker’s fees or honoraria from AbbVie, Amgen, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Roche, Sanofi, Sun Pharma, and Valeant. James Del Rosso has received grants as an investigator, honoraria for lecturing, and/or consulting fees from AbbVie, Amgen (Celgene), AOBiome, Aslan, Arbonne, Arcutis, Bausch Health (Ortho Derm), Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, Exeltis, Ferndale, Galderma, Incyte, IntraDerm, Johnson & Johnson, La Roche-Posay/L’Oréal, LEO Pharma, Menlo Therapeutics, Nektar, Pfizer, Pierre Fabre, Regeneron/Sanofi Genzyme, Sun Pharma, Theraplex, UCB Pharma, Unilever, and Verrica Pharmaceuticals. Linda Stein Gold is an investigator and/or consultant and/or speaker for: AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, Galderma, Incyte Corporation, Janssen, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, and UCB Pharma. Erin Johansson, Martin Dossenbach, Gaia Gallo, and Marta Casillas are employees and minor shareholders of Eli Lilly and Company, which funded this study. Buelent Akmaz is an employee of Almirall. Andrei Karlsson and Tristan Curteis are employees of Costello Medical, which was funded by Eli Lilly and Company to provide analytical services for this publication. Raj Chovatiya served as an advisor, consultant, speaker, and/or investigator for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Argenx, ASLAN Pharmaceuticals, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, Eli Lilly and Company, FIDE, Galderma, Genentech, GSK, Incyte, LEO Pharma, L’Oréal, Nektar Therapeutics, Novartis, Opsidio, Pfizer Inc., Regeneron, RAPT, Sanofi, Sitryx, and UCB.
Ethical Approval
This study is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Prior Presentation: This work was previously presented as poster presentations at the 2023 Fall Clinical Dermatology Conference and 2024 European Academy of Dermatology and Venereology conference.
References
- 1.Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 Suppl):S115–23. [PubMed] [Google Scholar]
- 2.Ständer S, Simpson EL, Guttman-Yassky E, et al. Clinical relevance of skin pain in atopic dermatitis. J Drugs Dermatol. 2020;19(10):921–6. 10.36849/jdd.2020.5498. [DOI] [PubMed] [Google Scholar]
- 3.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 4.Grant L, Seiding Larsen L, Trennery C, et al. Conceptual model to illustrate the symptom experience and humanistic burden associated with atopic dermatitis in adults and adolescents. Dermatitis. 2019;30(4):247–54. 10.1097/DER.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin M, Misery L, von Kobyletzki L, et al. Unveiling the true costs and societal impacts of moderate-to-severe atopic dermatitis in Europe. J Eur Acad Dermatol Venereol. 2022;36(Suppl 7):3–16. 10.1111/jdv.18168. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Investig Dermatol. 2015;135(1):56–66. 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 7.Newsom M, Bashyam AM, Balogh EA, Feldman SR, Strowd LC. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80(11):1041–52. 10.1007/s40265-020-01335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmariah SB, Smith JS, Merola JF. JAK in the [black] box: a dermatology perspective on systemic JAK inhibitor safety. Am J Clin Dermatol. 2022;23(4):427–31. 10.1007/s40257-022-00701-3. [DOI] [PubMed] [Google Scholar]
- 9.Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13(3):729–49. 10.1007/s13555-023-00892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace DV. Treatment options for moderate to severe atopic dermatitis. Allergy Asthma Proc. 2022;43(6):474–93. 10.2500/aap.2022.43.220076. [DOI] [PubMed] [Google Scholar]
- 11.Wan H, Jia H, Xia T, Zhang D. Comparative efficacy and safety of abrocitinib, baricitinib, and upadacitinib for moderate-to-severe atopic dermatitis: a network meta-analysis. Dermatol Ther. 2022;35(9):e15636. 10.1111/dth.15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon S, Kim K, Shin K, et al. The safety of systemic Janus kinase inhibitors in atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2023. 10.1111/jdv.19426. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. RINVOQ (upadacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s004lbl.pdf. Accessed 9 Nov 2023.
- 14.US Food and Drug Administration. CIBINQOTM (abrocitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf. Accessed 9 Nov 2023.
- 15.US Food and Drug Administration. OLUMIANT (baricitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s006lbl.pdf. Accessed 4 Nov 2024.
- 16.Caffarelli C, Giannetti A, Giannì G, Ricci G. Anti-inflammatory and biologic drugs for atopic dermatitis: a therapeutic approach in children and adolescents. Front Med (Lausanne). 2023;10:1214963. 10.3389/fmed.2023.1214963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. ADBRY (tralokinumab). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf. Accessed 8 Nov 2023.
- 18.European Medicines Agency. Adtralza (tralokinumab). https://www.ema.europa.eu/en/medicines/human/EPAR/adtralza. Accessed 8 Nov 2023.
- 19.European Medicines Agency. Dupixent (dupilumab) https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent. Accessed 8 Nov 2023.
- 20.US Food and Drug Administration. DUPIXENT (dupilumab) https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf. Accessed 8 Nov 2023.
- 21.Prajapati S, Fardos M, Desai AD, Feldman SR. The role of lebrikizumab in the treatment of atopic dermatitis in the adult population. Immunotherapy. 2023;15(13):981–91. 10.2217/imt-2023-0066. [DOI] [PubMed] [Google Scholar]
- 22.Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863-71.e11. 10.1016/j.jaad.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Paller AS, Flohr C, Eichenfield LF, et al. Safety and efficacy of lebrikizumab in adolescent patients with moderate-to-severe atopic dermatitis: a 52-week, open-label, phase 3 study. Dermatol Ther (Heidelb). 2023. 10.1007/s13555-023-00942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson EL, Gooderham M, Wollenberg A, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol. 2023;159(2):182–91. 10.1001/jamadermatol.2022.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388(12):1080–91. 10.1056/NEJMoa2206714. [DOI] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Ebglyss (lebrikizumab) https://www.ema.europa.eu/en/medicines/human/summaries-opinion/ebglyss. Accessed 8 Nov 2023.
- 27.US Food and Drug Administration. EBGLYSS (lebrikizumab) https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761306Orig1s000correctedlbl.pdf. Accessed 20 Sept 2024.
- 28.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–55. 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dias S, Ades A, Welton N, Jansen JNSA. Network meta-analysis for decision-making. Chichester: Wiley; 2018. [Google Scholar]
- 30.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10(4):277–303. 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 31.Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 2020;156(6):659–67. 10.1001/jamadermatol.2020.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta-analysis. Allergy. 2021;76(4):1053–76. 10.1111/all.14631. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg JI, Hong HC, Calimlim BM, et al. Comparative efficacy of targeted systemic therapies for moderate-to-severe atopic dermatitis without topical corticosteroids: an updated network meta-analysis. Dermatol Ther (Heidelb). 2023;13(10):2247–64. 10.1007/s13555-023-01000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverberg JI, Hong HC, Thyssen JP, et al. Comparative efficacy of targeted systemic therapies for moderate to severe atopic dermatitis without topical corticosteroids: systematic review and network meta-analysis. Dermatol Ther (Heidelb). 2022;12(5):1181–96. 10.1007/s13555-022-00721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverberg JI, Thyssen JP, Fahrbach K, et al. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: a systematic literature review and network meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(9):1797–810. 10.1111/jdv.17351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drucker AM, Lam M, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: living systematic review and network meta-analysis update. JAMA Dermatol. 2024. 10.1001/jamadermatol.2024.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Medicines Agency. Olumiant (baricitinib) https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf. Accessed 9 Nov 2023.
- 39.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: Wiley; 2019. [Google Scholar]
- 40.Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10(1):11–8. 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 41.Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74(2):288–94. 10.1016/j.jaad.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 42.Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83(3):839–46. 10.1016/j.jaad.2020.04.104. [DOI] [PubMed] [Google Scholar]
- 44.Dias S, Welton NJ, Sutton AJ, Ades A. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011. [PubMed]
- 45.Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU technical support document 3: heterogeneity: subgroups, meta-regression, bias and bias-adjustment. 2011. [PubMed]
- 46.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. 2014. [PubMed]
- 47.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10(4):325–37. 10.1023/A:1008929526011. [Google Scholar]
- 48.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. 2022.
- 49.Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Softw. 2005;12(3):1–16. [Google Scholar]
- 50.EUnetHTA Methodological Guidance, p 16. https://www.eunethta.eu/wp-content/uploads/2022/08/EUnetHTA-21-Deliverable-D4.3.2-Methodological-Guideline-on-Direct-and-indirect-comparisons-V1.0.pdf. Accessed 8 Nov 2023.
- 51.MRC Biostatistics Unit CU. Deviance information criterion: https://ams132-winter17-01.courses.soe.ucsc.edu/system/files/attachments/DIC-FAQ.pdf. Accessed 8 Nov 2023.
- 52.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Stat Methodol). 2002;64(4):583–639. 10.1111/1467-9868.00353. [Google Scholar]
- 53.Zhao Y, Wu L, Lu Q, et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2022;186(4):633–41. 10.1111/bjd.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156(4):411–20. 10.1001/jamadermatol.2020.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okragly AJ, Ryuzoji A, Wulur I, et al. Binding, neutralization and internalization of the interleukin-13 antibody, lebrikizumab. Dermatol Ther (Heidelb). 2023. 10.1007/s13555-023-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong M, Gao Q, Ren H, Zhong T. Comparative efficacy of targeted systemic therapies for pruritus in moderate-to-severe atopic dermatitis without topical treatment: a network meta-analysis. J Dermatol Treat. 2024;35(1):2432930. 10.1080/09546634.2024.2432930. [DOI] [PubMed] [Google Scholar]
- 57.Blauvelt A, Thyssen JP, Guttman-Yassky E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023;188(6):740–8. 10.1093/bjd/ljad022. [DOI] [PubMed] [Google Scholar]
- 58.Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(2):131–43. 10.1001/jamadermatol.2019.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–49. 10.1111/bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rand K, Ramos-Goñi JM, Akmaz B, Solé-Feu L, Armario-Hita JC. Matching-adjusted indirect comparison of the long-term efficacy maintenance and adverse event rates of lebrikizumab versus dupilumab in moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2024;14(1):169–82. 10.1007/s13555-023-01058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flohr C, Cork MJ, Ardern-Jones MR, et al. Efficacy and safety of abrocitinib monotherapy in adolescents and adults: a post hoc analysis of the phase 3 JAK1 atopic dermatitis efficacy and safety (JADE) REGIMEN clinical trial. J Dermatolog Treat. 2023;34(1):2200866. 10.1080/09546634.2023.2200866. [DOI] [PubMed] [Google Scholar]
- 62.Silverberg JI, Simpson EL, Wollenberg A et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157(6):691–9. 10.1001/jamadermatol.2021.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–13. 10.1001/jamadermatol.2022.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waligóra-Dziwak K, Dańczak-Pazdrowska A, Jenerowicz D. A comprehensive review of biologics in phase III and IV clinical trials for atopic dermatitis. J Clin Med. 2024. 10.3390/jcm13144001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein Gold L, Thaçi D, Thyssen JP, et al. Safety of lebrikizumab in adults and adolescents with moderate-to-severe atopic dermatitis: an integrated analysis of eight clinical trials. Am J Clin Dermatol. 2023. 10.1007/s40257-023-00792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beck LA, Deleuran M, Bissonnette R, et al. Dupilumab provides acceptable safety and sustained efficacy for up to 4 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2022;23(3):393–408. 10.1007/s40257-022-00685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferreira S, Torres T. Conjunctivitis in patients with atopic dermatitis treated with dupilumab. Drugs Context. 2020. 10.7573/dic.2020-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wollenberg A, Beck LA, de Bruin WM, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2022;186(3):453–65. 10.1111/bjd.20810. [DOI] [PubMed] [Google Scholar]
- 69.Gargiulo L, Ibba L, Malagoli P, et al. Management of patients affected by moderate-to-severe atopic dermatitis with JAK inhibitors in real-world clinical practice: an Italian Delphi consensus. Dermatol Ther (Heidelb). 2024;14(4):919–32. 10.1007/s13555-024-01135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haag C, Alexis A, Aoki V, et al. A practical guide to using oral Janus kinase inhibitors for atopic dermatitis from the International Eczema Council. Br J Dermatol. 2024;192(1):135–43. 10.1093/bjd/ljae342. [DOI] [PubMed] [Google Scholar]
- 71.Davis DMR, Drucker AM, Alikhan A, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90(2):e43–56. 10.1016/j.jaad.2023.08.102. [DOI] [PubMed] [Google Scholar]
- 72.Dias S, Welton N, Sutton AAA: NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2016. http://www.nicedsu.org.uk. Accessed 21 June 2023. [PubMed]
- 73.Sedeh FB, Henning MAS, Jemec GBE, Ibler KS. Comparative efficacy and safety of monoclonal antibodies and janus kinase inhibitors in moderate-to-severe atopic dermatitis: a systematic review and meta-analysis. Acta Derm Venereol. 2022;102:adv00764. 10.2340/actadv.v102.2075. [DOI] [PMC free article] [PubMed]
- 74.Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–32. 10.1001/jamadermatol.2022.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Study evaluating the mechanism of action of PF-04965842 monotherapy for moderate-to-severe atopic dermatitis (JADE MOA). https://www.clinicaltrials.gov/ct2/show/NCT03915496?term=abrocitinib&cond=Atopic+Dermatitis&draw=2&rank=4. Accessed 10 Oct 2023.
- 76.Study to evaluate Pf-04965842 in subjects with moderate to severe atopic dermatitis. https://clinicaltrials.gov/study/NCT02780167?tab=results&a=15. Accessed 10 Oct 2023.
- 77.Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52. 10.1016/s0140-6736(15)00388-8. [DOI] [PubMed] [Google Scholar]
- 78.Merola JF, Bagel J, Almgren P, et al. Tralokinumab does not impact vaccine-induced immune responses: results from a 30-week, randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2021;85(1):71–8. 10.1016/j.jaad.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 79.Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–84. 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 80.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. 10.1001/jamadermatol.2019.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paller AS, Flohr C, Cork M, et al. Efficacy and safety of tralokinumab in adolescents with moderate to severe atopic dermatitis: the phase 3 ECZTRA 6 randomized clinical trial. JAMA Dermatol. 2023;159(6):596–605. 10.1001/jamadermatol.2023.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or as supplementary information files.