Abstract
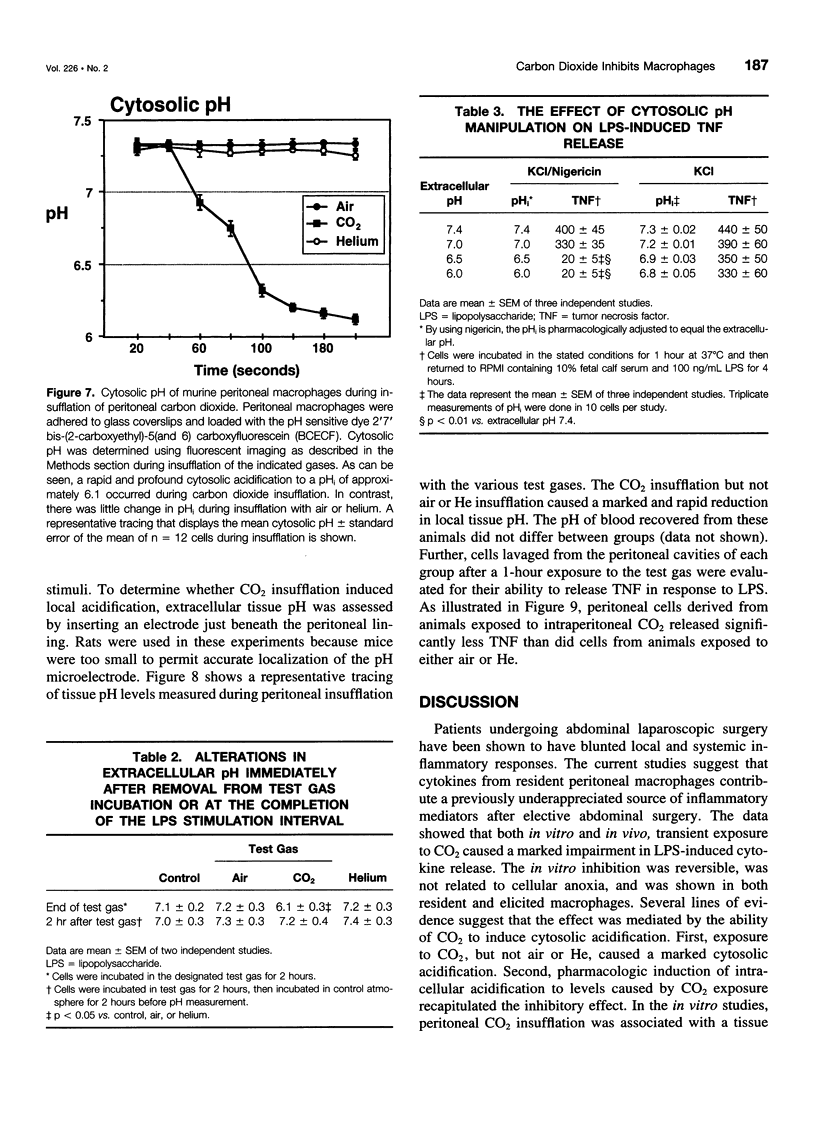
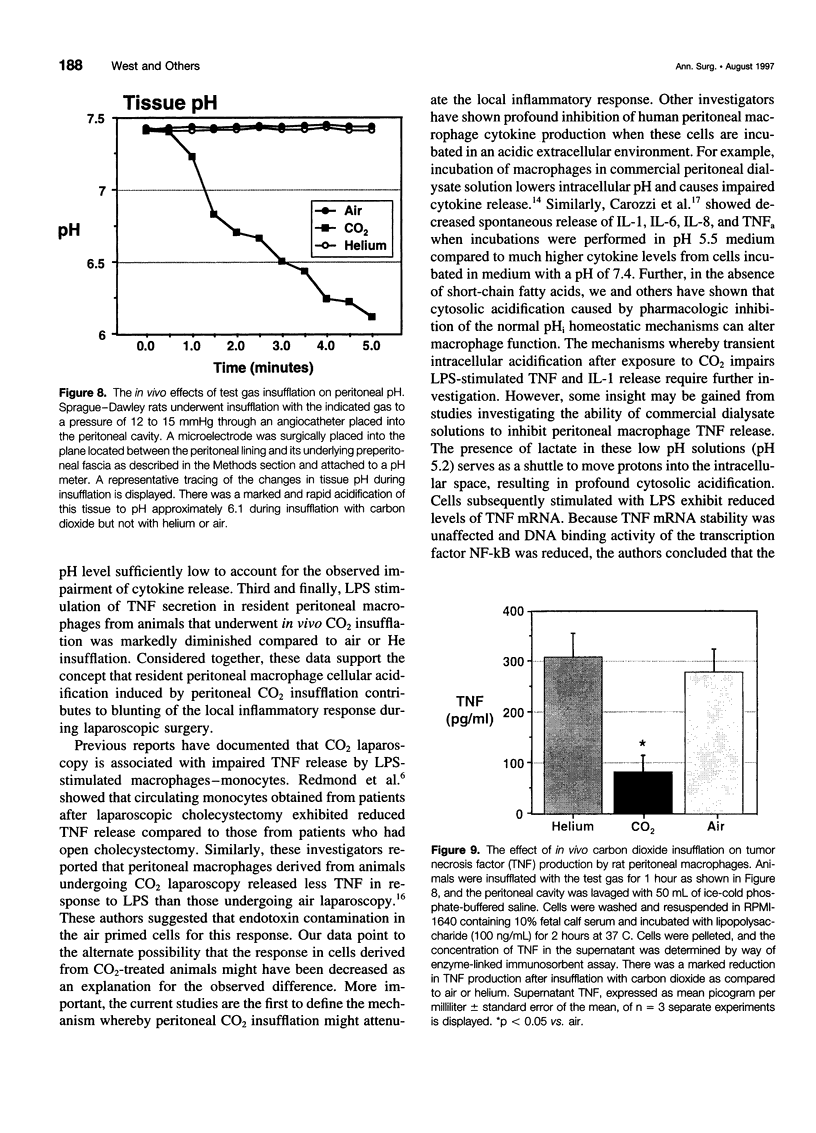
OBJECTIVE: The objective of this study was to determine the effect of carbon dioxide (CO2) on the function of peritoneal macrophages. SUMMARY BACKGROUND DATA: Laparoscopic surgery is associated with minimal pain, fever, and low levels of inflammatory cytokines. To understand the mechanisms involved, the authors investigated the effect of different gases on murine peritoneal macrophage intracellular pH and correlated these alterations with alterations in LPS-stimulated inflammatory cytokine release. METHODS: Peritoneal macrophages were incubated for 2 hours in air, helium, or CO2, and the effect of the test gas on immediate or next day lipopolysaccharide (LPS)-stimulated tumor necrosis factor (TNF) and interleukin-1 release compared. Cytosolic pH of macrophages exposed to test gases was measured using single-cell fluorescent imaging. The in vivo effects of test gases were determined in anesthetized rats during abdominal insufflation. RESULTS: Macrophages incubated in CO2 produced significantly less TNF and interleukin-1 in response to LPS compared to incubation in air or helium. Cytokine production returned to normal 24 hours later. Exposure to CO2, but not air or helium, caused a marked cytosolic acidification. Pharmacologic induction of intracellular acidification to similar levels reproduced the inhibitory effect. In vitro studies showed that CO2 insufflation lowered tissue pH and peritoneal macrophage LPS-stimulated TNF production. CONCLUSIONS: The authors propose that cellular acidification induced by peritoneal CO2 insufflation contributes to blunting of the local inflammatory response during laparoscopic surgery.
Full text
PDF

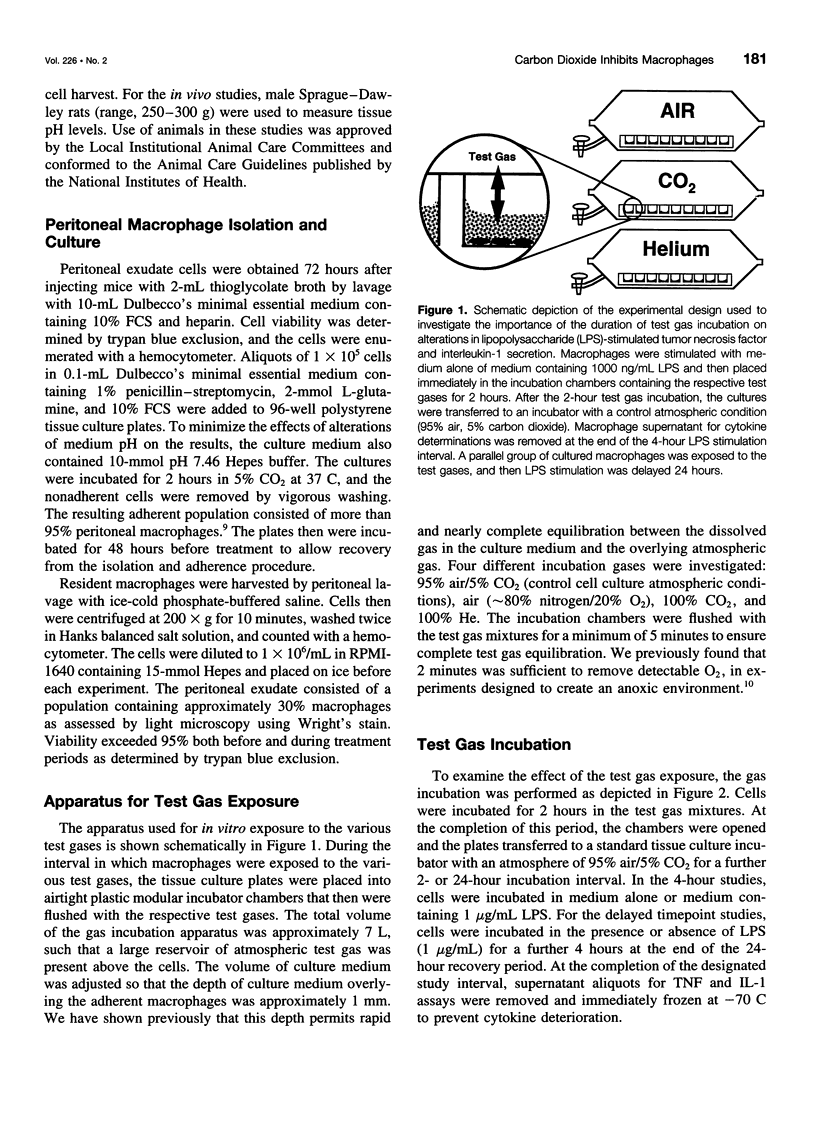


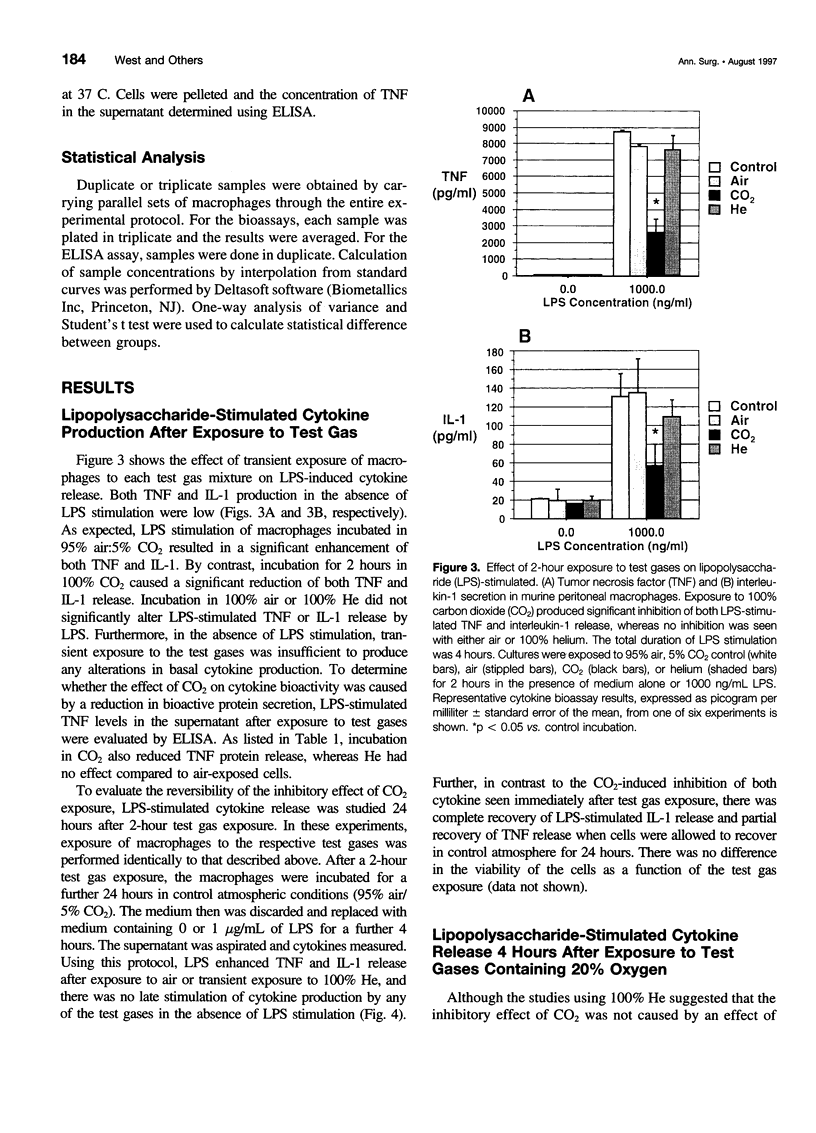
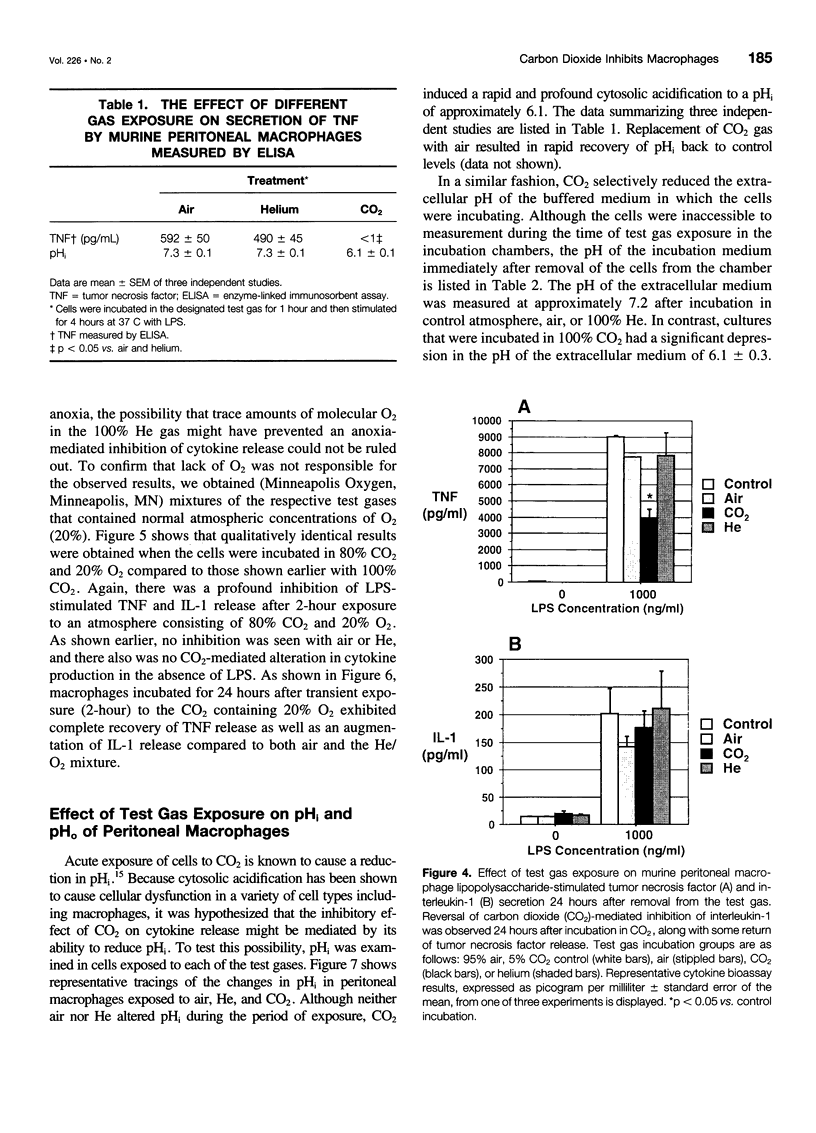
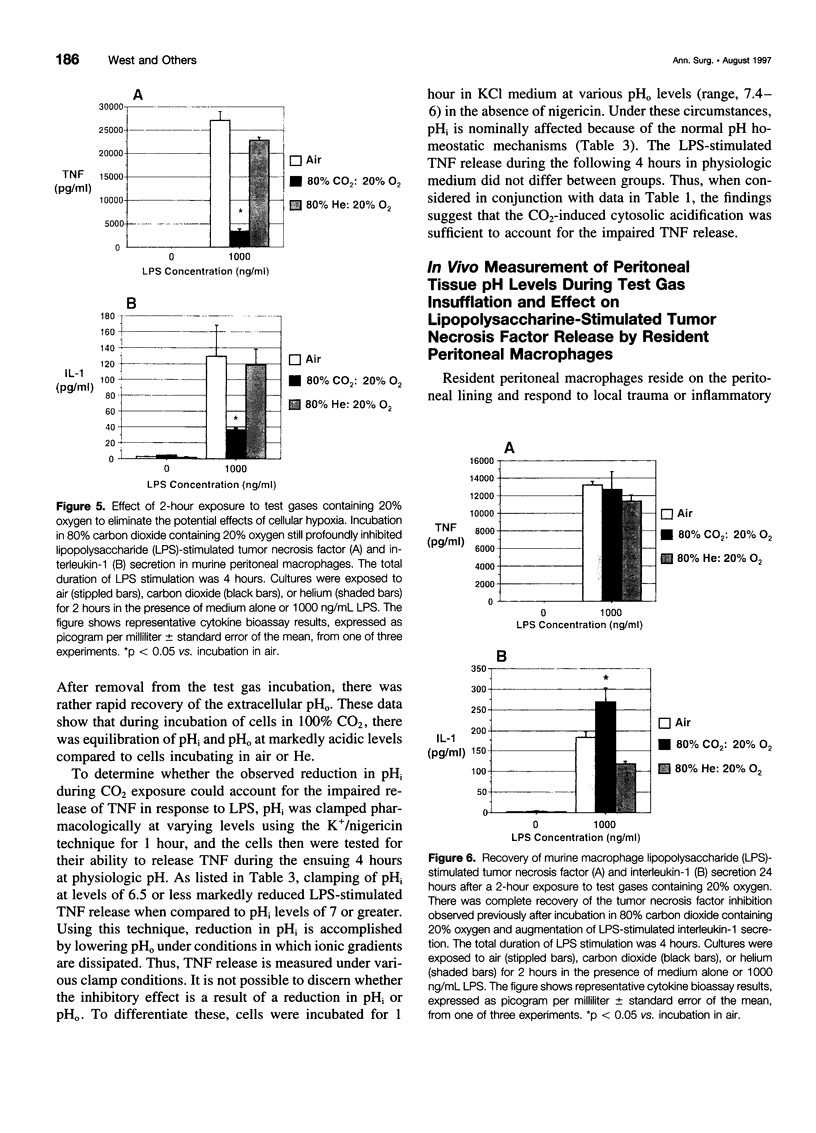


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B. Human lymphotoxin. Methods Enzymol. 1985;116:441–448. doi: 10.1016/s0076-6879(85)16035-0. [DOI] [PubMed] [Google Scholar]
- Barkun J. S., Wexler M. J., Hinchey E. J., Thibeault D., Meakins J. L. Laparoscopic versus open inguinal herniorrhaphy: preliminary results of a randomized controlled trial. Surgery. 1995 Oct;118(4):703–710. doi: 10.1016/s0039-6060(05)80038-8. [DOI] [PubMed] [Google Scholar]
- Bidani A., Heming T. A. Effects of bafilomycin A1 on functional capabilities of LPS-activated alveolar macrophages. J Leukoc Biol. 1995 Feb;57(2):275–281. doi: 10.1002/jlb.57.2.275. [DOI] [PubMed] [Google Scholar]
- Carozzi S., Caviglia P. M., Nasini M. G., Schelotto C., Santoni O., Pietrucci A. Peritoneal dialysis solution pH and Ca2+ concentration regulate peritoneal macrophage and mesothelial cell activation. ASAIO J. 1994 Jan-Mar;40(1):20–23. [PubMed] [Google Scholar]
- Collet D., Vitale G. C., Reynolds M., Klar E., Cheadle W. G. Peritoneal host defenses are less impaired by laparoscopy than by open operation. Surg Endosc. 1995 Oct;9(10):1059–1064. doi: 10.1007/BF00188987. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Douvdevani A., Abramson O., Tamir A., Konforty A., Isakov N., Chaimovitz C. Commercial dialysate inhibits TNF alpha mRNA expression and NF-kappa B DNA-binding activity in LPS-stimulated macrophages. Kidney Int. 1995 Jun;47(6):1537–1545. doi: 10.1038/ki.1995.217. [DOI] [PubMed] [Google Scholar]
- Douvdevani A., Rapoport J., Konforty A., Yulzari R., Moran A., Chaimovitz C. Intracellular acidification mediates the inhibitory effect of peritoneal dialysate on peritoneal macrophages. J Am Soc Nephrol. 1995 Aug;6(2):207–213. doi: 10.1681/ASN.V62207. [DOI] [PubMed] [Google Scholar]
- Glaser F., Sannwald G. A., Buhr H. J., Kuntz C., Mayer H., Klee F., Herfarth C. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995 Apr;221(4):372–380. doi: 10.1097/00000658-199504000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Characterization of the amiloride-sensitive Na+-H+ antiport of human neutrophils. Am J Physiol. 1986 Feb;250(2 Pt 1):C283–C291. doi: 10.1152/ajpcell.1986.250.2.C283. [DOI] [PubMed] [Google Scholar]
- Jatzko G. R., Lisborg P. H., Pertl A. M., Stettner H. M. Multivariate comparison of complications after laparoscopic cholecystectomy and open cholecystectomy. Ann Surg. 1995 Apr;221(4):381–386. doi: 10.1097/00000658-199504000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealy K., Gallagher H., Barry M., Lennon F., Traynor O., Hyland J. Physiological and metabolic responses to open and laparoscopic cholecystectomy. Br J Surg. 1992 Oct;79(10):1061–1064. doi: 10.1002/bjs.1800791024. [DOI] [PubMed] [Google Scholar]
- Ortega A. E., Hunter J. G., Peters J. H., Swanstrom L. L., Schirmer B. A prospective, randomized comparison of laparoscopic appendectomy with open appendectomy. Laparoscopic Appendectomy Study Group. Am J Surg. 1995 Feb;169(2):208–213. doi: 10.1016/s0002-9610(99)80138-x. [DOI] [PubMed] [Google Scholar]
- Papanastasiou-Diamandi A., Christopoulos T. K., Diamandis E. P. Ultrasensitive thyrotropin immunoassay based on enzymatically amplified time-resolved fluorescence with a terbium chelate. Clin Chem. 1992 Apr;38(4):545–548. [PubMed] [Google Scholar]
- Redmond H. P., Watson R. W., Houghton T., Condron C., Watson R. G., Bouchier-Hayes D. Immune function in patients undergoing open vs laparoscopic cholecystectomy. Arch Surg. 1994 Dec;129(12):1240–1246. doi: 10.1001/archsurg.1994.01420360030003. [DOI] [PubMed] [Google Scholar]
- Swallow C. J., Grinstein S., Sudsbury R. A., Rotstein O. D. Relative roles of Na+/H+ exchange and vacuolar-type H+ ATPases in regulating cytoplasmic pH and function in murine peritoneal macrophages. J Cell Physiol. 1993 Dec;157(3):453–460. doi: 10.1002/jcp.1041570304. [DOI] [PubMed] [Google Scholar]
- Vogt D. M., Curet M. J., Pitcher D. E., Martin D. T., Zucker K. A. Preliminary results of a prospective randomized trial of laparoscopic onlay versus conventional inguinal herniorrhaphy. Am J Surg. 1995 Jan;169(1):84–90. doi: 10.1016/s0002-9610(99)80114-7. [DOI] [PubMed] [Google Scholar]
- Watson R. W., Redmond H. P., McCarthy J., Burke P. E., Bouchier-Hayes D. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br J Surg. 1995 Aug;82(8):1060–1065. doi: 10.1002/bjs.1800820820. [DOI] [PubMed] [Google Scholar]
- West M. A., Keller G. A., Cerra F. B., Simmons R. L. Killed Escherichia coli stimulates macrophage-mediated alterations in hepatocellular function during in vitro coculture: a mechanism of altered liver function in sepsis. Infect Immun. 1985 Sep;49(3):563–570. doi: 10.1128/iai.49.3.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. A., Li M. H., Seatter S. C., Bubrick M. P. Pre-exposure to hypoxia or septic stimuli differentially regulates endotoxin release of tumor necrosis factor, interleukin-6, interleukin-1, prostaglandin E2, nitric oxide, and superoxide by macrophages. J Trauma. 1994 Jul;37(1):82–90. doi: 10.1097/00005373-199407000-00015. [DOI] [PubMed] [Google Scholar]




