Abstract
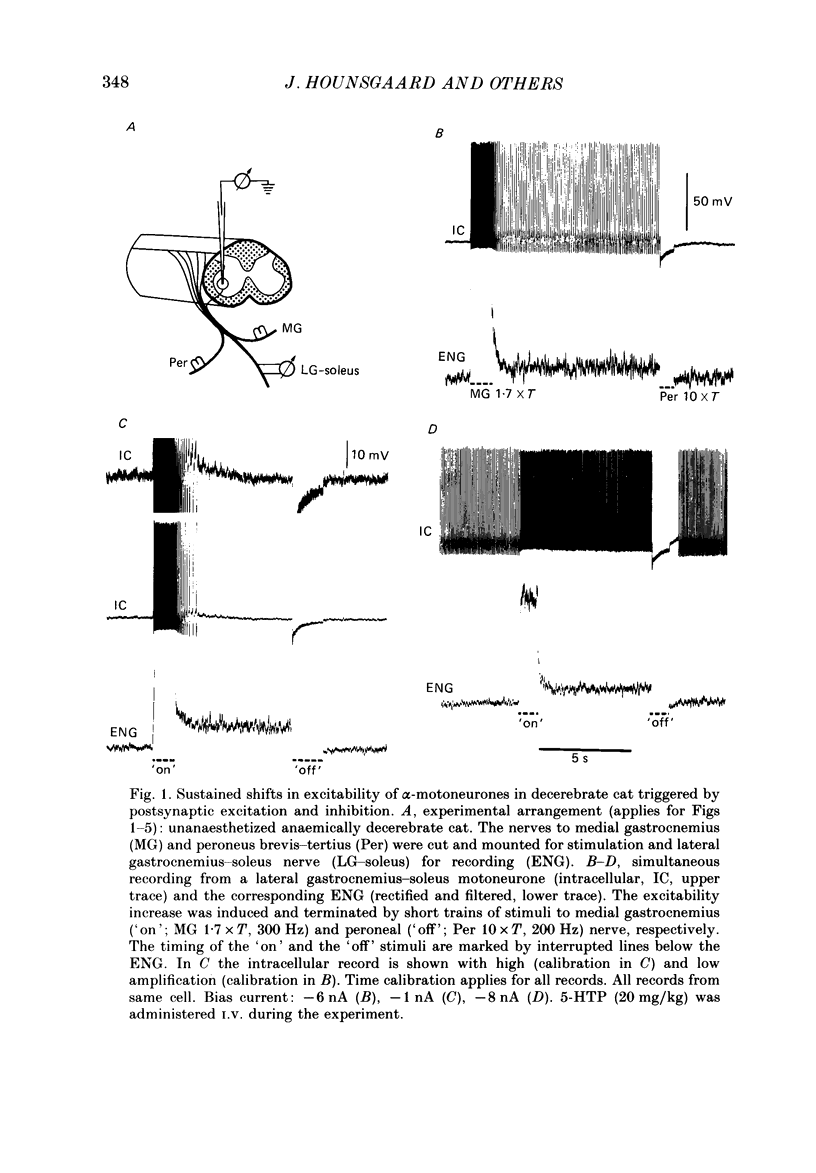
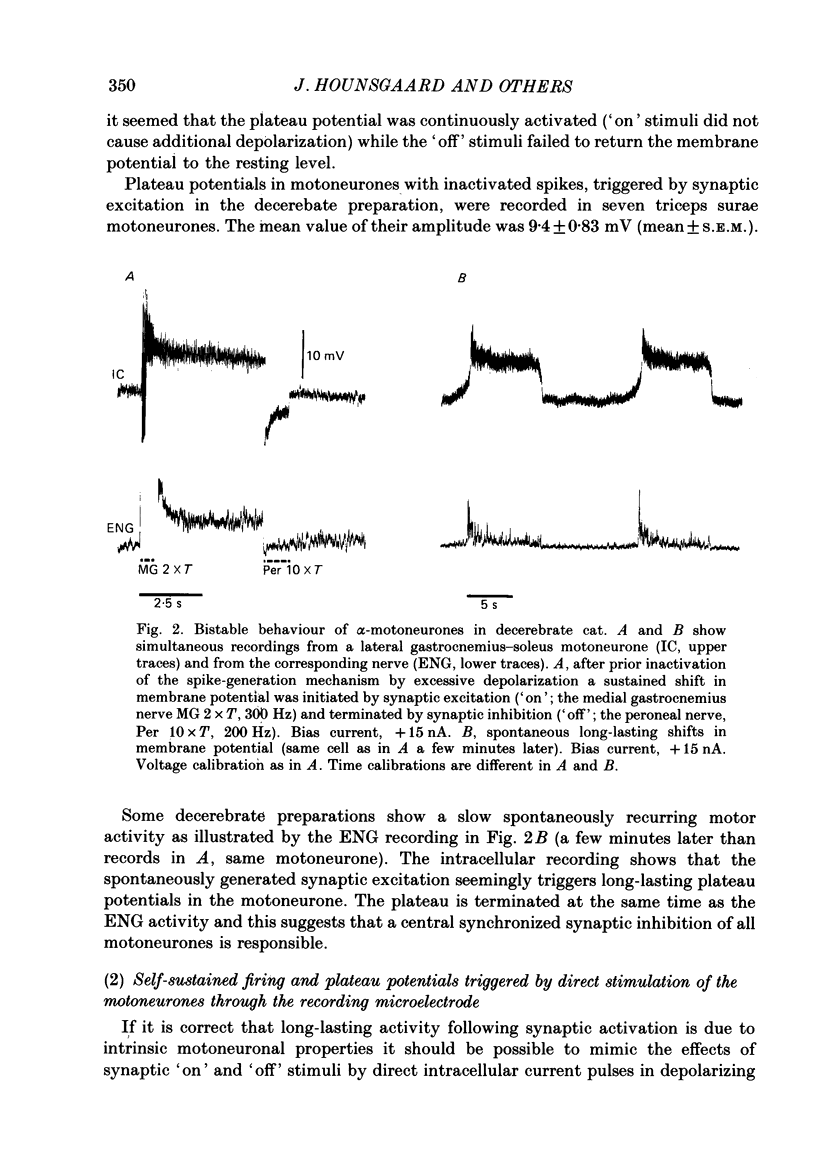
1. In the preceding paper (Crone, Hultborn, Kiehn, Mazieres & Wigström, 1988) it was shown that a short-lasting synaptic excitation ('on' stimulus) of extensor motoneurones (primarily triceps surae) in the decerebrate cat often resulted in a maintained excitability increase, which could be reset by a short-lasting inhibitory stimulus train ('off' stimulus). In the present experiments intracellular recording from triceps surae motoneurones and the electroneurogram (ENG activity) from triceps surae nerve branches were performed in parallel. 2. Sustained firing of individual triceps surae motoneurones was most often recorded in parallel with the maintained ENG activity following a synaptic 'on' stimulus. When the motoneurone was silenced, by a hyperpolarizing current through the microelectrode, there was no sign of on-going synaptic excitation during the maintained ENG activity following an 'on' stimulus. It was therefore suggested that voltage-dependent intrinsic properties of the motoneurones themselves could be responsible for the maintained firing. 3. In confirmation of this hypothesis it was found that short-lasting depolarizing current pulses through the recording microelectrode could trigger a self-sustained firing in the motoneurone provided that the bias current (i.e. the holding potential) was kept within certain limits. Hyperpolarizing current pulses terminated the firing. When the spike-generating mechanism was inactivated (by long-lasting excessive depolarization) similar depolarizing and hyperpolarizing current pulses could initiate and terminate plateau potentials in the motoneurones. By grading the depolarizing current pulses it was found that the plateau potentials were of all-or-none character, typically around 10 mV in amplitude. The two levels of excitability which can be triggered by short-lasting excitation and inhibition of the motoneurones is referred to as 'bistable' behaviour of the motoneurones. 4. After an acute spinal transection, in the unanaesthetized cat, the bistable behaviour of the motoneurones disappeared. However, it reappears following intravenous injection of the serotonin precursor 5-hydroxytryptophan (50-120 mg/kg). 5. Individual triceps surae motor units were recorded by selective EMG electrodes during tonic stretch reflexes in the decerebrate preparations. Based on an analysis of their firing pattern during lengthening and shortening (or vibration) of the muscle it is suggested that plateau potentials in motoneurones are recruited during the tonic stretch reflex. Furthermore, it is argued that a quantitatively important part of the depolarization of motoneurones during the tonic stretch reflex indeed originates from these plateau potentials.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
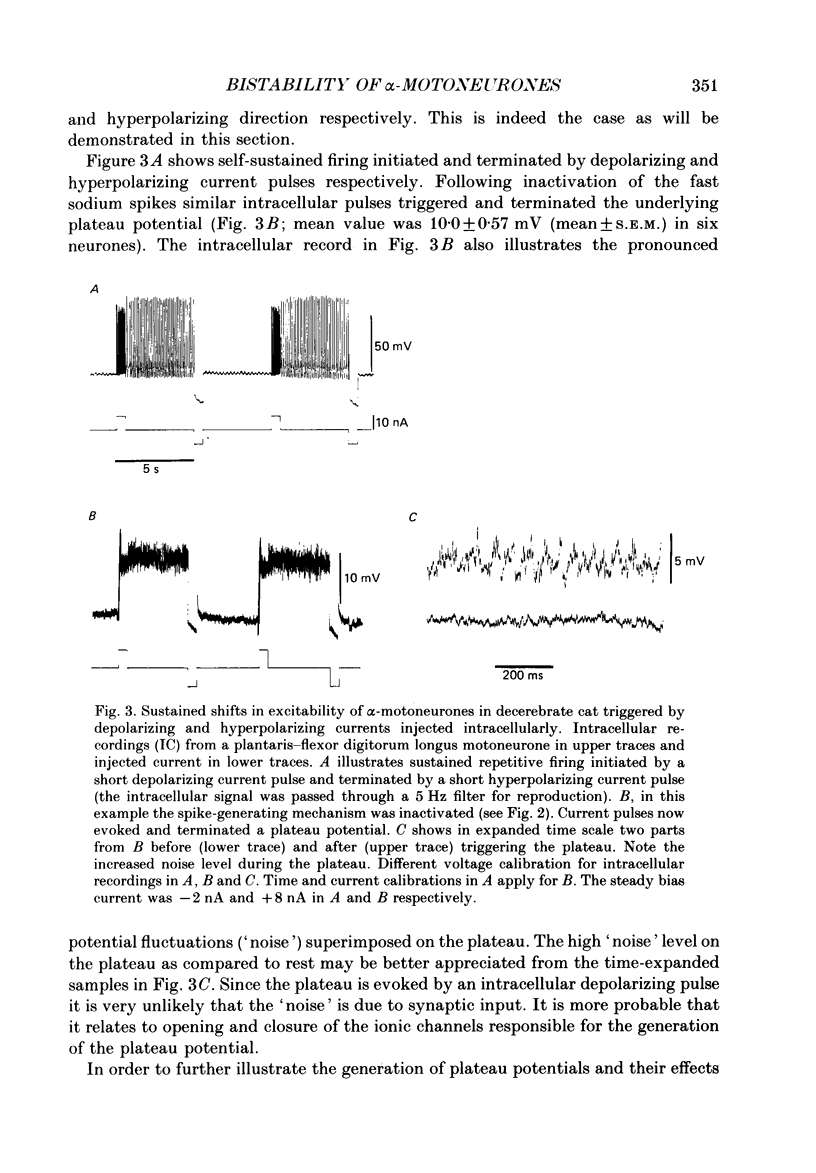
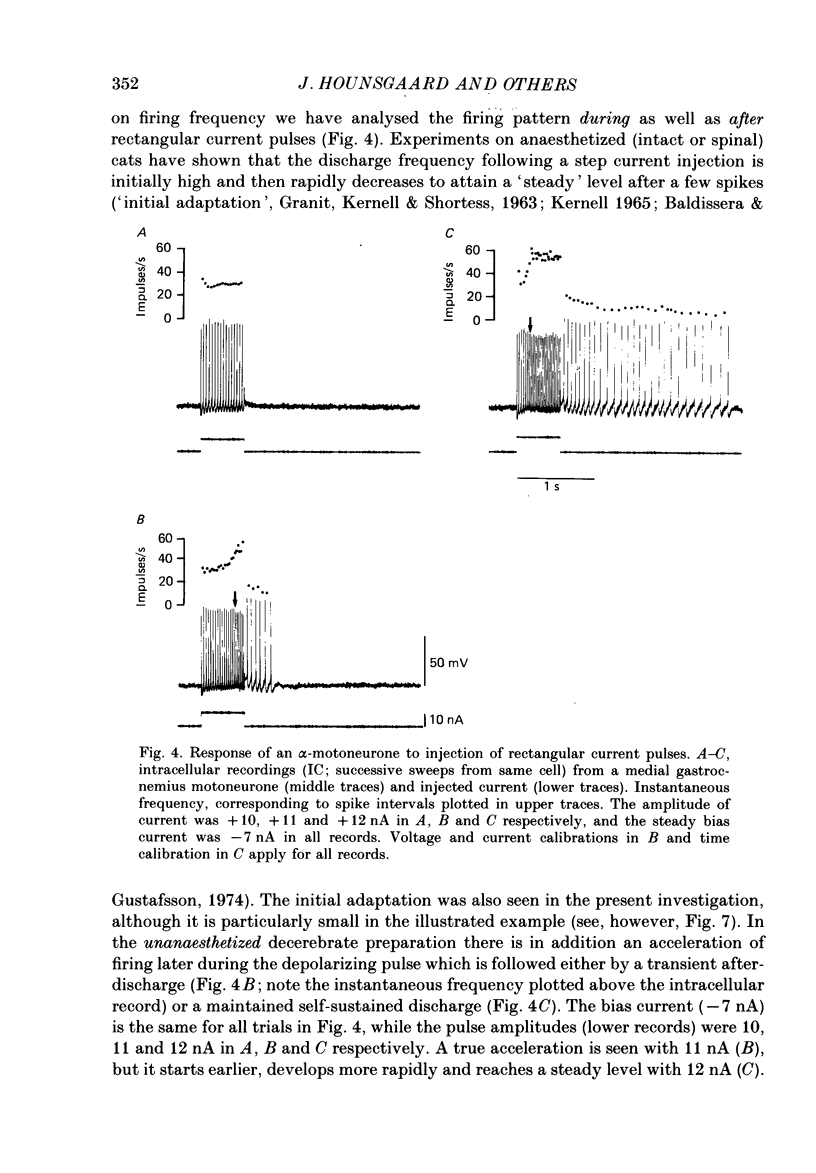
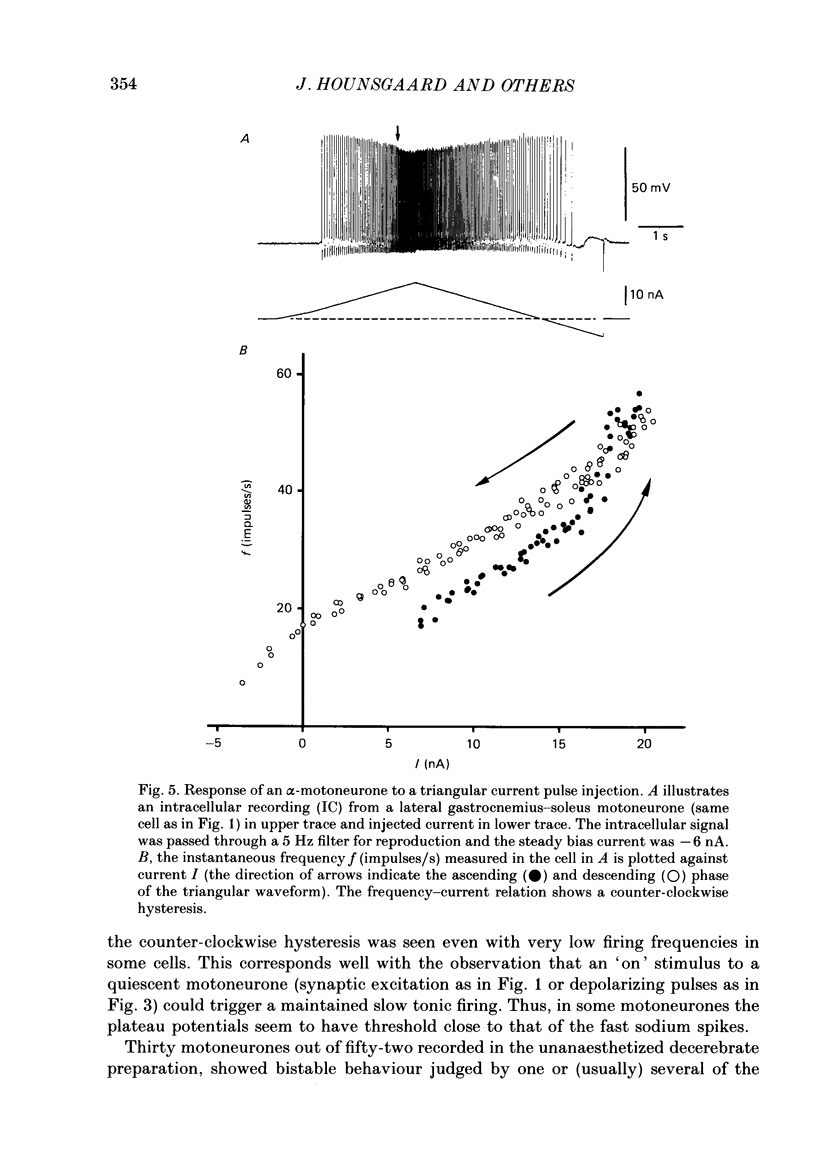
PDF




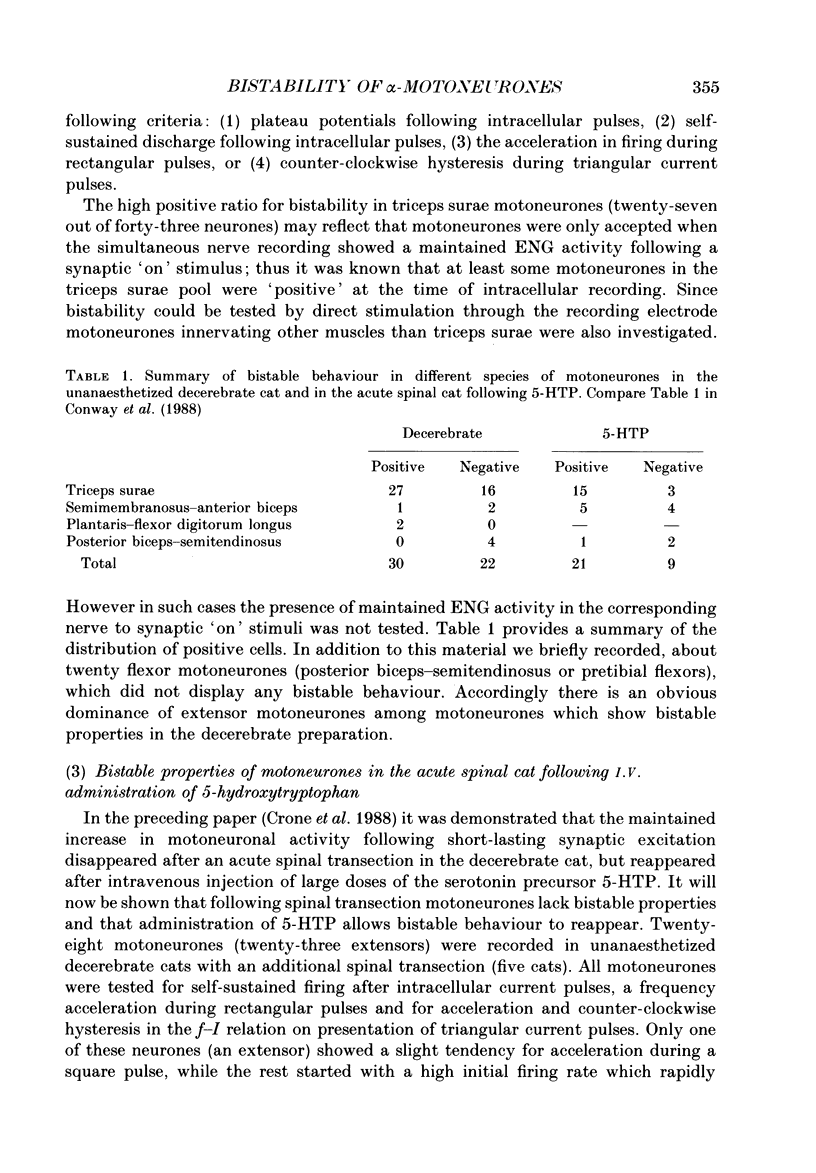
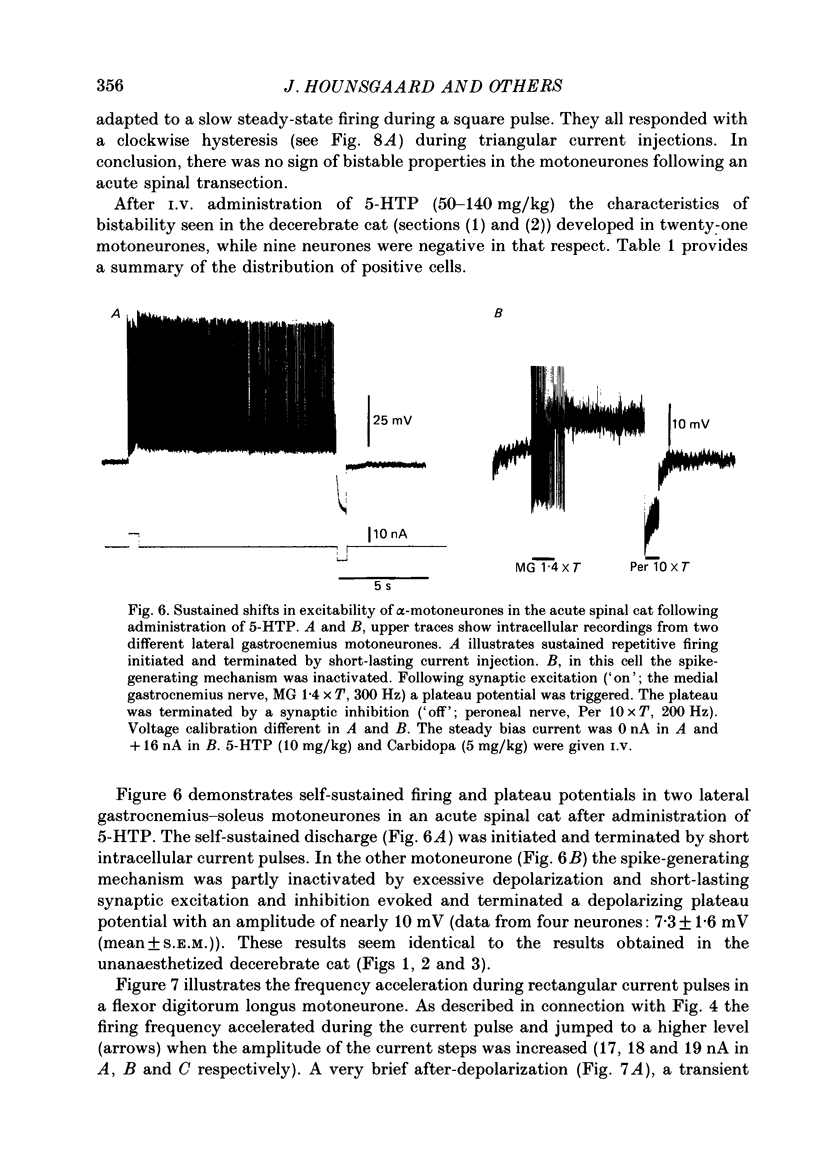
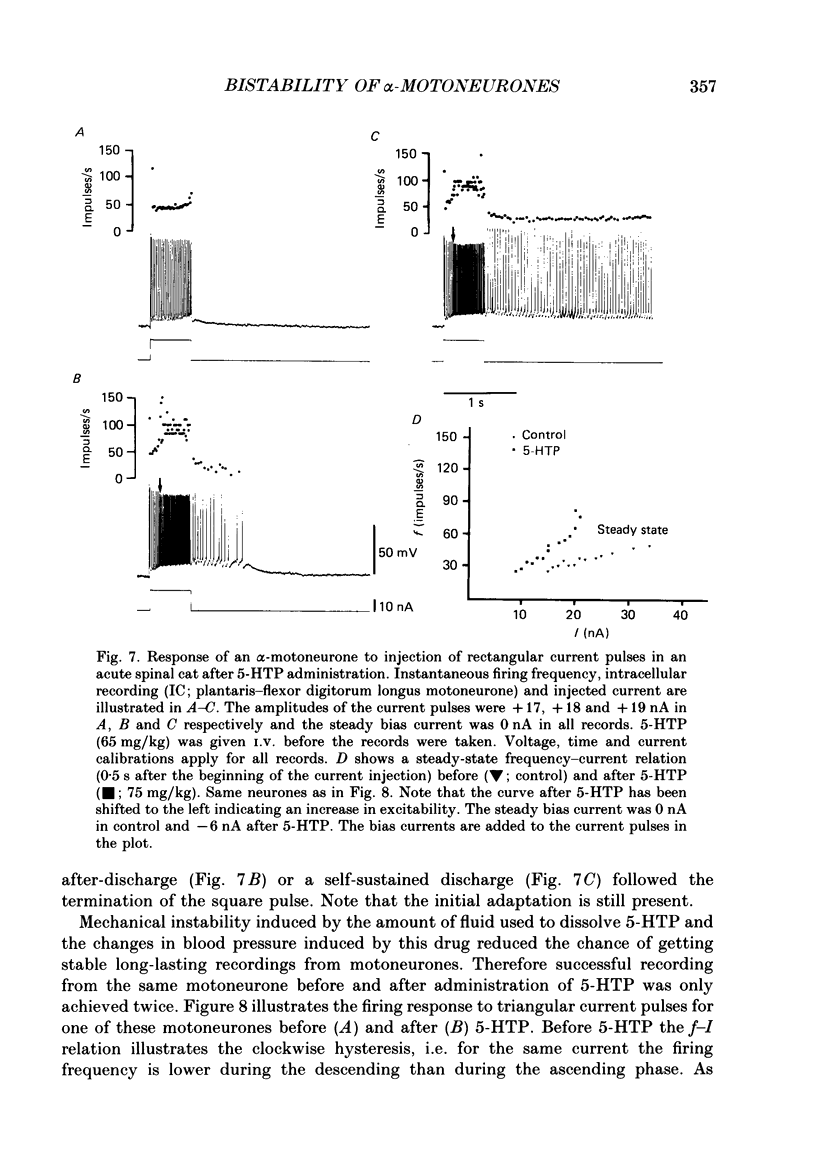

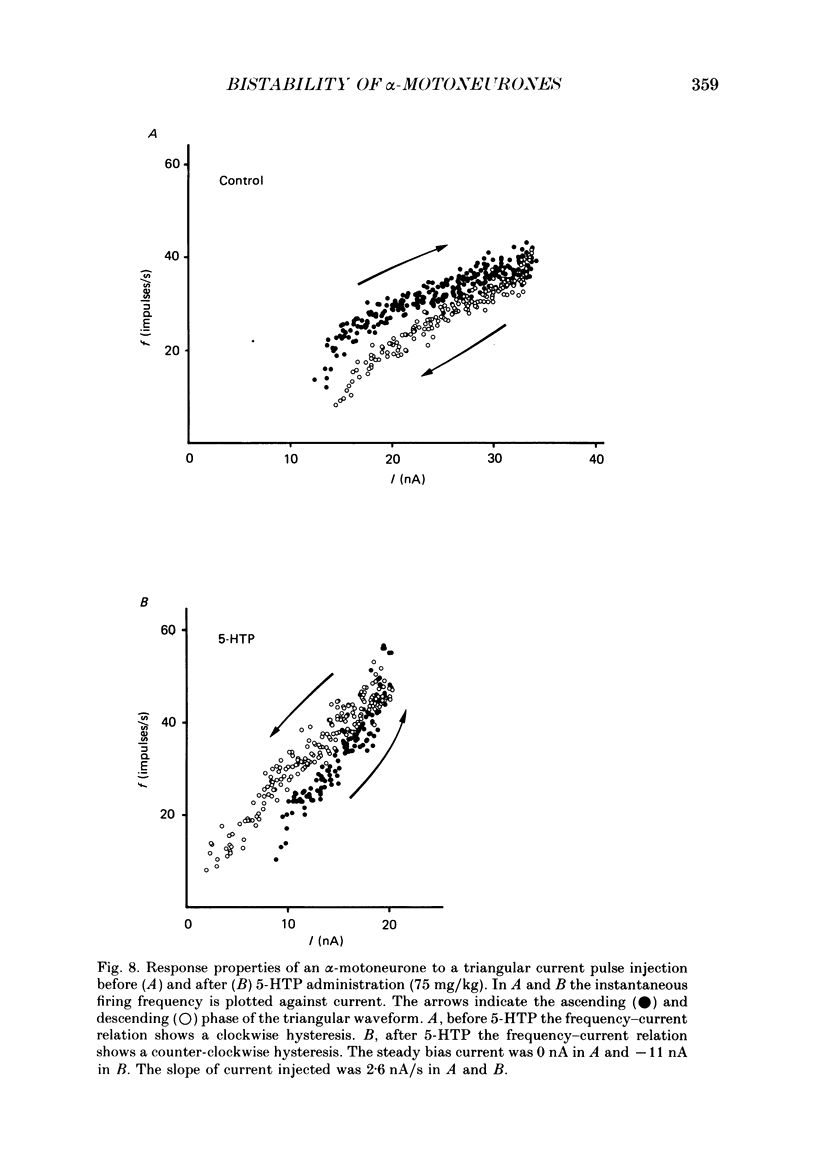






Images in this article
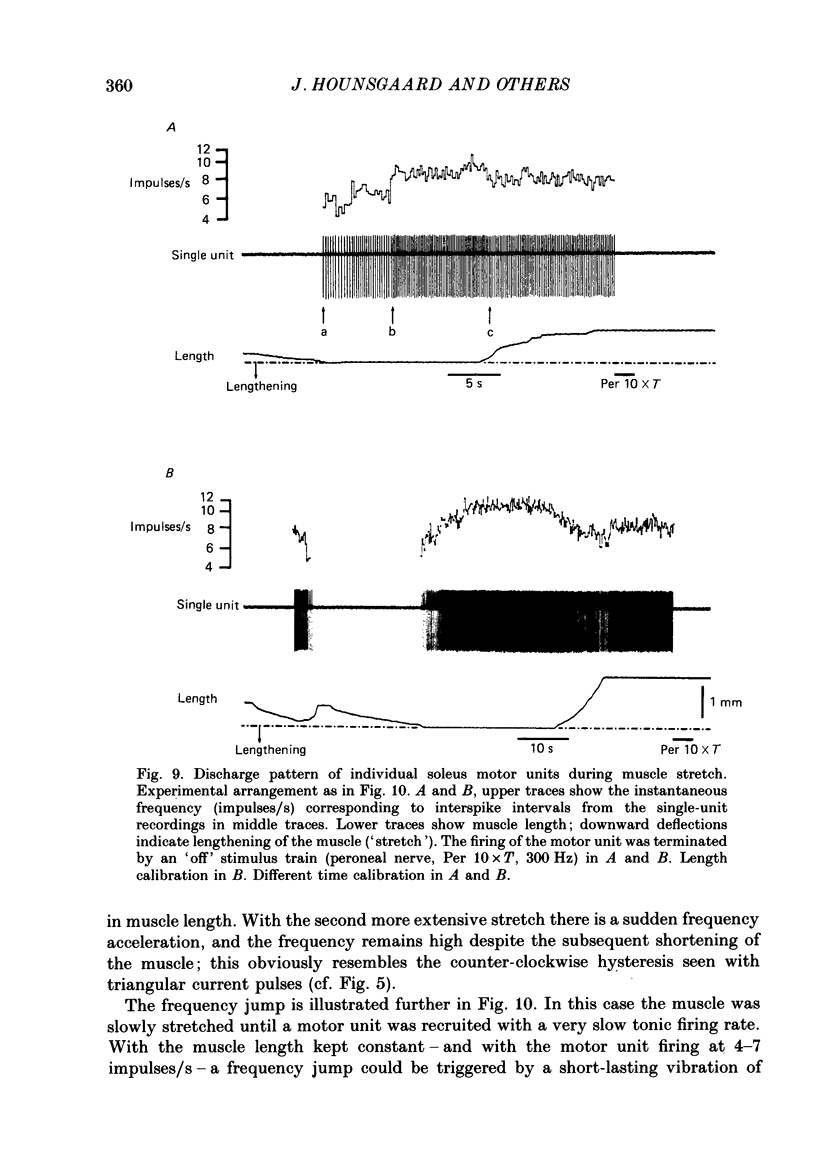
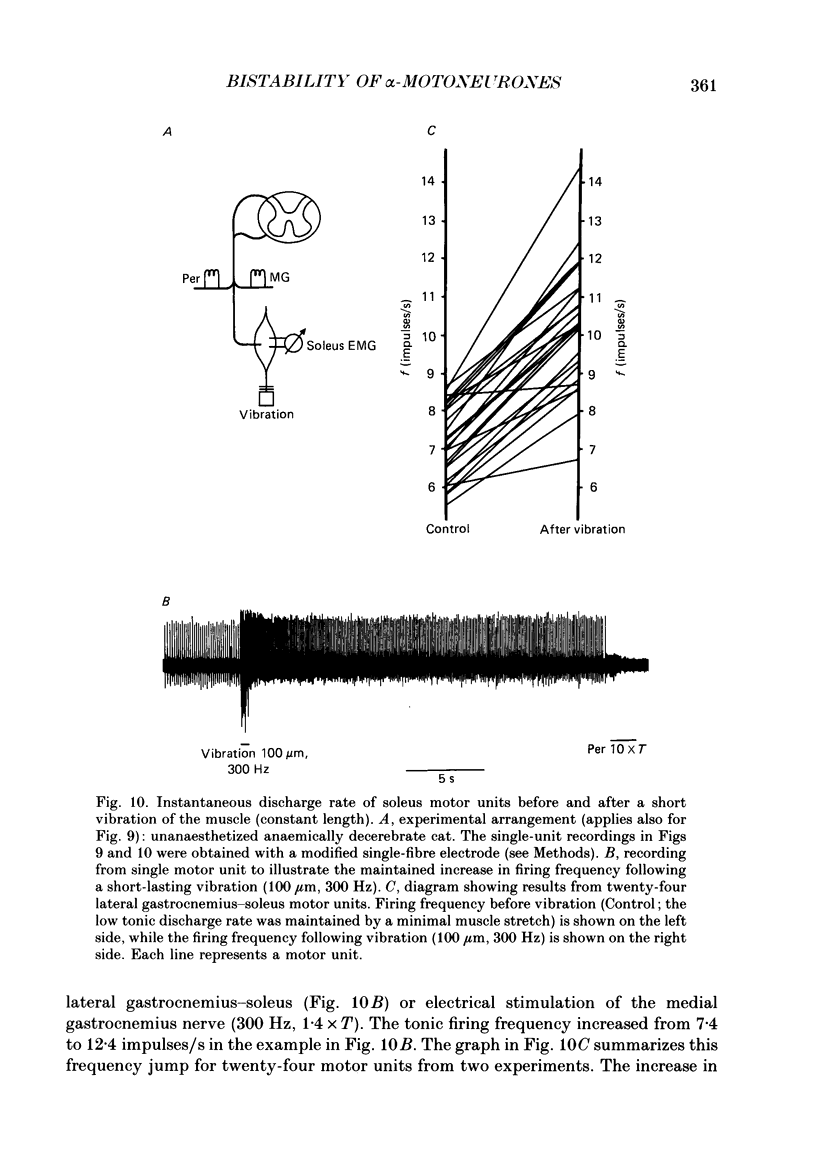
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldissera F., Gustafsson B. Afterhyperpolarization conductance time course in lumbar motoneurones of the cat. Acta Physiol Scand. 1974 Aug;91(4):512–527. doi: 10.1111/j.1748-1716.1974.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol. 1968 Jun;196(3):631–654. doi: 10.1113/jphysiol.1968.sp008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B. A., Hultborn H., Kiehn O., Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988 Nov;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo P. J., Rymer W. Z. Motor-unit activation patterns in lengthening and isometric contractions of hindlimb extensor muscles in the decerebrate cat. J Neurophysiol. 1982 May;47(5):782–796. doi: 10.1152/jn.1982.47.5.782. [DOI] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Kiehn O., Mazieres L., Wigström H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988 Nov;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Lundberg A., Ryall R. W. Is the tonic decerebrate inhibition of reflex paths mediated by monoaminergic pathways? Acta Physiol Scand. 1968 Jan-Feb;72(1):123–133. doi: 10.1111/j.1748-1716.1968.tb03834.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SHORTESS G. K. QUANTITATIVE ASPECTS OF REPETITIVE FIRING OF MAMMALIAN MOTONEURONES, CAUSED BY INJECTED CURRENTS. J Physiol. 1963 Oct;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S., Udo M. Motor unit activity and stiffness of the contracting muscle fibres in the tonic stretch reflex. Acta Physiol Scand. 1971 Mar;81(3):422–424. doi: 10.1111/j.1748-1716.1971.tb04916.x. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Prog Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57(2):422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- Kernell D., Monster A. W. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46(2):191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W., Zengel J. E., Lofton S. A., Fleshman J. W. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1982 Nov;48(5):1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980 Oct;44(4):827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980 Jun;43(6):1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt P., Crill W. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980 May;43(5):1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]