Abstract
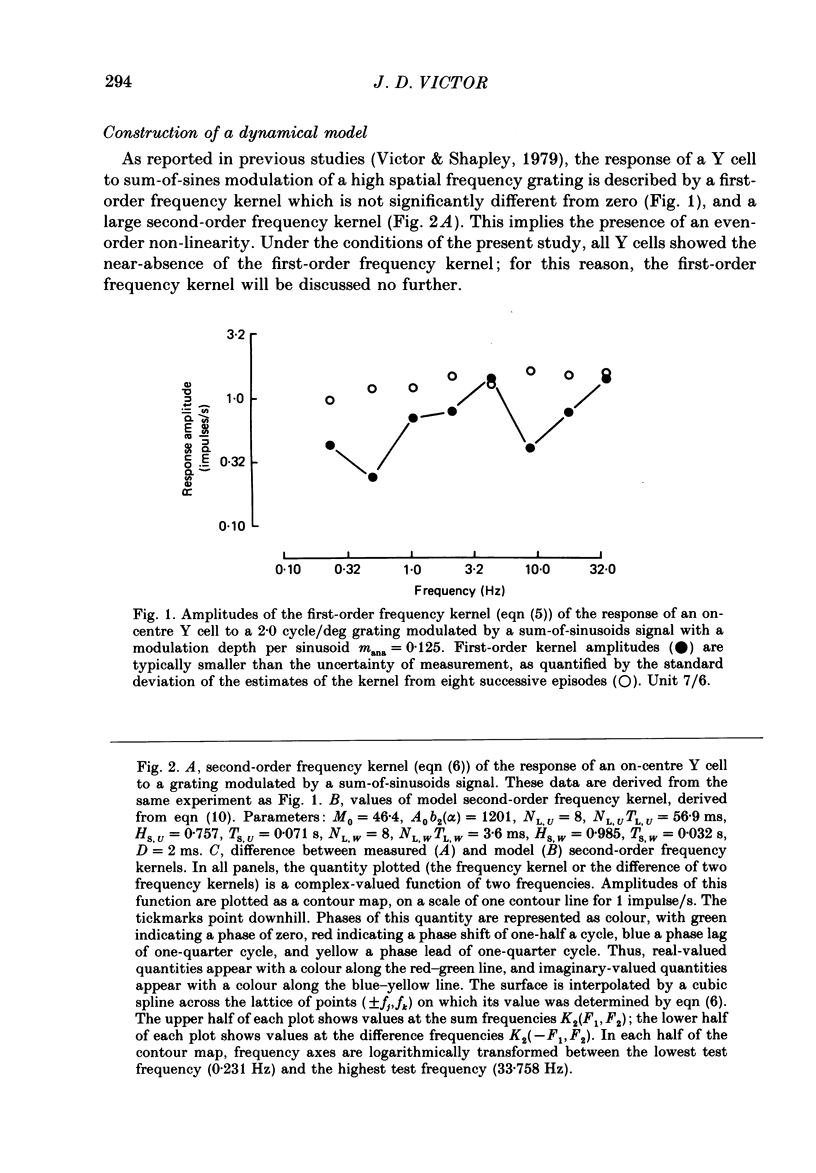
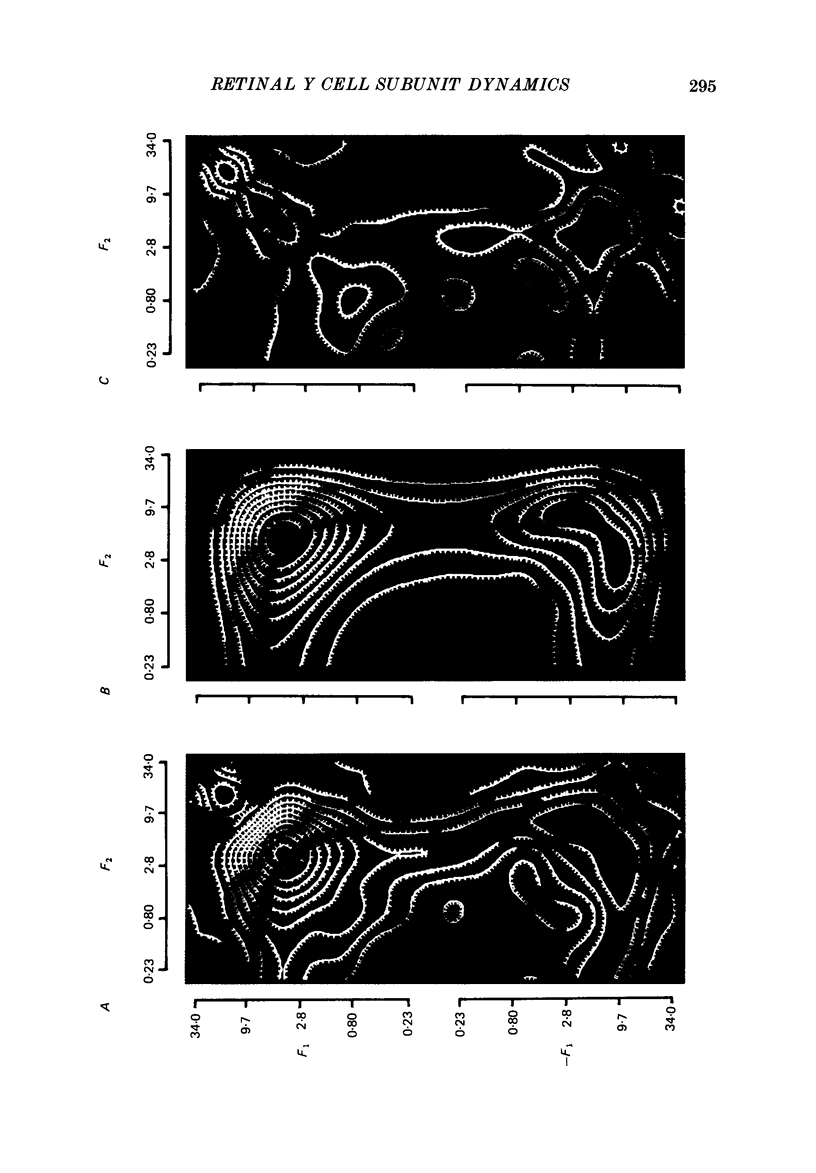
1. The dynamics of the subunit mechanism of individual cat Y retinal ganglion cells are investigated. In order to isolate the response of the non-linear subunit mechanism, the visual stimuli were sine gratings of a spatial frequency sufficiently high so that contrast reversal of the grating elicited no fundamental response in any spatial phase. For study of the non-linear subunit mechanism, the contrast of the spatial spine grating was varied in time by a temporal modulation signal, consisting of either a square wave or a sum of sinusoids. 2. The responses of twenty-three Y ganglion cells (sixteen on-centre, seven off-centre) to these two stimulus types were measured at a range of contrasts. Responses to the sum-of-sinusoids signal were characterized by the second-order frequency kernel. The overall size of the second-order frequency kernel was approximately proportional to contrast. The deviation from proportionality suggested a power-law scaling, with a power in the range 0.8-0.9. 3. Square-wave responses, as characterized by the post-stimulus histogram, demonstrated identical responses at both reversals of the grating. A similar contrast dependence was observed in the overall size of the square-wave responses. 4. In order to attempt to predict the square-wave responses from the sum-of-sinusoids responses, the second-order frequency kernel measured at each contrast level was fitted with a lumped linear-static non-linear-linear model. In eighteen of twenty-three cells (eleven on-centre, seven off-centre), this model provided an adequate description of the response to the sum-of-sinusoids stimulus. In these cells, the linear-static non-linear-linear model accurately reproduced the square-wave response. 5. In the remaining five ganglion cells (all on-centre), the second-order frequency kernel could not be fitted by a linear-static non-linear-linear model. This diversity of dynamical properties among Y cells was not apparent from the responses of these Y cells to the square-wave temporal stimulus. 6. In the eighteen Y ganglion cells that were fitted well with the linear-static non-linear-linear model, substantial variation of the dynamical parameters was found. However, there were systematic differences between the dynamics of the typical on-centre and off-centre ganglion cells. These differences relate to both linear stages of the model, and are not merely consequences of the lower firing rate of the off-centre cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





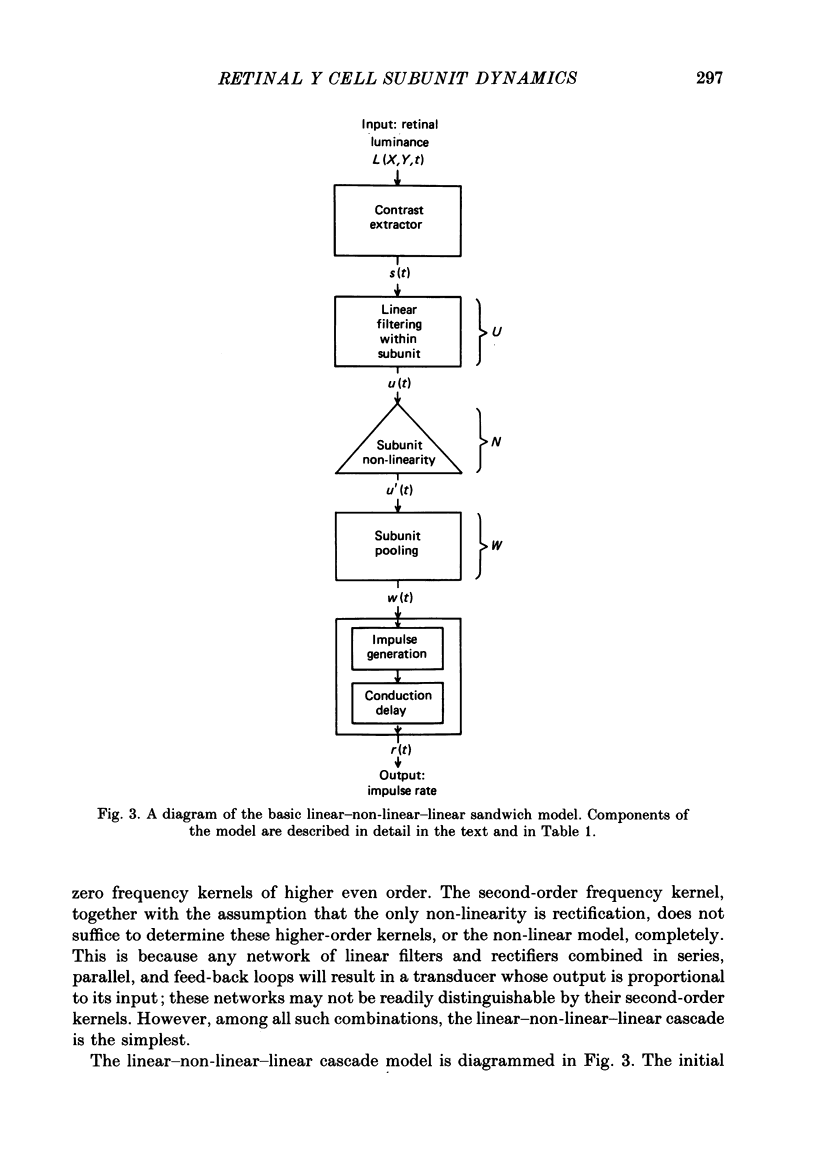



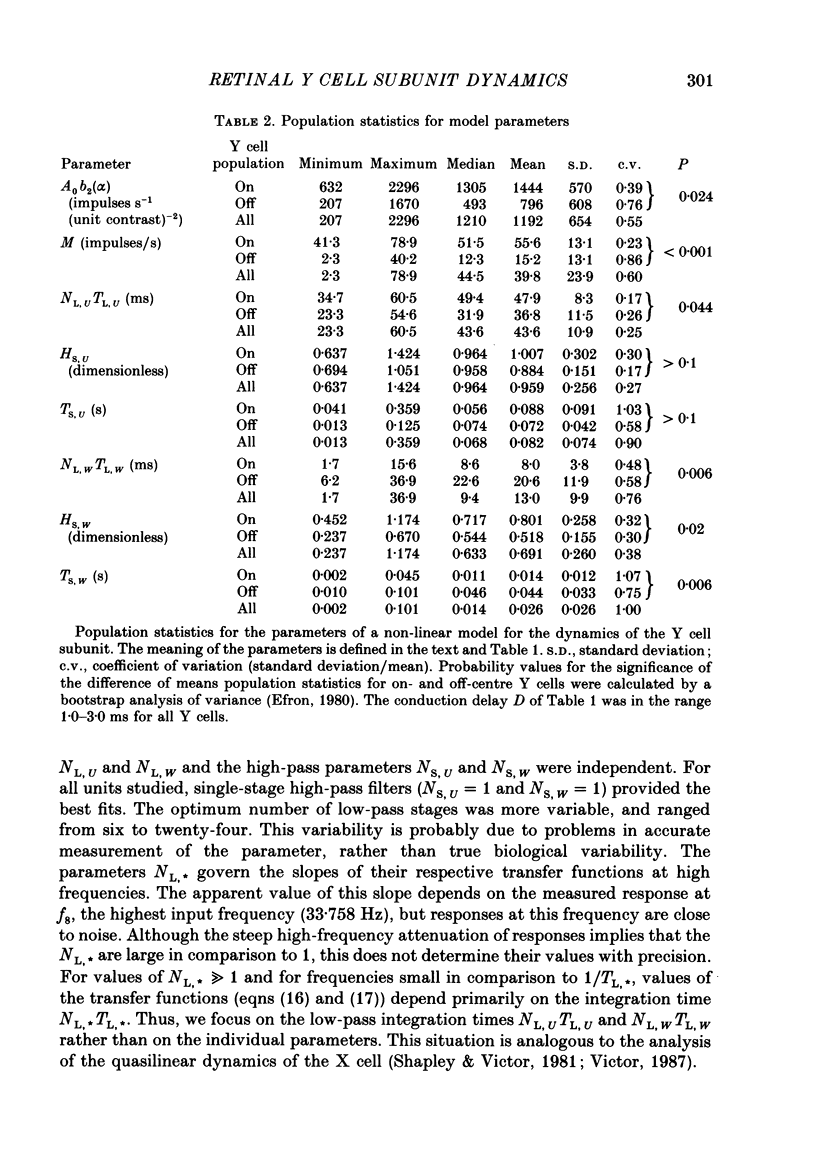

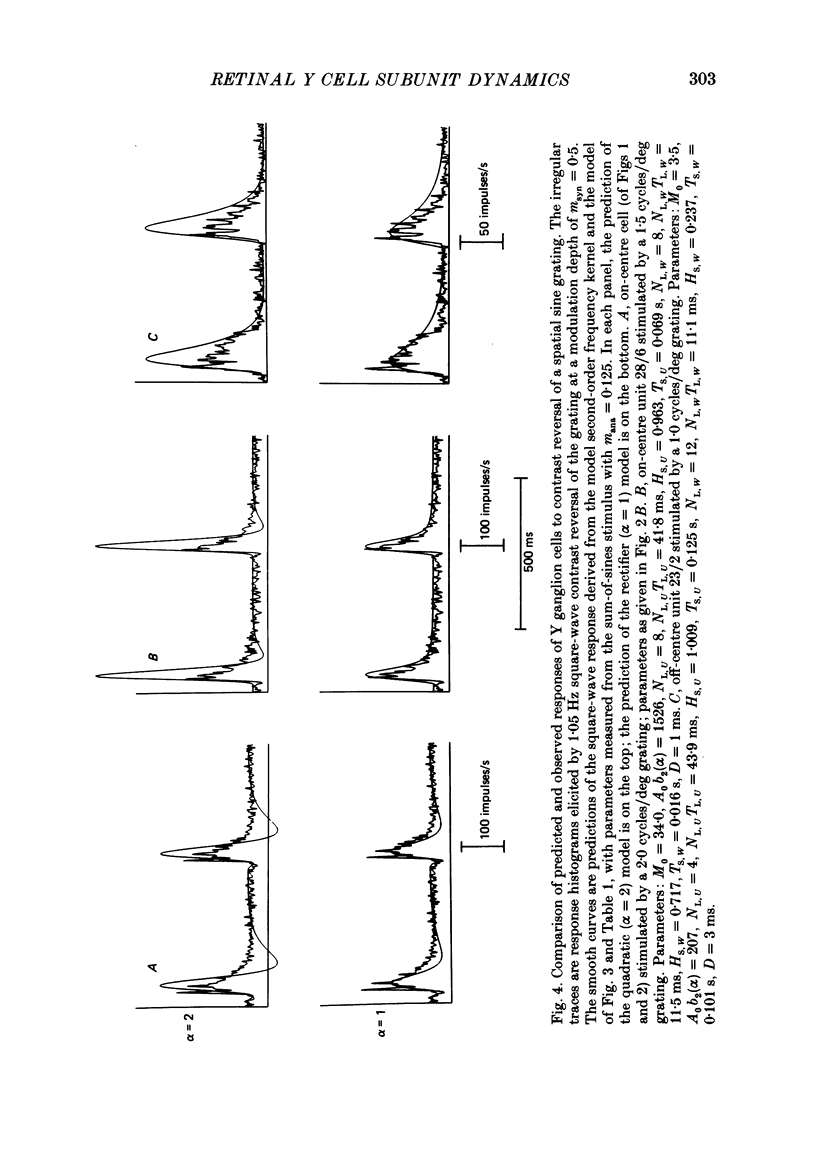

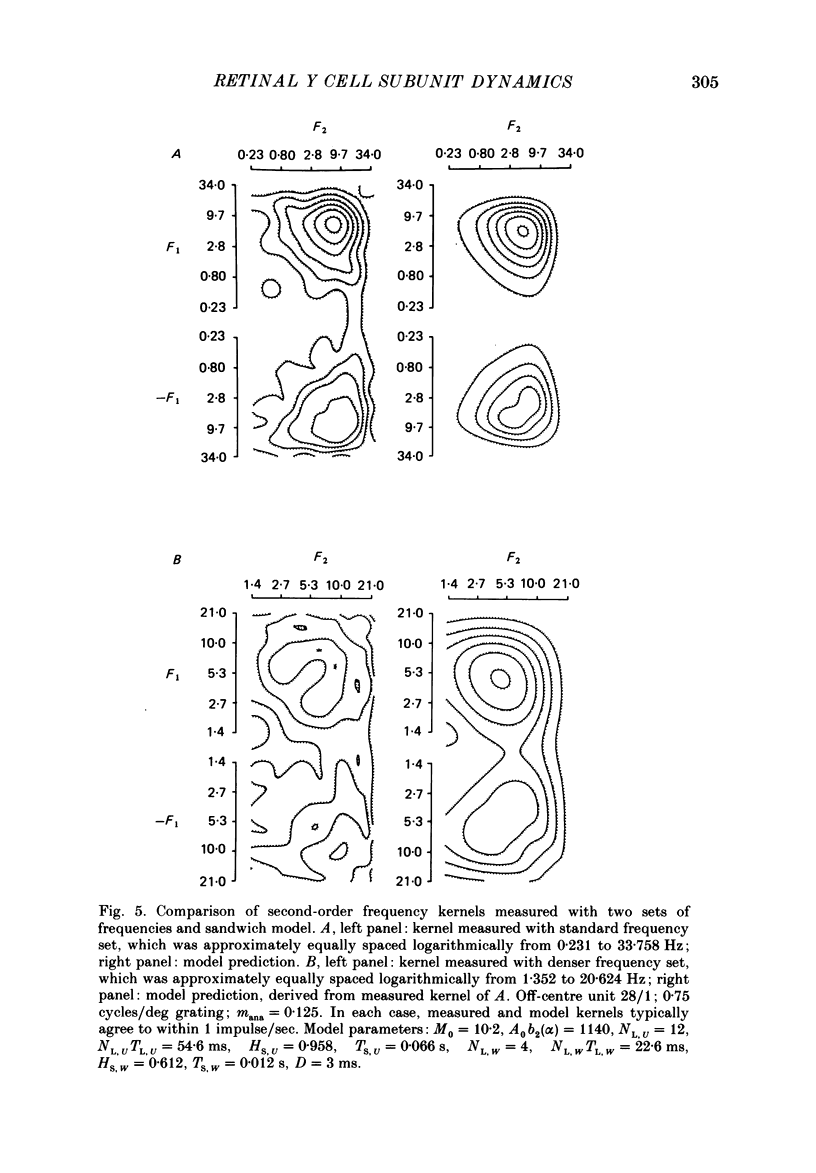




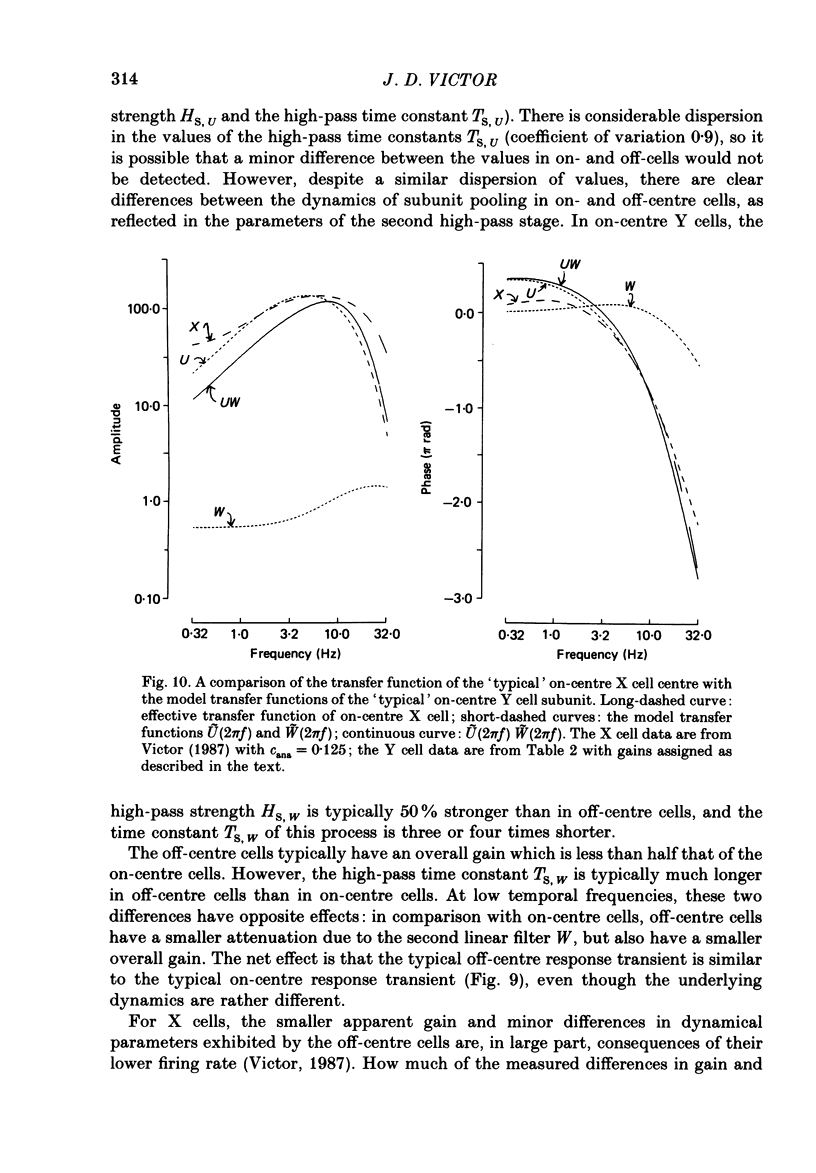
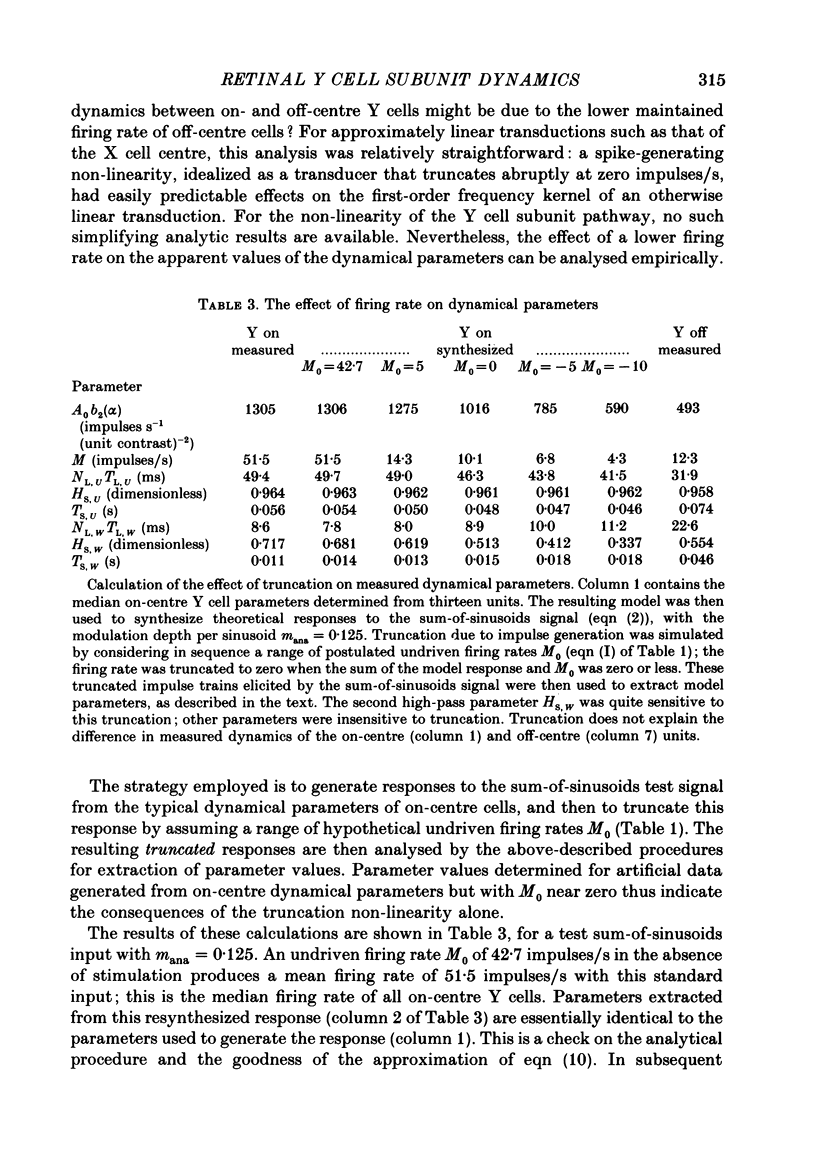





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. The origin of the S (slow) potential in the mammalian lateral geniculate nucleus. Exp Brain Res. 1984;55(1):111–116. doi: 10.1007/BF00240504. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20(7):561–594. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- McGuire B. A., Stevens J. K., Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984 Dec;4(12):2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K., Marmarelis P. Z., Chan R. Y. Morphological and functional identifications of catfish retinal neurons. III. Functional identification. J Neurophysiol. 1975 Jan;38(1):92–131. doi: 10.1152/jn.1975.38.1.92. [DOI] [PubMed] [Google Scholar]
- Nelson R., Famiglietti E. V., Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978 Mar;41(2):472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. How the contrast gain control modifies the frequency responses of cat retinal ganglion cells. J Physiol. 1981 Sep;318:161–179. doi: 10.1113/jphysiol.1981.sp013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the non-linear response of the Y cell. J Physiol. 1980 May;302:535–547. doi: 10.1113/jphysiol.1980.sp013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978 Dec;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Otsu K. Bipolar-amacrine transmission in the carp retina. Vision Res. 1973 Feb;13(2):295–307. doi: 10.1016/0042-6989(73)90108-9. [DOI] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M. The nonlinear pathway of Y ganglion cells in the cat retina. J Gen Physiol. 1979 Dec;74(6):671–689. doi: 10.1085/jgp.74.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J. D. The dynamics of the cat retinal X cell centre. J Physiol. 1987 May;386:219–246. doi: 10.1113/jphysiol.1987.sp016531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J., Shapley R. A method of nonlinear analysis in the frequency domain. Biophys J. 1980 Mar;29(3):459–483. doi: 10.1016/S0006-3495(80)85146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]