Abstract
Periodontitis, a prevalent and costly oral disease, remains incompletely understood in its etiopathogenesis. The conventional model attributes it to pathogenic bacteria, but emerging evidence suggests dysbiosis involving bacteria, herpesviruses, and an exaggerated host immune response. Among herpesviruses, Epstein-Barr virus (EBV) closely links to severe periodontitis, yet the mechanisms underlying EBV-related pathogenesis remain elusive. This study examined the presence, methylation patterns, and infection states of EBV in gingival tissues from healthy patients and those with periodontitis. It also assessed gene expression differences associated with EBV through whole-genome transcriptomic profiling in healthy and periodontitis-affected tissues. EBV DNA was found at similar frequencies in healthy and periodontitis tissues, suggesting common EBV infection even before disease manifestation. In healthy tissues, mostly unmethylated EBV genomes indicated lytic infection in gums, consistent with the literature on lytic EBV spread in epithelia and continual significant virus release in the saliva of healthy carriers. Conversely, EBV DNA in periodontitis tissues showed both methylated and unmethylated patterns, suggesting a mix of latent and lytic genomes. This indicates the coexistence of latent EBV in B-cells and lytic EBV in plasma cells (PCs), linking EBV presence with both cell types in periodontitis. Whole-genome transcriptomic analysis revealed distinct expression profiles in EBV-positive periodontitis tissues, with upregulated genes associated with inflammatory/immune responses and B-cell and PC markers, while downregulated genes were related to epithelial structure and organization. The EBV-positive periodontitis signature differed distinctly from that of EBV-positive healthy gums, eliciting only a typical viral-induced immune response. These findings provide new insights into EBV physiopathology in the gum, notably assigning a direct etiopathogenetic contribution to EBV in periodontitis. The results suggest a model where EBV can commonly, and apparently asymptomatically, spread in healthy gingiva but may also aggravate inflammation in the context of gum dysbiosis, involving infiltration of B-cells and PCs and loss of epithelial integrity.
Keywords: periodontal diseases, periodontium, herpesvirus 4, host-pathogen interactions, inflammation, plasma cells
Introduction
Periodontitis, an infection-driven inflammatory disease, damages tooth-supporting tissues, causing tooth loss, masticatory dysfunction, nutritional deficiencies, and reduced quality of life (Könönen et al. 2019). Linked to >50 systemic diseases (Beck et al. 2019) and various cancers (Michaud et al. 2018), periodontitis is a significant global health burden with >1 billion severe periodontitis cases worldwide (Jain et al. 2024). Current treatments offer only stabilization, highlighting the need for better solutions.
Despite its impact, the etiopathogenesis of periodontitis remains unclear. The conventional model blames bacteria, but an emerging hypothesis suggests complex interactions among bacteria, host immune responses, and human herpesviruses (HHVs; Slots 2015). Notably, Epstein-Barr virus (EBV) has been detected in periodontal lesions at varying levels, depending on the severity of periodontitis and detection procedures (Slots 2015; Tonoyan et al. 2021). It is plausible that bacteria and HHVs collaborate in periodontal lesions to exacerbate immune responses and damage the periodontium (Slots 2007; Chen et al. 2020), gaining credibility with increasing evidence of viral-bacterial synergy in various diseases (Zhang et al. 2024). These polymicrobial synergisms offer new insights into pathogenesis and therapies.
EBV, a prevalent γ-HHV, infects approximately 95% of people asymptomatically, primarily targeting B-cells and oral epithelial cells (ECs) via saliva transmission (Thorley-Lawson 2001). It establishes latent infection in B-cells, with sporadic lytic reactivation associated with terminal B-cell differentiation into plasma cells (PCs; Thorley-Lawson 2015). Long-term latent EBV infection is linked to lymphomas and carcinomas (Ayee et al. 2020). EBV is also implicated in several autoimmune diseases (Houen and Trier 2021), including as a direct culprit in multiple sclerosis (Bjornevik et al. 2022).
Our prior research uncovered significant insights into EBV’s role in periodontitis. We found that EBV commonly infected periodontal junctional ECs (Vincent-Bugnas et al. 2013; Tonoyan et al. 2024). Moreover, while it was established that periodontitis lesions contain numerous PCs (Li et al. 2020), we showed that these PCs were mostly EBV positive (Olivieri et al. 2020). These studies marked the first evidence of active EBV presence in periodontal lesions, suggesting that it worsens local inflammation and contributes to periodontitis pathogenesis. However, is EBV the primary suspect?
This study aims to detail differential gene expression signatures and related pathways linked to EBV presence in both periodontitis-affected and healthy gingiva, providing foundational insight into EBV’s role in periodontitis.
Materials and Methods
Donors, Gingival Tissue Sampling, and Nucleic Acid Extraction
Biomedical sample collection was conducted under DC-2022-5040 as approved by the Comité de Protection des Personnes Sud Méditerannée, and informed consent was obtained from all participants.
A total of 54 participants were recruited: 27 patients with periodontitis and 27 healthy controls (Appendix Table 1). Periodontitis diagnosis was based on established criteria (Caton et al. 2018). Patients with periodontitis displayed tooth sites with probing depth ≥6 mm, clinical attachment level ≥5 mm, and bleeding on probing. Healthy controls exhibited no signs of periodontal disease or gum inflammation.
Periodontitis-affected tissues were collected as surgical waste during periodontal therapy. Healthy gingival tissues were collected from the area just posterior to the second molar at the maxillary tuberosity region by carefully elevating the sulcus epithelium and periodontal attachment with a periosteal elevator in healthy donors. These tissues were considered surgical waste during gingival graft procedures for aesthetic purposes.
Tissues were homogenized and total RNA/DNA extracted with Qiagen kits. The concentration and purity of DNA/RNA were assessed by a SimpliNano spectrophotometer (Biochrom), and RNA integrity was confirmed with an Agilent Bioanalyzer. RNA samples with an RNA integrity number (RIN) ≥6 were selected for transcriptomic analysis.
EBV Detection
Extracted DNA was tested for the presence and quantity of EBV load via the Argene EBV quantification kit (Biomérieux), according to the manufacturer’s instructions. Following the EBV detection, all patient samples were categorized as EBV-negative and EBV-positive.
Methylation-Specific Quantitative Polymerase Chain Reaction for Latent vs Lytic EBV
EBV-positive samples (Appendix Tables 1 and 2) underwent methylation-specific quantitative polymerase chain reaction (methyl-qPCR) to assess methylation levels, distinguishing latent and lytic states (Borde et al. 2022).
Transcriptome Microarrays
Sixteen tissue samples underwent transcriptomic analysis: 4 healthyEBV-negative (H_NEG), 4 healthy EBV-positive (H_EBV), 4 periodontitis EBV-negative (P_NEG), and 4 periodontitis EBV-positive (P_EBV). Briefly, RNA was transcribed to cDNA and labeled by the single-color Low Input Quick Amp Labeling Kit (Agilent). Labeled cRNA was hybridized onto SurePrint G3 Human Gene Expression (8 × 60 K) microarrays, with fluorescent signals detected by Agilent’s scanner. Data were analyzed with limma and normalized by the quantile method. Pathway analysis was conducted by Ingenuity Pathway Analysis (IPA; Qiagen; Fall Release 2022) software. The microarray data are deposited in the GEO database (GSE223328).
High-Throughput Microfluidics-Based Reverse Transcription–qPCR for Target Gene Validation
Sixteen samples from the microarray assay and 16 new independent samples (Appendix Table 1) were used to validate the transcriptomic microarray results. Selected genes were further analyzed by microfluidics-based reverse transcription–qPCR (RT-qPCR) technology on the Biomark system (Standard BioTools), following the manufacturer’s instructions and previously described methods (Fassy et al. 2021). Thirty-one genes related to tissue markers, 30 immune and inflammatory genes, 5 B-cell and 6 PC markers, and 2 reference genes (GAPDH and RPLP0) were selected to confirm the expression patterns observed with microarray.
Statistics
EBV prevalence percentage and 95% confidence intervals (CIs) were calculated via the Clopper-Pearson method. A chi-square (χ2) test assessed EBV’s association with periodontitis. Differences in viral loads were evaluated with a Mann-Whitney U test. Pearson correlation was used for expression correlation between microarray and RT-qPCR. Two-way analysis of variance (ANOVA) with a Tukey’s test analyzed gene expression differences between P_EBV and P_NEG, with normality assessed via a Kolmogorov-Smirnov test. P < 0.05 was considered statistically significant.
See Appendix methods for more details.
Results
Prevalence of EBV in Health and Periodontitis
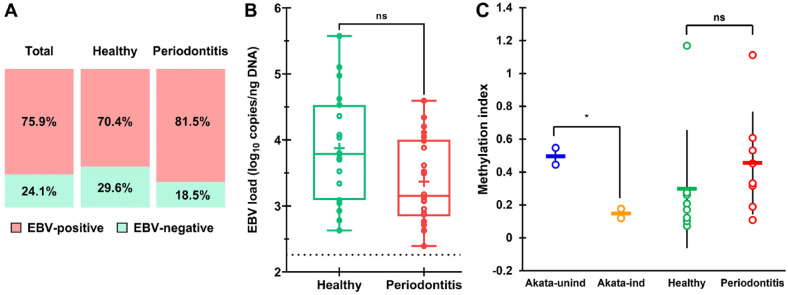
EBV was detected in 75.9% (95% CI, 62.4%–86.5%) of gingival tissue samples among total participants (Fig. 1A). EBV prevalence was slightly higher in periodontitis tissues than in healthy controls (81.5% vs 70.4%) but was not statistically different (P = 0.34). EBV loads in EBV-positive tissues varied (Appendix Table 1), with median loads of 3.8 and 3.2 log10 copies/ng of DNA in healthy and periodontitis states, respectively, showing no significant difference between the conditions (U = 136, P = 0.057; Fig. 1B). EBV quantities varied across periodontitis stages and grades, but differences were not statistically significant (Appendix Figure 1).
Figure 1.
Presence of EBV in periodontitis and healthy gingiva. (A) Prevalence of EBV in study participants (total, healthy, and periodontitis). (B) Comparison of the EBV load (log10 copies/ng of DNA) in healthy (n = 19) and periodontitis (n = 22) cases that tested positive in both samples. (C) Mean methylation index in uninduced (latent) and anti-IgG–induced (lytic) Akata cells; in tissues of periodontally healthy (n = 8) and periodontitis patients (n = 8). Each dot represents one individual patient. Data are expressed as (B) median (interquartile range (IQR); 95% CI) and (C) mean ± SD. *P < 0.05. EBV, Epstein-Barr virus; ns, not significant.
EBV genome methylation levels were examined to discern their latent or lytic states. For validation, methylation levels were assessed in EBV-infected Akata cells during latency and lytic reactivation induced by antisurface IgG. The mean methylation index dropped from 0.5 in uninduced Akata cells to 0.15 in induced ones (Fig. 1C), consistent with the findings of Borde et al. (2022). These results confirm that methyl-qPCR distinguishes latent from lytic states. In EBV-positive healthy patients, a low average methylation index indicated predominantly lytic EBV, with only 1 patient showing a high index, suggesting latent EBV (Fig. 1C, Appendix Table 2). In contrast, patients with periodontitis had both methylated and unmethylated EBV genomes, indicating a mix of latent and lytic EBV.
Identification of Up- and Downregulated Genes
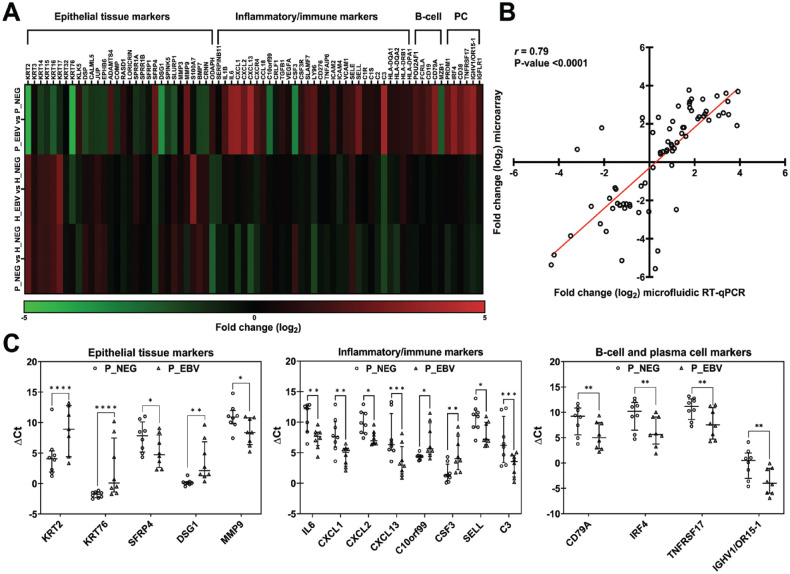
Microarray-based transcriptomic analysis of healthy and periodontitis-affected gingival tissues with and without EBV presence was conducted to understand EBV’s role in periodontitis. The microarray results were subjected to IPA analysis, and differentially expressed genes (DEGs) were identified. The highest number of DEGs was in EBV-infected periodontitis tissues (P_EBV vs P_NEG), followed by EBV-negative periodontitis tissues (P_NEG vs H_NEG) and EBV-infected healthy tissues (H_EBV vs H_NEG; Fig. 2A). These results imply that EBV could have caused an extra burden on the host in addition to the periodontal disease state itself.
Figure 2.
Overview of gene expression profiles. (A) Transcriptomic microarray analysis with the number of differentially expressed upregulated and downregulated genes in EBV-positive periodontitis tissues (P_EBV vs P_NEG), periodontally healthy EBV-positive tissues (H_EBV vs H_NEG), and gingival tissues affected by periodontal disease (P_NEG vs H_NEG). Log2 fold change |FC| ≥0.7 and P ≤ 0.05 cutoffs were implemented. (B) Significantly upregulated and downregulated genes identified through transcriptomic microarray analysis in EBV-positive periodontitis (P_EBV vs P_NEG), encompassing changes within epithelial tissue markers, inflammatory and immune response, as well as B-cell and plasma cell profiles. Red indicates upregulation, while green represents downregulation. (C) Venn diagram with top modulated genes for the 3 scenarios. Log2 |FC| ≥2 and P≤0.05 cutoffs were implemented. EBV, Epstein-Barr virus; H, healthy; NEG, negative; P, periodontitis.
When we examined EBV’s impact in periodontitis (P_EBV vs P_NEG; Fig. 2B; Appendix Data Set 1), significant upregulation occurred in inflammatory molecules (IL1B, IL6, CXCL1, CXCL2, CXCL13, CSF3, ICAM4), B-cell markers (MZB1, POU2AF1, CD19, FCRLA, FCRL5, CD79A, TCL1A), and PC markers (TNFRSF17, IRF4, PRDM1, CD38, and numerous immunoglobulin transcripts), indicating an intensified inflammatory/immune response. Conversely, genes involved in epithelial cell development, keratinization, and cell adhesion were downregulated (BMP7, CALML5, CDHR1, COMP, DLX3, DSG1, DSP, JUP, KLK5, KLK7, KLK11, KRT2, KRT3, KRT6B, KRT6C, KRT14, KRT15, KRT16, KRT17, KRT31, KRT32, KRT76, LORICRIN, SLURP1, SPRR1B, etc.), indicating epithelial tissue degradation and injury. This tissue degradation–associated cohort also included upregulated matrix metalloproteinases (MMP3, MMP9, ADAMTS4).
Second, when we investigated EBV’s impact in healthy gingiva (H_EBV vs H_NEG; Appendix Data Set 1), significantly upregulated DEGs featured S100A7, IGFLR1, and HLA-DQA1, which are associated with immune/inflammatory responses, with SERPINB11 (cellular protease protection) being the only downregulated DEG.
Third, as a control, we compared the states of health and periodontal disease in EBV-negative samples (P_NEG vs H_NEG; Appendix Data Set 1) and found that significantly upregulated DEGs included RPS4Y1 and RPS4Y2 ribosomal protein genes, THBS4 (cell-to-cell/matrix interactions), MT4 (regulates zinc metabolism in stratified epithelial differentiation), STMN2 (regulates osteogenesis), and SFRP2 (cell-cell signaling). Significantly downregulated genes were ANGPTL4 (negative regulation of endothelial cell apoptosis), PLAT (cell migration and tissue remodeling), and PADI1 (late stages of epidermal differentiation).
Overall, DEG analysis showed poor overlap across the 3 scenarios, indicating distinct patterns linked to EBV presence and/or absence in healthy and/or periodontitis-affected gum. Specifically, only 2 genes overlapped between P_EBV vs P_NEG and P_NEG vs H_NEG, being expressed in opposing directions (Fig. 2C).
Validation of Microarray Gene Expression by Microfluidics-Based RT-qPCR
The expression trends of selected genes identified as DEGs by microarray were validated by microfluidics-based RT-qPCR. Results confirmed downregulation of epithelial tissue markers and upregulation in inflammatory/immune response and B-cell and PC markers for P_EBV vs P_NEG (Fig. 3A). Pearson correlation analysis revealed a significant positive relationship between the expression levels obtained by both techniques (r[72] = 0.79, P < 0.001; Fig. 3B). Nearly all genes (93.6% tissue, 90% inflammatory/immune, 80% B-cell, and 100% PC profiles) exhibited regulation in the same direction (up or down) in P_EBV vs P_NEG as measured by both techniques. The RT-qPCR confirmed statistical significance for most of the top-modulated genes, spanning epithelial tissue, inflammatory/immune response, and B-cell and PC markers (Fig. 3C). These results validate the microarray analysis in a larger cohort using microfluidic RT-qPCR, demonstrating mutual support between the 2 methods.
Figure 3.
Validation of transcriptomic microarray results with microfluidics-based RT-qPCR. (A) Heat map generated from transcriptomic microfluidic RT-qPCR analysis comparing log2 FC levels of gene expressions within epithelial tissue markers, inflammatory/immune response, and B-cell and plasma cell profiles across 3 scenarios. Red implies upregulation; green, downregulation; and black, no changes in gene expression levels. (B) Gene expression correlation between microfluidic RT-qPCR and transcriptomic microarray data. The linear regression line, Pearson correlation coefficient (r), and P value are indicated. Each point corresponds to the log2 FC of a single gene. (C) Statistically significantly modulated genes from tissue markers, immune and inflammatory response, and B-cell and plasma cell profiles in P_EBV vs P_NEG scenario determined by microfluidic RT-qPCR. Scatter plots of ΔCt values (normalized according to 2 reference genes, GAPDH and RPLP0) of individual samples in P_NEG and P_EBV groups. Symbols represent individual participants (n = 8 in each group); midline, median; and error bars, IQR. Significant differences between P_EBV and P_NEG were tested by Tukey’s multiple-comparison test. *P < 0.05. **P < 0.001. ***P < 0.0001. ****P < 0.00001. EBV, Epstein-Barr virus; H, healthy; NEG, negative; P, periodontitis; PC, plasma cell; RT-qPCR, reverse transcription–quantitative polymerase chain reaction.
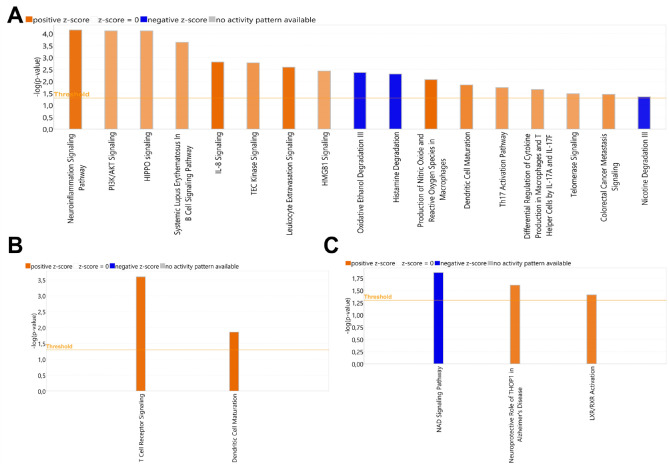
Identification of Canonical Pathways
IPA analysis was conducted to assess canonical pathway (CP) enrichments in each scenario (Appendix Data Set 2). In P_EBV vs P_NEG, 169 significant pathways were identified. The most pronounced activations (determined by z-score metric) were observed in pathways related to cellular immune response and cytokine signaling (Fig. 4A), which could lead to chronic inflammation, gingival tissue degradation, and necrosis. In H_EBV vs H_NEG, 54 significant CPs were identified. Only 2 CPs, both categorized under cellular immune response, were notably activated (Fig. 4B). In P_NEG vs H_NEG, among the 40 significantly involved CPs, the activated pathways included those related to neuroprotection and regulation of lipid metabolism and inflammation, while the inhibited pathway was associated with immune/inflammatory response (Fig. 4C). These findings suggest distinct pathway dynamics between periodontitis-affected and healthy gingival tissues in the absence or presence of EBV.
Figure 4.
Enriched canonical pathways. (A–C) Significant canonical pathways predicted to be activated or inhibited in P_EBV vs P_NEG, H_EBV vs H_NEG, and P_NEG vs H_NEG. The heights of the bars represent the significance level of the pathways based on the right-tailed Fisher’s exact test scored as −log(P value) ≥1.3, which is depicted by the orange threshold line. Shades of orange or blue indicate that the pathway is activated (z-score ≥2) or inhibited (z-score ≤2), respectively. EBV, Epstein-Barr virus; H, healthy; NEG, negative; P, periodontitis.
Discussion
Identifying true pathogens and the underlying biological processes during the transition from periodontal health to disease remains elusive. Transcriptomic analysis has emerged as a leading method for studying diseases at the molecular level and uncovering mechanisms. Several studies have performed transcriptomic analyses on gingival tissues from patients with periodontitis and reported gene expression variations in periodontitis-affected vs healthy tissues related to inflammation, antimicrobial humoral response, antigen presentation, leukocyte transendothelial migration, cell communication, apoptosis, tissue degradation, response to wound healing, proliferation, regulation of metabolic processes, signal transduction, angiogenesis, etc. (Demmer et al. 2008; Abe et al. 2011; Davanian et al. 2012; Becker et al. 2014; Lundmark et al. 2015; Kim et al. 2016). Papapanou et al. (2009) even coupled 2 key drivers of periodontitis: host and microbial factors. They investigated if bacterial content in the periodontal pocket affected gene expression in nearby gingival tissues, finding a strong correlation. Nevertheless, no studies to date have investigated gingival tissue gene expression specifically in response to EBV infection in both healthy and periodontitis tissues.
This study reveals the widespread presence of EBV in both periodontitis-affected and healthy gingival tissues. EBV was detected in 82% of periodontitis cases and, unexpectedly, 70% of healthy tissues as well, highlighting its ubiquitous occurrence in gingival tissues regardless of inflammation. While EBV presence in healthy gingiva was previously suggested (Vincent-Bugnas et al. 2013), most studies reported higher prevalence in periodontitis (Slots and Slots 2019). This discrepancy between studies may stem from several factors, such as variations in detection methods, a lack of standardized procedures, and differences in sample types and sources. A consensus on EBV detection and quantification in the periodontal samples is lacking due to the diverse amplification methods and EBV genes employed across studies (Tonoyan et al. 2021), complicating comparisons. Here, we employed a standard qPCR assay, commonly used for detecting EBV in biological samples (Engelmann et al. 2018), to directly identify EBV in tissues. Another key aspect of this study was the use of tissue lysates, which offer better sensitivity for the unbiased detection of viral infections as compared with sulcular fluid or gingival smears, as they provide a concentrated source of viral genetic material. As an illustration of how sample type and detection method influence EBV prevalence, our earlier study (Vincent-Bugnas et al. 2013) detected EBV in ECs collected via cytospin from periodontal pockets and healthy sulci using Epstein-Barr virus-encoded RNA in situ hybridization, with occurrences of 26.45% and 13.2%, respectively, lower than in this study.
Epigenetic modifications, such as DNA methylation of EBV gene promoters, significantly influence the entire process of EBV infection, specifically governing the transition between latent and lytic states (Zhang et al. 2022). To investigate EBV’s latent/lytic state in gingival tissues, this study employed methyl-qPCR, a method not previously documented for detecting latent/lytic EBV in gingival tissues. It is worth mentioning that EBV plays a crucial role in B-cell biology: it infects naïve B-cells and persists latently in memory B-cells. EBV replication occurs in terminally differentiated B-cells, namely PCs, where EBV transitions from latency to lytic cycle, releasing virions for dissemination. The presence of EBV in periodontitis is thus closely associated with the existence of both EBV-positive B-cells and PCs. Interestingly, we observed a similar proportion of latent and lytic EBV genomes in patients with periodontitis, suggesting the coexistence of latent EBV in B-cells and lytic EBV in PCs within the gingival tissues of these patients. Conversely, in healthy gingival tissues, a predominant lytic form of EBV was observed. Using the same method, Borde et al. (2022) similarly detected lytic EBV DNA in the saliva of EBV-positive healthy donors. Lytic EBV in healthy gingiva implies EBV spreading in epithelia, as EBV infection is primarily lytic in ECs (Eichelberg et al. 2019). This supports prior findings, showing that healthy EBV carriers release significant virus levels in saliva, highlighting ECs’ crucial role (Hadinoto et al. 2009). Although the sample size was relatively small and larger cohort analyses are necessary to validate these findings, our results highlight the distinction between the predominantly lytic state in healthy gingival tissues as compared with the balanced lytic/latent state in periodontitis. These observations imply divergent pathogenesis profiles in EBV infection between healthy and periodontitis tissues.
In a comparison of EBV-positive and EBV-negative healthy tissues, activated pathways mainly involved the cellular immune response, with DEGs mostly related to the major histocompatibility complex (Appendix Data Set 2). This suggests that immune response activation in EBV-positive healthy tissues may function as a defense mechanism against viral challenge rather than direct pathology. Conversely, altered immune-related pathways in EBV presence in healthy tissues may signal disease onset. Our results indicate that EBV can be silently present in apparently healthy tissues, likely maintained by a local equilibrium or immune tolerance between the virus and host. Disruptions to this balance, potentially from bacterial dysbiosis and/or environmental factors, could shift EBV from a silent presence to a more detrimental role by altering local immunity. However, these speculations require further investigation.
When periodontal disease was compared with health, several pathways associated with tissue damage and immune/inflammatory responses were observed, aligning with existing literature and expectations, as they reflect the pathologic processes characteristic of periodontitis.
The scenario of EBV in periodontitis was much more complex. Although CP categories in P_EBV vs P_NEG resembled those of P_NEG vs H_NEG, the sets of DEGs comprising each CP were extensive yet somewhat grouped into specific cohorts. Generally, inflammatory molecules involve cytokines, growth factors, enzymes, and complement-related molecules (Bagyinszky et al. 2017). And so, in the data set of P_EBV vs P_NEG, a wide range of mentioned inflammatory molecules was highly upregulated, such as interleukins, chemokines, colony-stimulating factors, adhesion molecules, complement-related molecules, etc. Further assessment of the transcriptomic profile of P_EBV vs P_NEG revealed panels of upregulated B-cell and PC signature genes, as well as extensive immunoglobulin transcripts that should correspond to an abundant PC infiltration. These data largely overlapped with gene expression patterns of known B-cell and PC profiles (Streicher et al. 2014), confirming the infiltration of B-cells and PCs into EBV-harboring periodontitis tissues. Interestingly, these data also confirmed our previous results that periodontal lesions were abundant with EBV-infected PCs (Olivieri et al. 2020). The presence of PCs in periodontitis has long been considered a sign of disease progression (Mahanonda et al. 2016). As we formerly proposed (Tonoyan et al. 2019), EBV-positive PCs in periodontitis may contribute to the disease through various mechanisms. These include local differentiation of EBV-infected B-cells, leading to autoantibody production. EBV-infected PCs, alongside immunoglobulin production, secrete proinflammatory molecules (e.g., IL1B, IL8, CXCL1, CXCL2), triggering significant inflammation. This heightened inflammatory response may disrupt host immune defenses, potentially leading to bacterial superinfection. The idea that EBV escalates PC infiltration in periodontitis lesions is groundbreaking, prompting further exploration of the pathogenic roles of PCs and EBV in periodontitis.
Gingival tissue destruction, another feature of periodontitis, was also evident in P_EBV vs P_NEG. Interestingly, we found DEGs known to be involved in epithelial cell development, keratinization, and cell adhesion, such as downregulated kallikreins, numerous keratins, small proline-rich proteins, desmogleins, desmoplakins, junction plakoglobins, cartilage oligomeric matrix proteins, and upregulated matrix metalloproteinases. These data suggest that the presence of EBV in periodontitis lesions may increase epithelial tissue destruction and injury. These transcriptomic results also agree with our previous study (Vincent-Bugnas et al. 2013), indicating that periodontal ECs were commonly infected with EBV and prone to apoptosis, potentially compromising epithelial integrity and contributing to periodontal destruction. Obviously, in periodontitis, the abundant clusters of EBV-infected PCs in close vicinity with ECs should greatly favor infection of periodontal epithelia and EBV oral spreading.
In conclusion, this study explored EBV’s impact on both healthy and periodontitis-affected tissues through gene expression profiles, revealing potential molecular mechanisms underlying EBV’s involvement in periodontitis pathogenesis. In particular, the continuous presence of lytic EBV in healthy gingiva may suggest the presence of yet unknown tolerogenic mechanisms that facilitate EBV persistence without eliciting inflammation. Our transcriptomic results support this assumption, revealing that EBV provoked only typical host immune surveillance against the viral challenge and no inflammatory cascade in periodontally healthy tissues. Consistent with the literature, in periodontitis-affected tissues, the host bioprocesses linked to immune/inflammatory response and tissue degradation were documented. While in periodontitis-affected tissues, the presence of EBV significantly intensified these processes: EBV exacerbated the deleterious state of the disease by recruiting an army of inflammatory molecules, summoning assistance from B-cells and PCs, and precipitating tissue destruction.
One limitation of this study is that, although tissues were classified as healthy or periodontitis based on clinical signs, their cellular composition remained heterogeneous and uncertain. Future studies should use cell type–specific analyses to better understand EBV infection in the diverse cellular environment of periodontitis-affected tissues. Additionally, the small sample size in this study highlights the need for future research to include larger cohorts. Another limitation is the lack of data on bacterial infection status: while we focused on EBV, understanding its interaction with bacterial pathogens would provide a more comprehensive view of disease mechanisms. Future research should further incorporate pathogenic bacteria into the periodontitis jigsaw puzzle, establishing a model that encompasses the interplay among bacteria, EBV, and host immune response. Hence, we propose a tentative model of periodontitis: EBV-positive PCs infiltrate the periodontitis lesions, leading to EBV replication, massive inflammation, suppression of immune surveillance, and superinfection by pathogenic bacteria, thus managing periodontitis pathogenesis (Fig. 5). These findings shed new light on periodontitis pathogenesis, marking the first direct involvement of EBV in periodontitis pathogenesis. The results could revolutionize disease management by suggesting unconventional treatments, such as antivirals for EBV-positive patients.
Figure 5.
Proposed model of EBV-associated pathogenesis in periodontitis in the context of bacterial-viral-host synergistic interactions. This model suggests that episodic overgrowth of periodontopathic bacteria in the gingiva may trigger transient inflammation (gingivitis). If left untreated, this inflammation disrupts the balance between the host immune system and endogenous EBV, which persists within the tissues. Persistent EBV facilitates viral evasion and exacerbates the infection. Increased viral activity leads to heightened local immune and inflammatory responses, worsening the inflammatory condition, promoting B-cell and plasma cell infiltration, and advancing epithelial tissue degradation. EBV-induced impairment of host defenses results in bacterial superinfection, culminating in destructive periodontitis. This model underscores the intricate interplay among host immune responses, EBV, and bacterial dysbiosis, challenging traditional paradigms that attribute periodontal disease solely to bacterial infection. EBV, Epstein-Barr virus.
Author Contributions
L. Tonoyan, A. Doglio, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; C. Mounier, J. Fassy, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, contributed to data acquisition and interpretation, critically revised the manuscript; B. Mari, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345241303138 for Unveiling the Etiopathogenic Role of Epstein-Barr Virus in Periodontitis by L. Tonoyan, C. Mounier, J. Fassy, S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, B. Mari and A. Doglio in Journal of Dental Research
Supplemental material, sj-xlsx-2-jdr-10.1177_00220345241303138 for Unveiling the Etiopathogenic Role of Epstein-Barr Virus in Periodontitis by L. Tonoyan, C. Mounier, J. Fassy, S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, B. Mari and A. Doglio in Journal of Dental Research
Supplemental material, sj-xlsx-3-jdr-10.1177_00220345241303138 for Unveiling the Etiopathogenic Role of Epstein-Barr Virus in Periodontitis by L. Tonoyan, C. Mounier, J. Fassy, S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, B. Mari and A. Doglio in Journal of Dental Research
Acknowledgments
The authors thank Prof. Vincent Marechal for helpful advice regarding methylation-specific quantitative polymerase chain reaction assays.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement 896201 and from GIRCI-Méditerrannée (PHRC 2020, HERPARO project).
ORCID iDs: L. Tonoyan  https://orcid.org/0000-0002-4393-3228
https://orcid.org/0000-0002-4393-3228
A. Doglio  https://orcid.org/0000-0002-5456-5598
https://orcid.org/0000-0002-5456-5598
A supplemental appendix to this article is available online.
References
- Abe D, Kubota T, Morozumi T, Shimizu T, Nakasone N, Itagaki M, Yoshie H. 2011. Altered gene expression in leukocyte transendothelial migration and cell communication pathways in periodontitis-affected gingival tissues. J Periodontal Res. 46(3):345–353. [DOI] [PubMed] [Google Scholar]
- Ayee R, Ofori MEO, Wright E, Quaye O. 2020. Epstein Barr virus associated lymphomas and epithelia cancers in humans. J Cancer. 11(7):1737–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagyinszky E, Giau VV, Shim K, Suk K, An SSA, Kim S. 2017. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J Neurol Sci. 376:242–254. [DOI] [PubMed] [Google Scholar]
- Beck JD, Papapanou PN, Philips KH, Offenbacher S. 2019. Periodontal medicine: 100 years of progress. J Dent Res. 98(10):1053–1062. [DOI] [PubMed] [Google Scholar]
- Becker ST, Beck-Broichsitter BE, Graetz C, Dörfer CE, Wiltfang J, Häsler R. 2014. Peri-implantitis versus periodontitis: functional differences indicated by transcriptome profiling. Clin Implant Dent Relat Res. 16(3):401–411. [DOI] [PubMed] [Google Scholar]
- Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, et al. 2022. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 375(6578):296–301. [DOI] [PubMed] [Google Scholar]
- Borde C, Quignon F, Amiel C, Gozlan J, Marechal V, Brissot E. 2022. Methyl-qPCR: a new method to investigate Epstein-Barr virus infection in post-transplant lymphoproliferative diseases. Clin Epigenetics. 14(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS. 2018. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J Periodontol. 89 Suppl 1:S1–S8. [DOI] [PubMed] [Google Scholar]
- Chen C, Feng P, Slots J. 2020. Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol 2000. 82(1):42–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanian H, Stranneheim H, Båge T, Lagervall M, Jansson L, Lundeberg J, Yucel-Lindberg T. 2012. Gene expression profiles in paired gingival biopsies from periodontitis-affected and healthy tissues revealed by massively parallel sequencing. PLOS One. 7(9):e46440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Behle JH, Wolf DL, Handfield M, Kebschull M, Celenti R, Pavlidis P, Papapanou PN. 2008. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 79(11):2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberg MR, Welch R, Guidry JT, Ali A, Ohashi M, Makielski KR, McChesney K, Van Sciver N, Lambert PF, Keleș S, et al. 2019. Epstein-Barr virus infection promotes epithelial cell growth by attenuating differentiation-dependent exit from the cell cycle. mBio. 10(4):e01332-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann I, Alidjinou EK, Lazrek M, Pouillaude J-M, Ogiez J, Rose F, Duhamel A, Dewilde A, Hober D. 2018. Comparison of two commercial quantitative PCR assays for EBV DNA detection and their correlation with the first WHO International Standard for EBV. J Med Microbiol. 67(4):529–536. [DOI] [PubMed] [Google Scholar]
- Fassy J, Lacoux C, Leroy S, Noussair L, Hubac S, Degoutte A, Vassaux G, Leclercq V, Rouquié D, Marquette C-H, et al. 2021. Versatile and flexible microfluidic qPCR test for high-throughput SARS-CoV-2 and cellular response detection in nasopharyngeal swab samples. PLOS One. 16(4):e0243333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. 2009. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLOS Pathog. 5(7):e1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houen G, Trier NH. 2021. Epstein-Barr virus and systemic autoimmune diseases. Front Immunol. 11:587380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Dutt U, Radenkov I, Jain S. 2024. WHO’s global oral health status report 2022: actions, discussion and implementation. Oral Dis. 30(2):73–79. [DOI] [PubMed] [Google Scholar]
- Kim Y-G, Kim M, Kang JH, Kim HJ, Park J-W, Lee J-M, Suh J-Y, Kim J-Y, Lee J-H, Lee Y. 2016. Transcriptome sequencing of gingival biopsies from chronic periodontitis patients reveals novel gene expression and splicing patterns. Hum Genomics. 10(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könönen E, Gursoy M, Gursoy UK. 2019. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med. 8(8):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Z, Wang Z. 2020. Differential immune cell infiltrations between healthy periodontal and chronic periodontitis tissues. BMC Oral Health. 20(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark A, Davanian H, Båge T, Johannsen G, Koro C, Lundeberg J, Yucel-Lindberg T. 2015. Transcriptome analysis reveals mucin 4 to be highly associated with periodontitis and identifies pleckstrin as a link to systemic diseases. Sci Rep. 5(1):18475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanonda R, Champaiboon C, Subbalekha K, Sa-Ard-Iam N, Rattanathammatada W, Thawanaphong S, Rerkyen P, Yoshimura F, Nagano K, Lang NP, et al. 2016. Human memory B cells in healthy gingiva, gingivitis, and periodontitis. J Immunol. 197(3):715–725. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Lu J, Peacock-Villada AY, Barber JR, Joshu CE, Prizment AE, Beck JD, Offenbacher S, Platz EA. 2018. Periodontal disease assessed using clinical dental measurements and cancer risk in the ARIC study. JNCI J Natl Cancer Inst. 110(8):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri CV, Raybaud H, Tonoyan L, Abid S, Marsault R, Chevalier M, Doglio A, Vincent-Bugnas S. 2020. Epstein-Barr virus-infected plasma cells in periodontitis lesions. Microb Pathog. 143:104128. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, Handfield M, Pavlidis P, Demmer RT. 2009. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol. 9(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. 2007. Herpesviral-bacterial synergy in the pathogenesis of human periodontitis. Curr Opin Infect Dis. 20(3):278–283. [DOI] [PubMed] [Google Scholar]
- Slots J. 2015. Periodontal herpesviruses: prevalence, pathogenicity, systemic risk. Periodontol 2000. 69(1):28–45. [DOI] [PubMed] [Google Scholar]
- Slots J, Slots H. 2019. Periodontal herpesvirus morbidity and treatment. Periodontol 2000. 79(1):210–220. [DOI] [PubMed] [Google Scholar]
- Streicher K, Morehouse CA, Groves CJ, Rajan B, Pilataxi F, Lehmann KP, Brohawn PZ, Higgs BW, McKeever K, Greenberg SA, et al. 2014. The plasma cell signature in autoimmune disease. Arthritis Rheumatol. 66(1):173–184. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. 2001. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 1(1):75–82. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. 2015. EBV persistence—introducing the virus. Curr Top Microbiol Immunol. 390 Pt 1:151–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoyan L, Chevalier M, Vincent-Bugnas S, Marsault R, Doglio A. 2021. Detection of Epstein-Barr virus in periodontitis: a review of methodological approaches. Microorganisms. 9(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoyan L, Olivieri CV, Chevalier M, Marsault R, Doglio A. 2024. Detection of Epstein-Barr virus infection in primary junctional epithelial cell cultures. J Oral Microbiol. 16(1):2301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoyan L, Vincent-Bugnas S, Olivieri C-V, Doglio A. 2019. New viral facets in oral diseases: the EBV paradox. Int J Mol Sci. 20(23):5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Bugnas S, Vitale S, Mouline CC, Khaali W, Charbit Y, Mahler P, Prêcheur I, Hofman P, Maryanski JL, Doglio A. 2013. EBV infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PLoS One. 8(12):e80336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang R, Xie Z. 2022. The roles of DNA methylation on the promotor of the Epstein-Barr virus (EBV) gene and the genome in patients with EBV-associated diseases. Appl Microbiol Biotechnol. 106(12):4413–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Xu T, Zeng L, Liu F, Huang X, Liu Q. 2024. Interactions among microorganisms open up a new world for anti-infectious therapy. FEBS J. 291(8):1615–1631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345241303138 for Unveiling the Etiopathogenic Role of Epstein-Barr Virus in Periodontitis by L. Tonoyan, C. Mounier, J. Fassy, S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, B. Mari and A. Doglio in Journal of Dental Research
Supplemental material, sj-xlsx-2-jdr-10.1177_00220345241303138 for Unveiling the Etiopathogenic Role of Epstein-Barr Virus in Periodontitis by L. Tonoyan, C. Mounier, J. Fassy, S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, B. Mari and A. Doglio in Journal of Dental Research
Supplemental material, sj-xlsx-3-jdr-10.1177_00220345241303138 for Unveiling the Etiopathogenic Role of Epstein-Barr Virus in Periodontitis by L. Tonoyan, C. Mounier, J. Fassy, S. Leymarie, S. Mouraret, P. Monneyron, S. Vincent-Bugnas, B. Mari and A. Doglio in Journal of Dental Research