Abstract
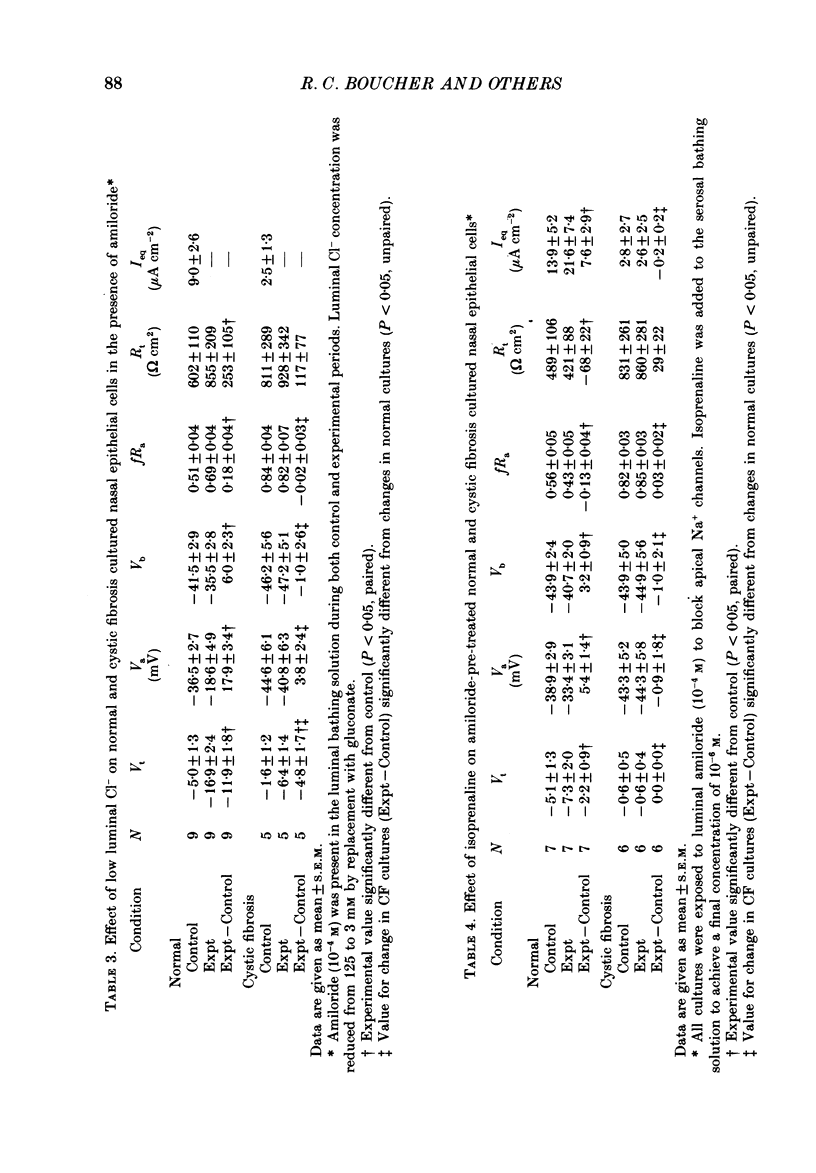
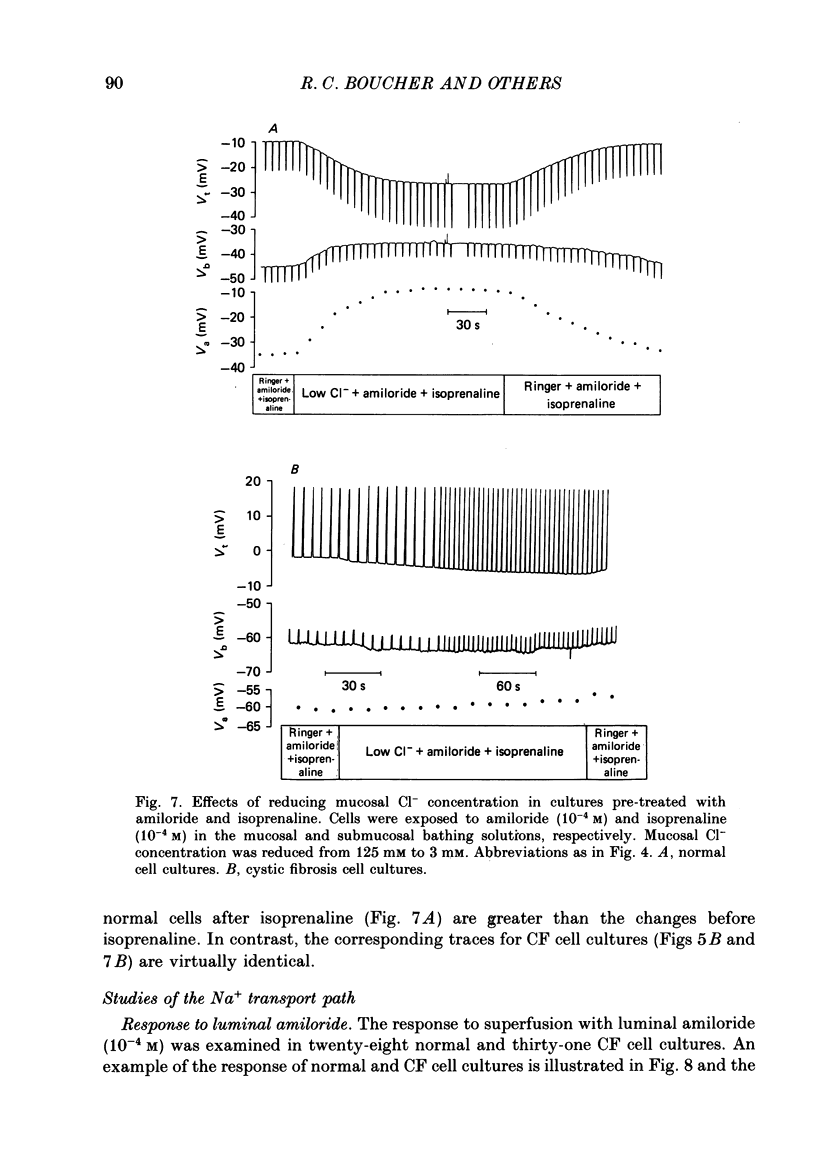
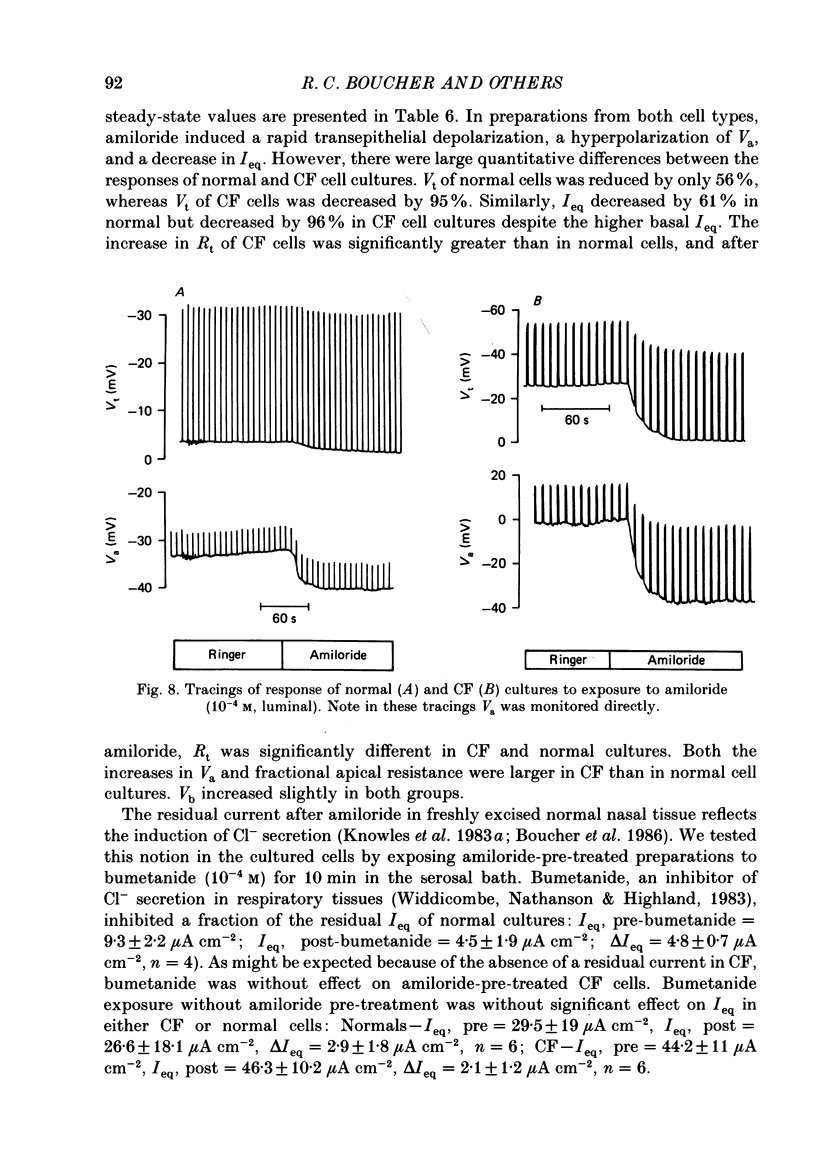
1. Employing a primary cell culture system and intracellular microelectrodes, we quantitated and compared the Na+ and Cl- pathways in apical membranes of normal and cystic fibrosis (CF) human airway epithelia. 2. Like the transepithelial difference (PD) in situ, the PD of CF epithelia in culture (-27 +/- 4 mV, mean +/- S.E.M.; n = 28) exceeded the PD of normal epithelia (-10 +/- 1 mV; n = 22). The raised PD principally reflected an increase in the rate of active transport (equivalent short circuit, Ieq) for CF epithelia (61 +/- 9 microA cm-2) as compared with normal epithelia (23 +/- 3 microA cm-2). No significant differences in transepithelial resistance were detected. 3. As indicated by ion replacement studies (gluconate for Cl-), the apical membrane of normal cells exhibits an apical membrane Cl- conductance (GCl) that can be activated by isoprenaline. CF cells do not exhibit an apical membrane GCl, nor can a GCl be activated by isoprenaline. 4. CF cells exhibited a larger amiloride-sensitive Ieq and amiloride-sensitive apical membrane conductance (GNa) than normal cells. Further, the amiloride-sensitive Ieq was increased by isoprenaline in CF but not normal airway epithelia. 5. Equivalent circuit analysis yielded evidence for a more positive electromotive force (EMF) across the apical membrane and a more negative EMF across the basolateral membrane of CF cells as compared with normal cells. Baseline resistances of the apical (Ra) and basolateral (Rb) membranes did not differ for normal and CF cells. 6. Estimates of the resistance of the paracellular path to ion flow (Rs) by equivalent circuit analysis or ion substitution detected no differences in Rs between CF and normal cells. 7. We conclude that abnormalities in both cellular Cl- permeability (reduced) and Na+ permeability (increased) are characteristic of the cultured CF respiratory epithelial cell. These data suggest that a defect in the regulation of apical membrane permeabilities is a central feature of this disease.
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Cheng E. Effect of catecholamines on ion transport in dog tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):397–403. doi: 10.1152/jappl.1979.47.2.397. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Gatzy J. T. Characteristics of sodium transport by excised rabbit trachea. J Appl Physiol Respir Environ Exerc Physiol. 1983 Dec;55(6):1877–1883. doi: 10.1152/jappl.1983.55.6.1877. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986 Nov;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton C. U., Lawson E. E., Boucher R. C., Gatzy J. T. Bioelectric properties and ion transport of airways excised from adult and fetal sheep. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1542–1549. doi: 10.1152/jappl.1983.55.5.1542. [DOI] [PubMed] [Google Scholar]
- Cotton C. U., Stutts M. J., Knowles M. R., Gatzy J. T., Boucher R. C. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J Clin Invest. 1987 Jan;79(1):80–85. doi: 10.1172/JCI112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Fuchs W., Larsen E. H., Lindemann B. Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J Physiol. 1977 May;267(1):137–166. doi: 10.1113/jphysiol.1977.sp011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981 Dec 17;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M., Murray G., Shallal J., Askin F., Ranga V., Gatzy J., Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- Nagel W., Reinach P. Mechanism of stimulation by epinephrine of active transepithelial Cl transport in isolated frog cornea. J Membr Biol. 1980 Aug 21;56(1):73–79. doi: 10.1007/BF01869354. [DOI] [PubMed] [Google Scholar]
- Quinton P. M., Bijman J. Higher bioelectric potentials due to decreased chloride absorption in the sweat glands of patients with cystic fibrosis. N Engl J Med. 1983 May 19;308(20):1185–1189. doi: 10.1056/NEJM198305193082002. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Passive electrical properties of toad urinary bladder epithelium. Intercellular electrical coupling and transepithelial cellular and shunt conductances. J Gen Physiol. 1974 Jul;64(1):1–25. doi: 10.1085/jgp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorofsky S. R., Field M., Fozzard H. A. Electrophysiology of Cl secretion in canine trachea. J Membr Biol. 1983;72(1-2):105–115. doi: 10.1007/BF01870318. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Steele R. E., Preston A. S., Johnson J. P., Handler J. S. Porous-bottom dishes for culture of polarized cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C136–C139. doi: 10.1152/ajpcell.1986.251.1.C136. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Cotton C. U., Yankaskas J. R., Cheng E., Knowles M. R., Gatzy J. T., Boucher R. C. Chloride uptake into cultured airway epithelial cells from cystic fibrosis patients and normal individuals. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6677–6681. doi: 10.1073/pnas.82.19.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Nathanson I. T., Highland E. Effects of "loop" diuretics on ion transport by dog tracheal epithelium. Am J Physiol. 1983 Nov;245(5 Pt 1):C388–C396. doi: 10.1152/ajpcell.1983.245.5.C388. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Welsh M. J., Finkbeiner W. E. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A. Intracellular Na+ activity as a function of Na+ transport rate across a tight epithelium. Biophys J. 1980 Apr;30(1):181–186. doi: 10.1016/S0006-3495(80)85086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Yankaskas J., Cheng E., Knowles M. R., Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985 Aug;132(2):311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- Yankaskas J. R., Cotton C. U., Knowles M. R., Gatzy J. T., Boucher R. C. Culture of human nasal epithelial cells on collagen matrix supports. A comparison of bioelectric properties of normal and cystic fibrosis epithelia. Am Rev Respir Dis. 1985 Dec;132(6):1281–1287. doi: 10.1164/arrd.1985.132.6.1281. [DOI] [PubMed] [Google Scholar]
- Yankaskas J. R., Knowles M. R., Gatzy J. T., Boucher R. C. Persistence of abnormal chloride ion permeability in cystic fibrosis nasal epithelial cells in heterologous culture. Lancet. 1985 Apr 27;1(8435):954–956. doi: 10.1016/s0140-6736(85)91728-3. [DOI] [PubMed] [Google Scholar]



