Abstract
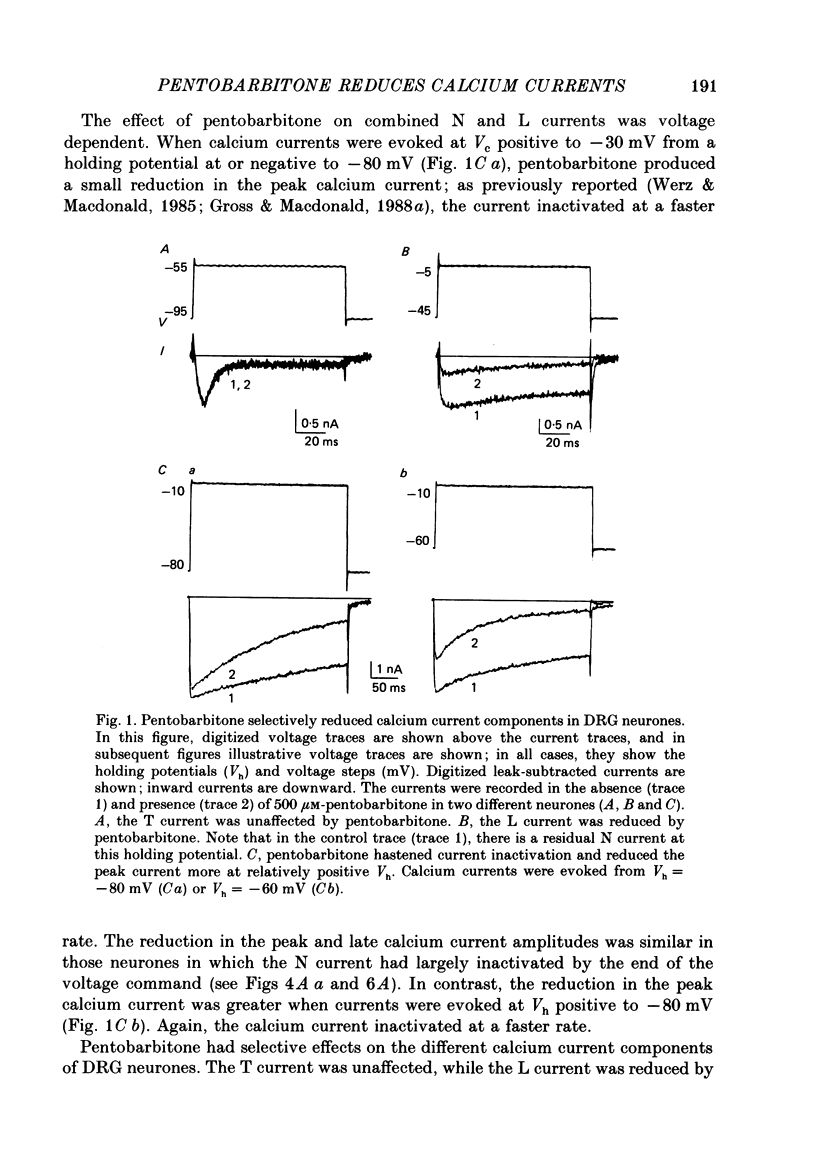
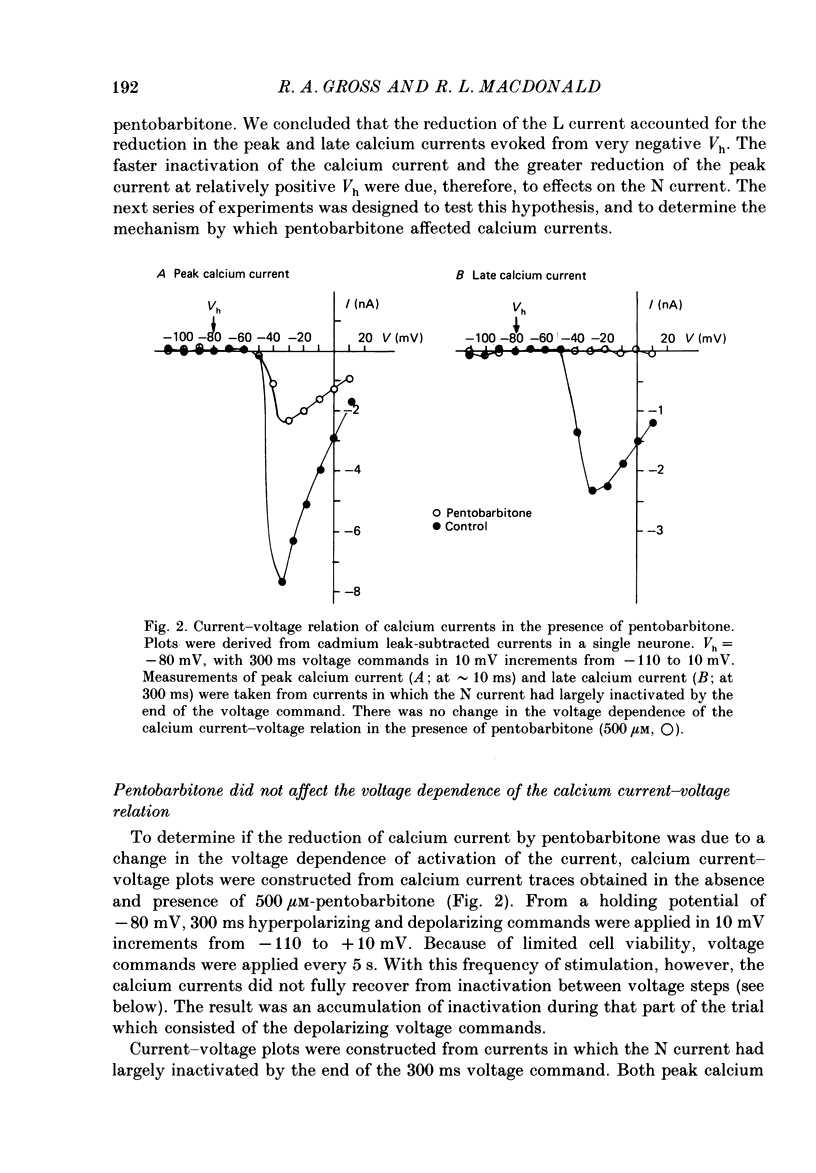
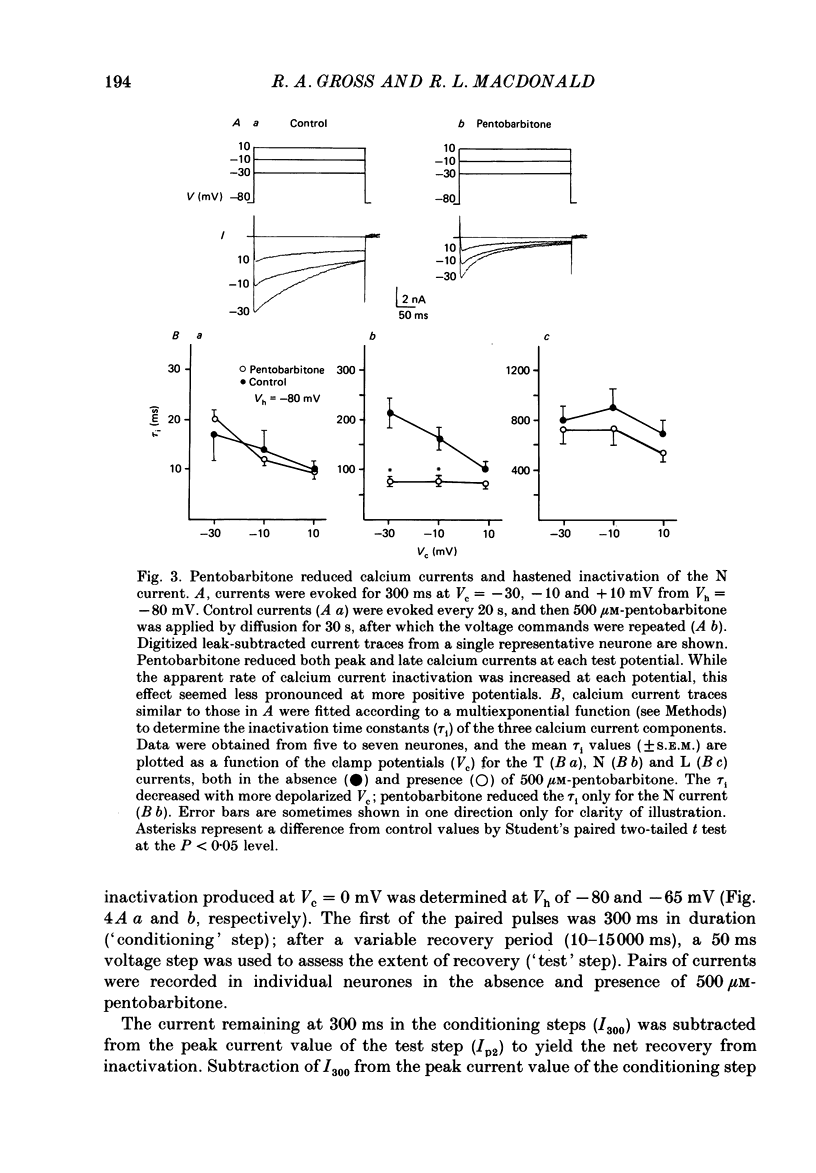
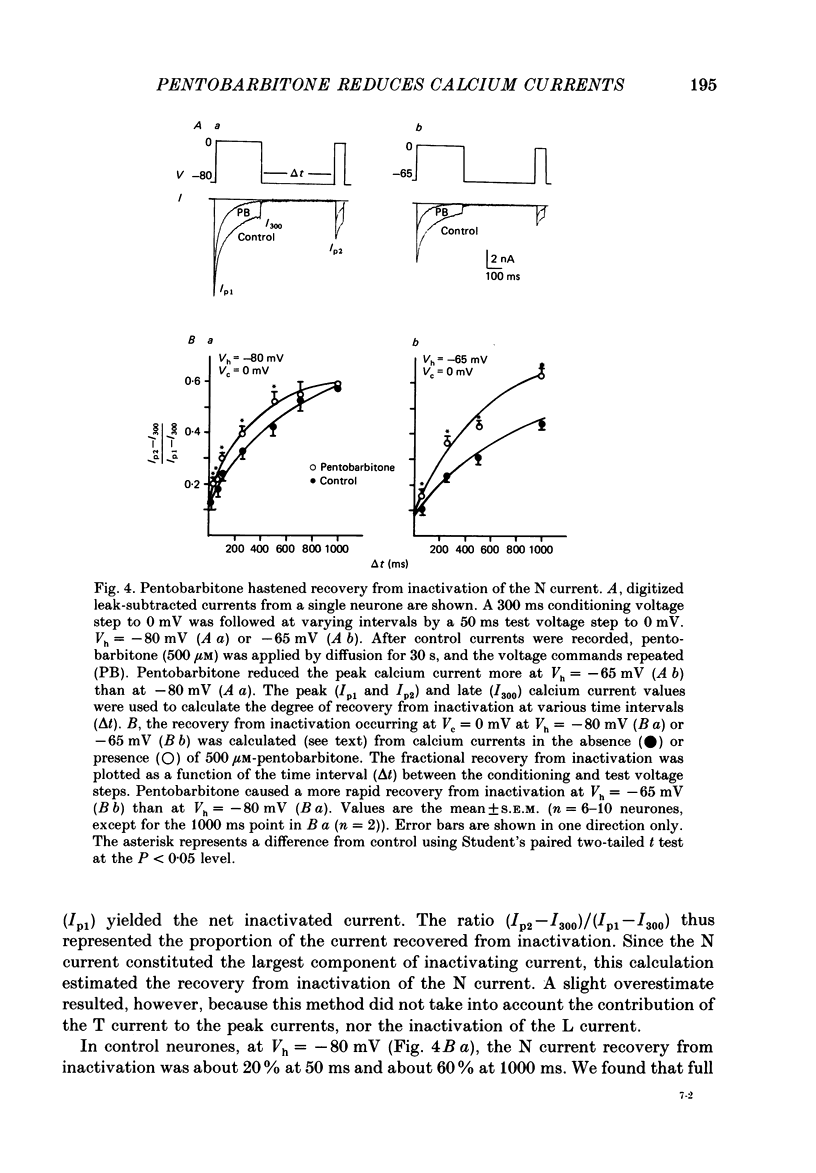
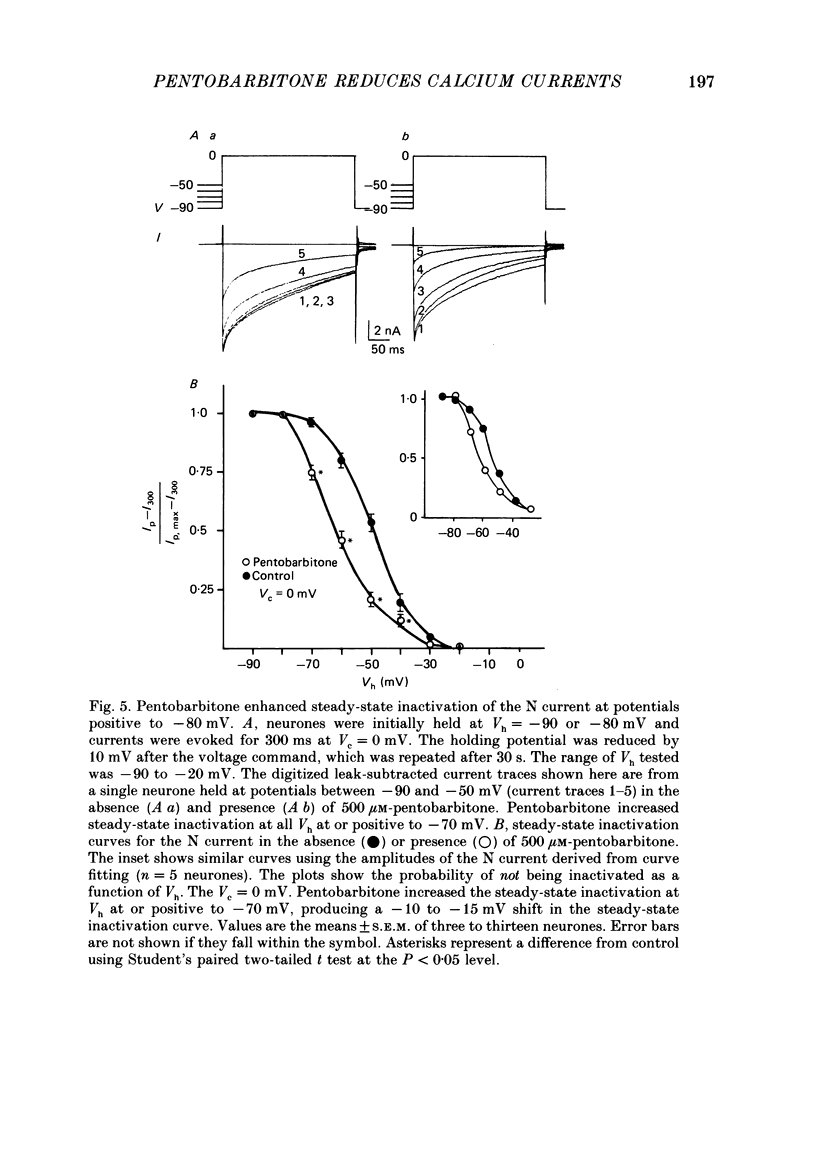
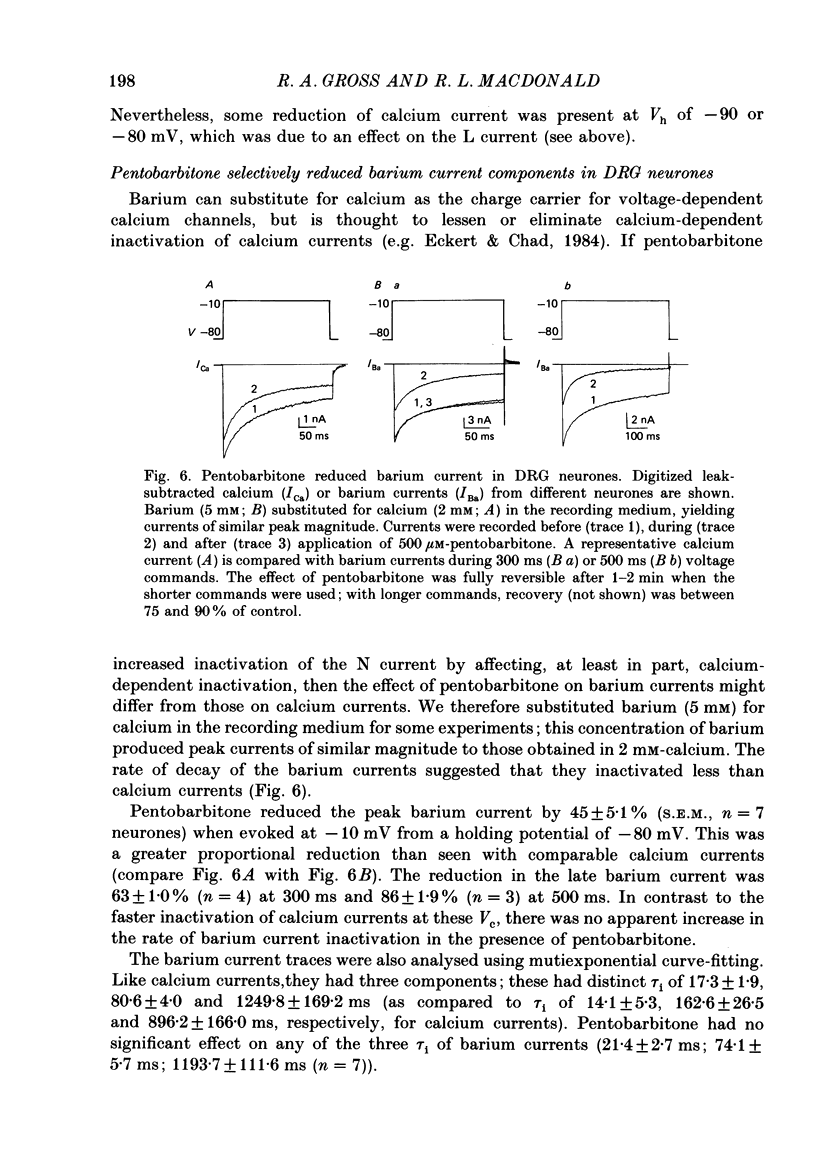
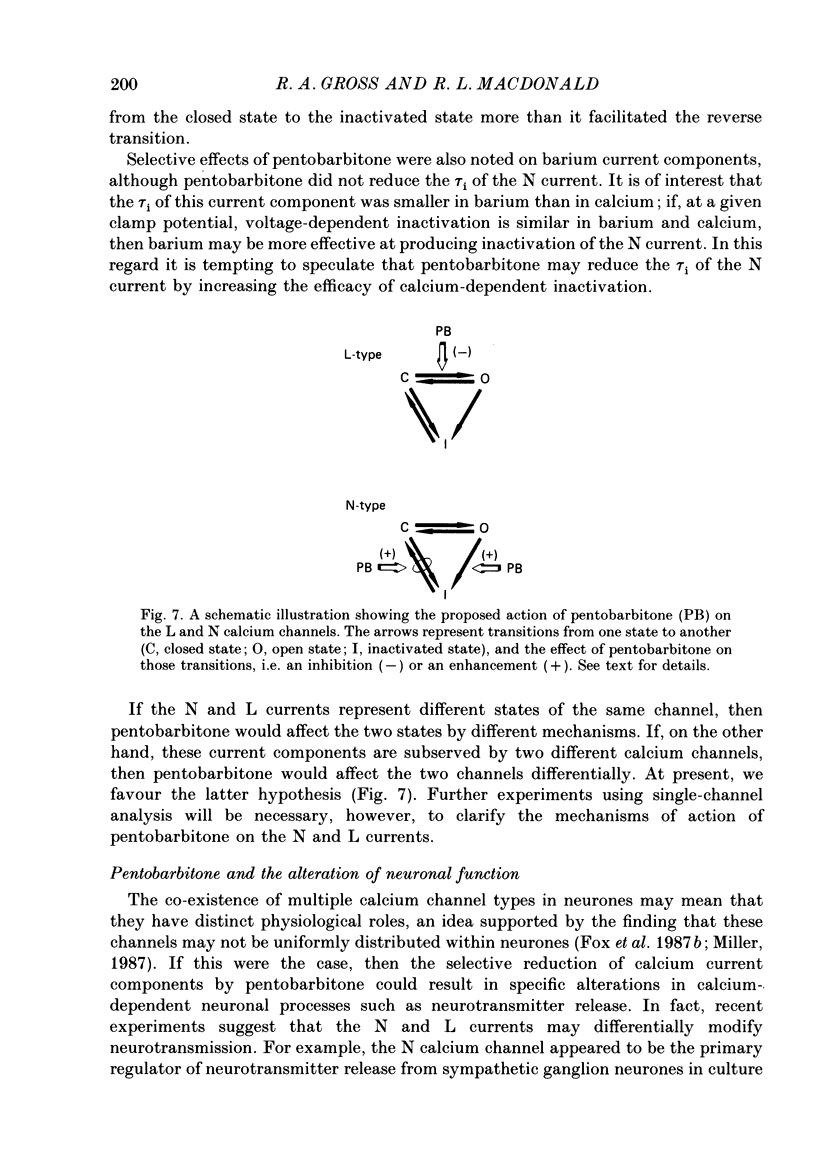
1. Using the single-electrode voltage clamp technique, three calcium current components were recorded at 35 degrees C from mouse dorsal root ganglion (DRG) neurones in culture. A transient low-threshold calcium current (T current) was recorded at clamp potentials (Vc) positive to -60 mV. Holding potentials (Vh) at or negative to -90 mV were required to fully remove inactivation. A large transient high-threshold calcium current component (N current) was recorded at Vc positive to -40 mV. Vh at or negative to -80 mV removed all steady-state inactivation. A slowly inactivating high-threshold calcium current component (L current) was recorded at Vc positive to -30 mV. Inactivation was removed by Vh at or negative to -60 mV. When currents were evoked at Vc positive to -20 mV from Vh negative to -60 mV, all three calcium current components were present. 2. Pentobarbitone (500 microM) had no effect on the isolated T current, but reduced the isolated L current 50-100% when evoked at Vc of -20 to 0 mV from Vh of -50 mV. Pentobarbitone had voltage-dependent effects on calcium currents containing all three calcium current components. Pentobarbitone produced small and equal reductions of the peak and late (greater than or equal to 300 ms) calcium currents evoked at -20 to 0 mV from Vh at or negative to -80 mV, but at more positive Vh there was a greater reduction in the peak current. The rate of current inactivation was increased in the presence of pentobarbitone. 3. Current-voltage plots were constructed from currents recorded in the absence and presence of 500 microM-pentobarbitone. Pentobarbitone reduced the magnitude of the calcium current without affecting the voltage dependence of the current-voltage relation. 4. Calcium current traces were fitted with a multiexponential function to determine the amplitudes and inactivation time constants (tau i) of the three calcium current components. Inactivation time constants decreased with more positive Vc for all three calcium current components. Pentobarbitone reduced only those tau i corresponding to the N current. 5. Recovery from inactivation of the N current was determined using a two-pulse protocol. In control neurones, recovery from inactivation occurring at 0 mV was slower at Vh = -65 mV than at Vh = -80 mV. In the presence of pentobarbitone, recovery from inactivation was faster, and occurred at a similar rate at both potentials. 6. Steady-state inactivation curves for the N current were derived from neurones in the absence and presence of pentobarbitone.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Barbiturate inhibition of calcium uptake by depolarized nerve terminals in vitro. Mol Pharmacol. 1975 May;11(3):369–378. [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Single low-voltage-activated calcium channels in chick and rat sensory neurones. J Physiol. 1987 May;386:571–601. doi: 10.1113/jphysiol.1987.sp016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRiemer S. A., Strong J. A., Albert K. A., Greengard P., Kaczmarek L. K. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985 Jan 24;313(6000):313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W. Evidence for two voltage-dependent calcium currents in the membrane of the ciliate Stylonychia. J Physiol. 1984 Oct;355:137–159. doi: 10.1113/jphysiol.1984.sp015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Adams P. R. Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res. 1982 Jul 22;244(1):135–144. doi: 10.1016/0006-8993(82)90911-8. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Barbiturates and nifedipine have different and selective effects on calcium currents of mouse DRG neurons in culture: a possible basis for differing clinical actions. The 1987 S. Weir Mitchell award. Neurology. 1988 Mar;38(3):443–451. doi: 10.1212/wnl.38.3.443. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5469–5473. doi: 10.1073/pnas.84.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Reduction of the same calcium current component by A and C kinases: differential pertussis toxin sensitivity. Neurosci Lett. 1988 May 16;88(1):50–56. doi: 10.1016/0304-3940(88)90314-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Barbiturate reduction of calcium-dependent action potentials: correlation with anesthetic action. Brain Res. 1982 Mar 18;236(1):157–171. doi: 10.1016/0006-8993(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Ikemoto Y., Mitsuiye T., Ishizuka S. Reduction of the voltage-dependent calcium current in Aplysia neurons by pentobarbital. Cell Mol Neurobiol. 1986 Sep;6(3):293–305. doi: 10.1007/BF00711115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Mills J. S. Calmodulin. Med Res Rev. 1986 Jul-Sep;6(3):341–363. doi: 10.1002/med.2610060304. [DOI] [PubMed] [Google Scholar]
- Kalant H., Grose W. Effects of ethanol and pentobarbital on release of acetylcholine from cerebral cortex slices. J Pharmacol Exp Ther. 1967 Dec;158(3):386–393. [PubMed] [Google Scholar]
- Leslie S. W., Friedman M. B., Wilcox R. E., Elrod S. V. Acute and chronic effects of barbituartes on depolarization-induced calcium influx into rat synaptosomes. Brain Res. 1980 Mar 10;185(2):409–417. doi: 10.1016/0006-8993(80)91078-1. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. L., Skerritt J. H., Werz M. A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J Physiol. 1986 Jan;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Werz M. A. Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J Physiol. 1986 Aug;377:237–249. doi: 10.1113/jphysiol.1986.sp016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Bryant S. H. Pentobarbital: presynaptic effect in the squid giant synapse. Experientia. 1977 Apr 15;33(4):487–488. doi: 10.1007/BF01922226. [DOI] [PubMed] [Google Scholar]
- Nishi K., Oyama Y. Accelerating effects of pentobarbitone on the inactivation process of the calcium current in Helix neurones. Br J Pharmacol. 1983 Jul;79(3):645–654. doi: 10.1111/j.1476-5381.1983.tb10001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Hirning L. D., Leeman S. E., Miller R. J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6656–6659. doi: 10.1073/pnas.83.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S. G., Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986 Jan;83(1):184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Strong J. A., Fox A. P., Tsien R. W., Kaczmarek L. K. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987 Feb 19;325(6106):714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Barbiturates decrease voltage-dependent calcium conductance of mouse neurons in dissociated cell culture. Mol Pharmacol. 1985 Sep;28(3):269–277. [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Dual actions of phorbol esters to decrease calcium and potassium conductances of mouse neurons. Neurosci Lett. 1987 Jul 9;78(1):101–106. doi: 10.1016/0304-3940(87)90569-6. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Phorbol esters: voltage-dependent effects on calcium-dependent action potentials of mouse central and peripheral neurons in cell culture. J Neurosci. 1987 Jun;7(6):1639–1647. doi: 10.1523/JNEUROSCI.07-06-01639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



