Abstract
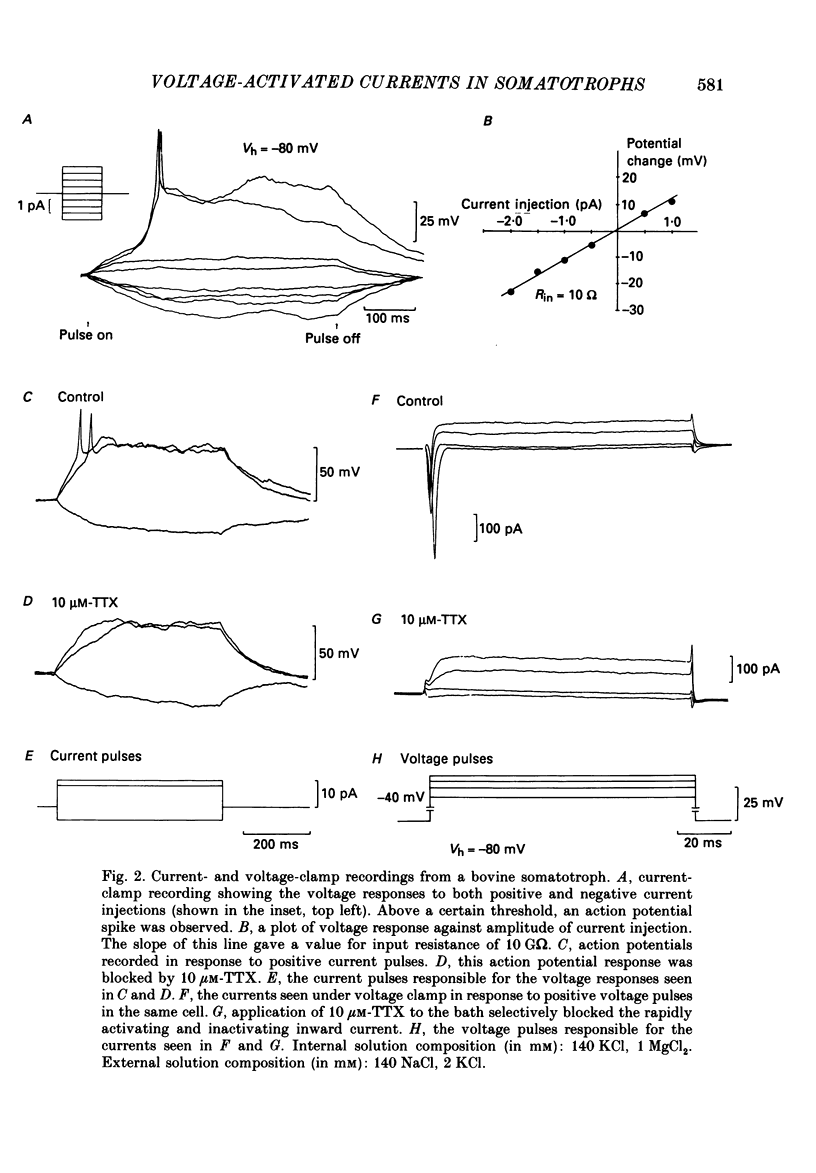
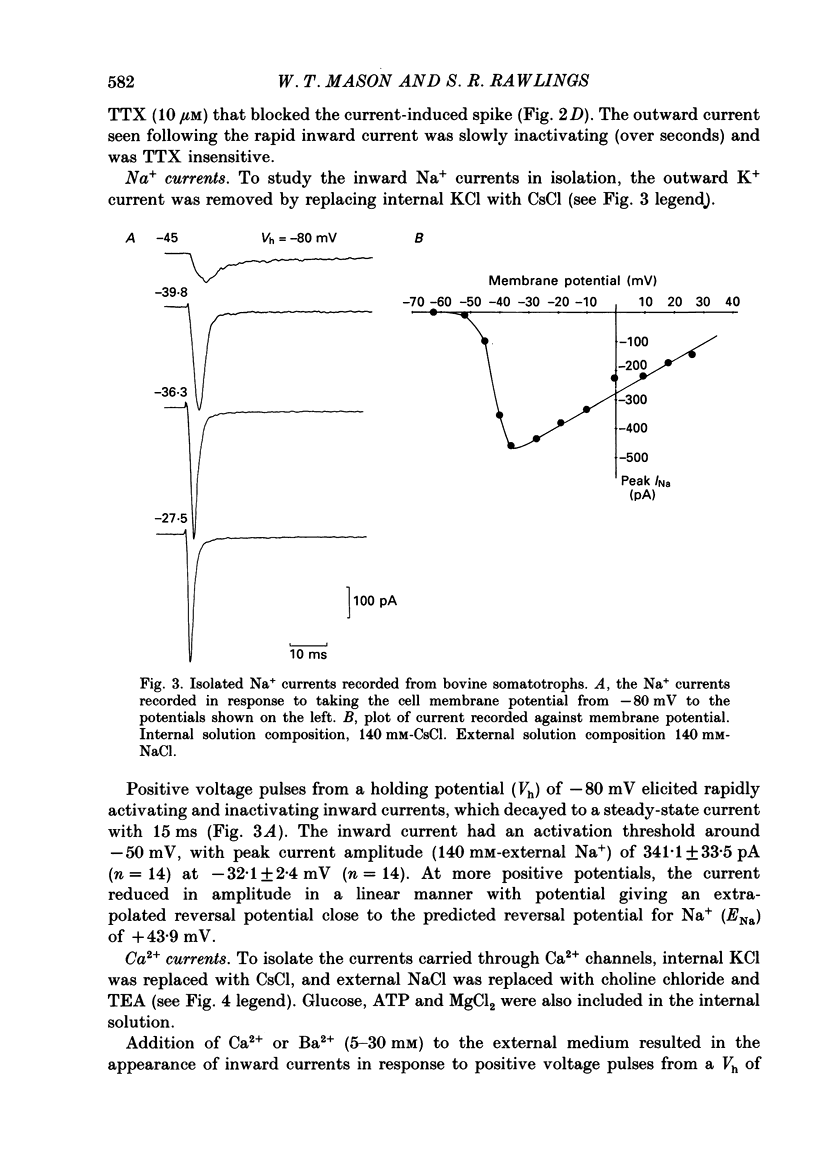
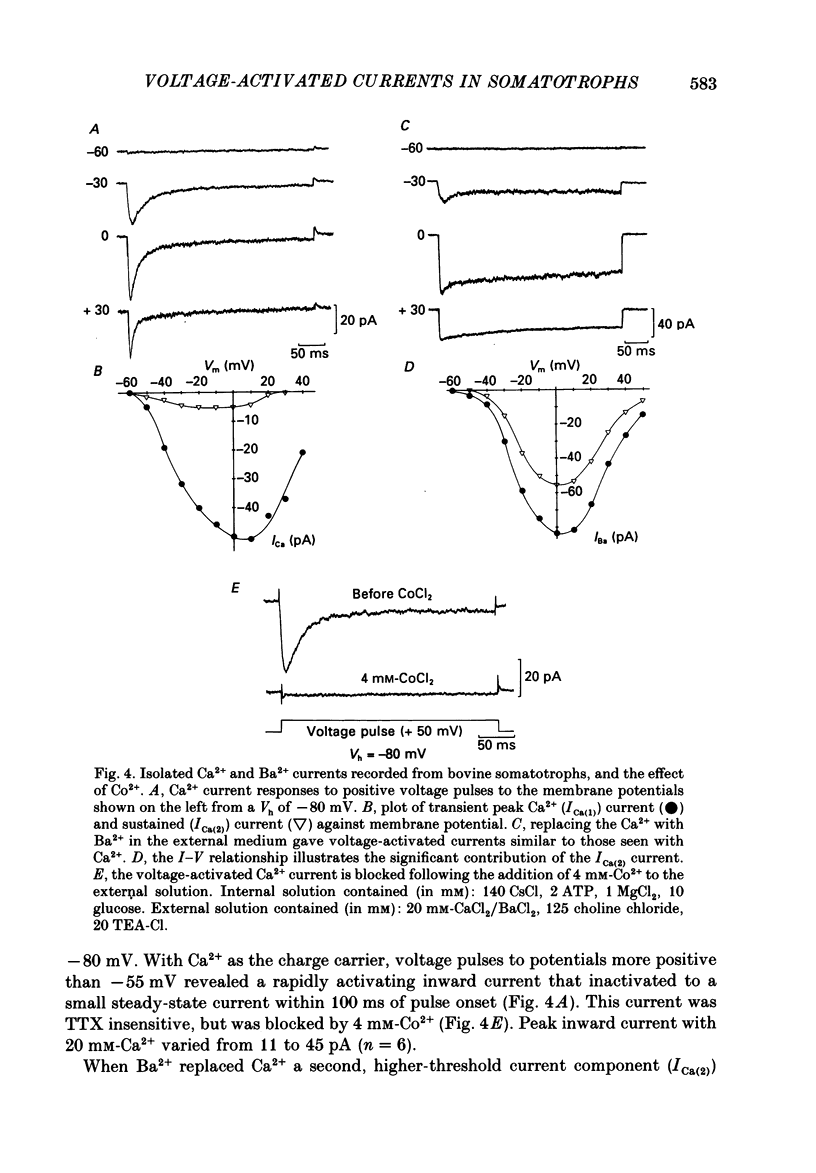
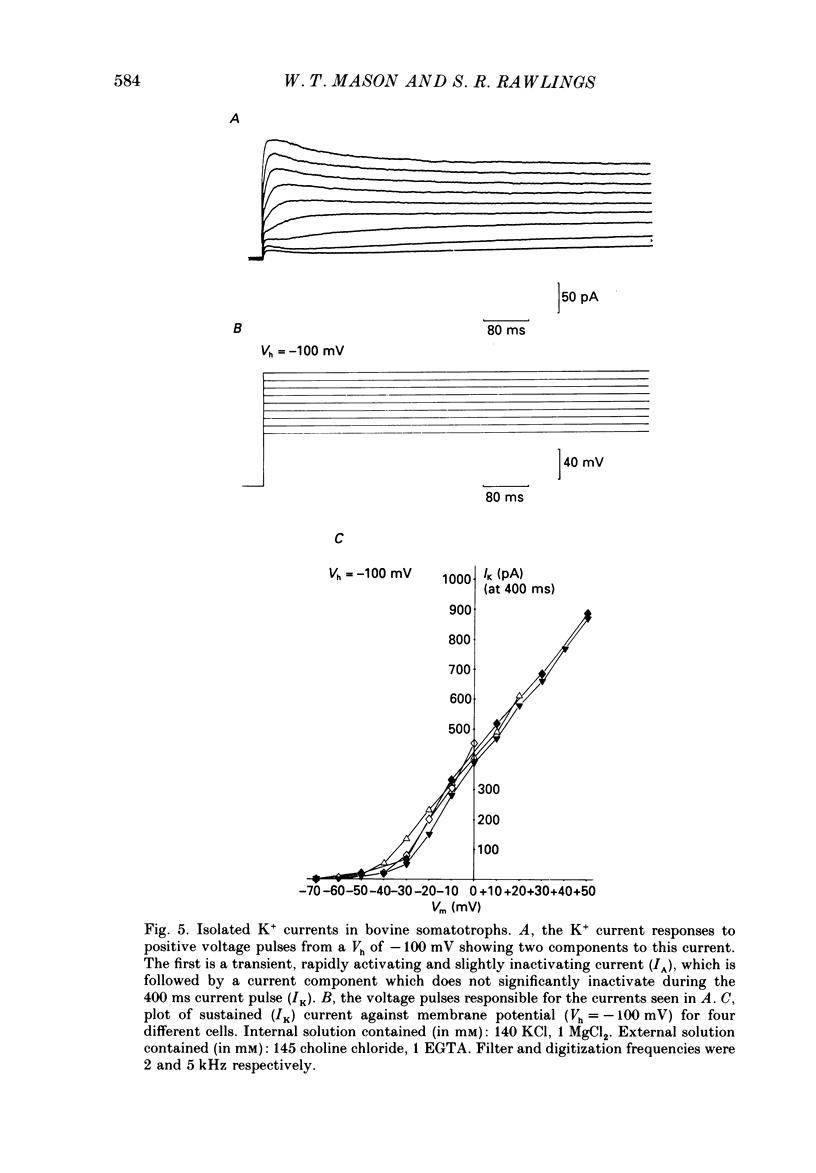
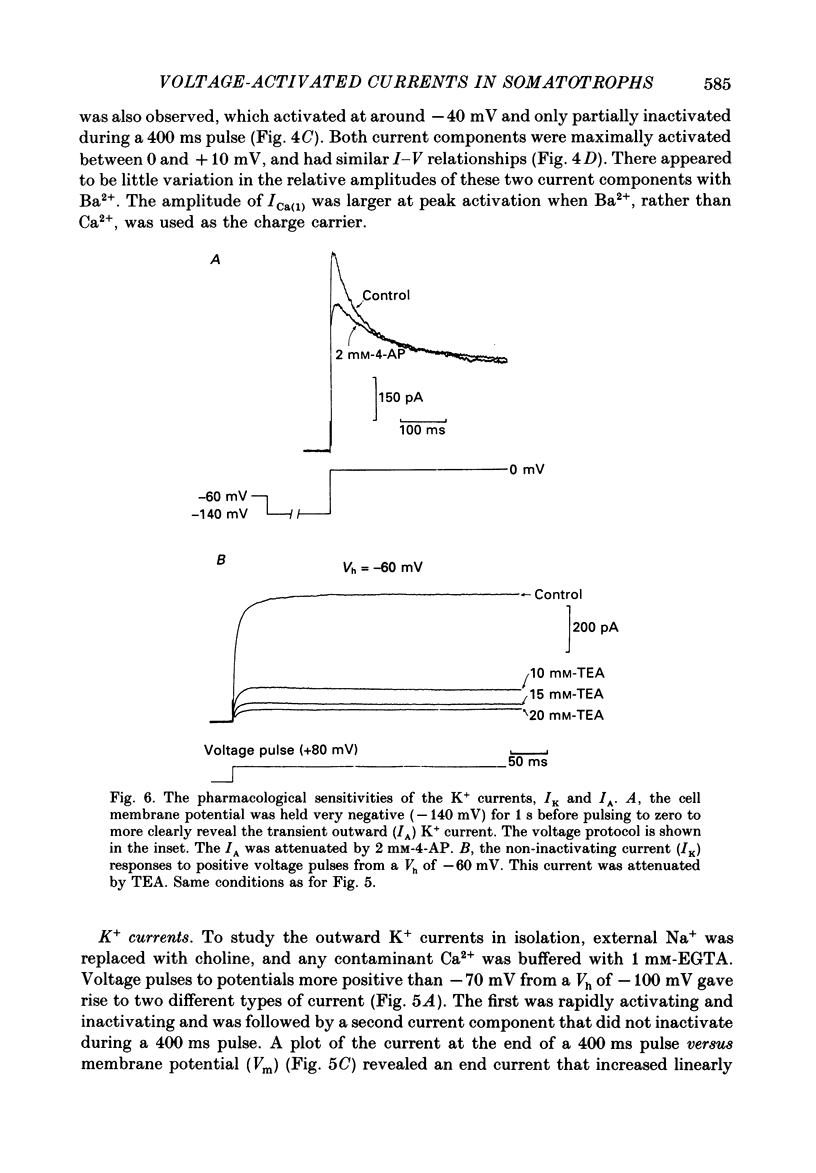
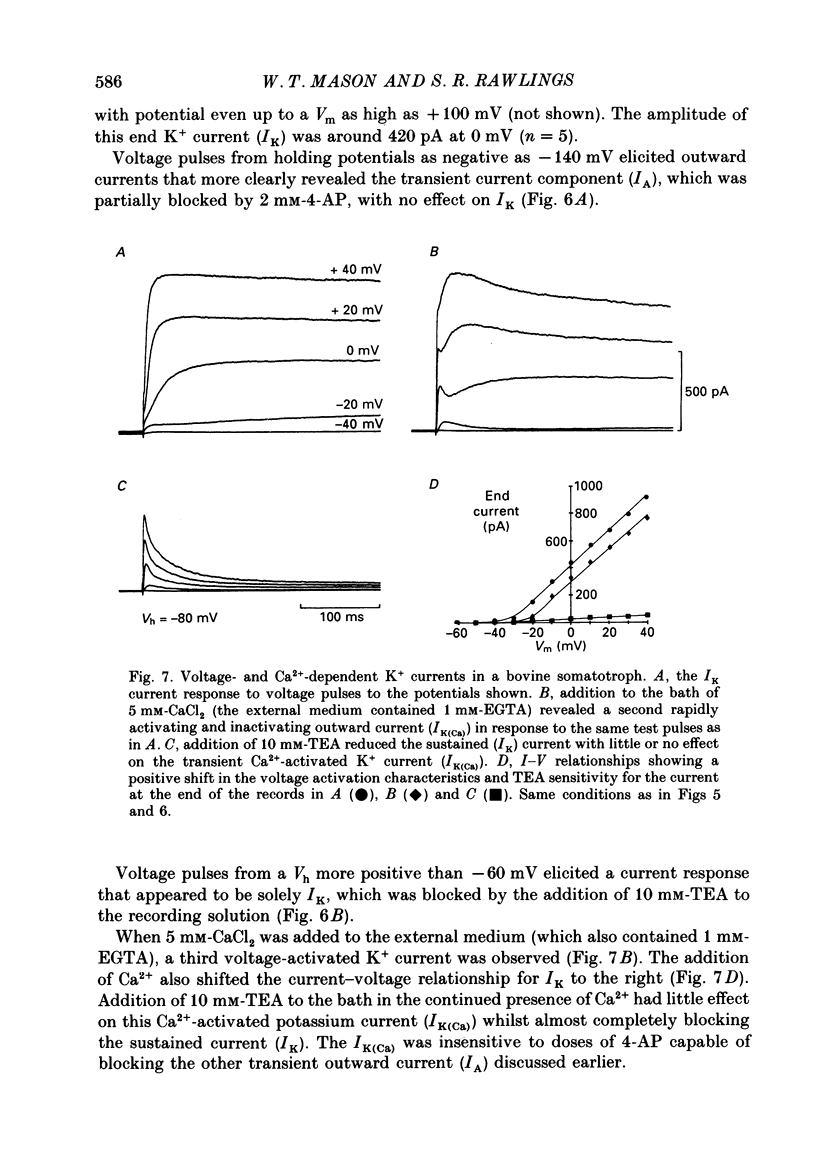
1. The whole-cell mode of the patch-clamp technique was used to record voltage-activated cationic currents in immunocytochemically identified bovine somatotrophs. 2. In current-clamp mode, cells had a resting membrane potential of -83.0 +/- 4.5 mV, and an input resistance of 8.4 +/- 2.1 G omega. Cells rarely fired action potentials spontaneously, but fired one to three action potentials in response to a suprathreshold current pulse. 3. Under voltage clamp, in Ca2+-free media, the action potential was shown to be composed of a TTX-sensitive inward Na+ current and an outward K+ current. 4. The isolated Na+ current had a threshold of approximately -50 mV, and rapidly activated and then inactivated to a small steady-state current. Peak Na+ current amplitude with 140 mM-external Na+ was 341.1 +/- 33.5 pA (n = 14) at a membrane potential of -32.1 +/- 2.4 mV (n = 14). 5. With Ca2+ or Ba2+ (5-30 mM) as the only membrane-permeable cation, voltage pulses to potentials more positive than -55 mV from a holding potential of -80 mV revealed a rapidly activating current component that was followed by a second, very slowly inactivating, current component, most clearly seen with Ba2+. Both components were maximally activated between 0 and +10 mV, were TTX insensitive, but were blocked by 4 mM-Co2+. 6. Three components of the isolated K+ current were identified (IA, IK and IK(Ca] by their voltage sensitivity, Ca2+ dependence and their response to 4-aminopyridine (4-AP) and tetraethylammonium (TEA). 7. Growth hormone (GH)-releasing hormone (GHRH) applied to cells under whole-cell voltage clamp had no effect on either steady-state or voltage-activated ionic currents. This is probably due to dialysis of cytoplasmic compounds vital for GHRH activation of the cell. 8. Both basal and GHRH-stimulated GH secretion were unaffected by TTX, implying that the Na+ action potential is not critical for such release. In contrast the Ca2+ channel blocker Co2+ attenuated GH release in both cases. The K+ channel blocker TEA stimulated GH release above basal and GHRH-stimulated levels.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Dufy B., Owen D. G., Segal M. Excitable membrane properties of cultured central nervous system neurons and clonal pituitary cells. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):259–268. doi: 10.1101/sqb.1983.048.01.028. [DOI] [PubMed] [Google Scholar]
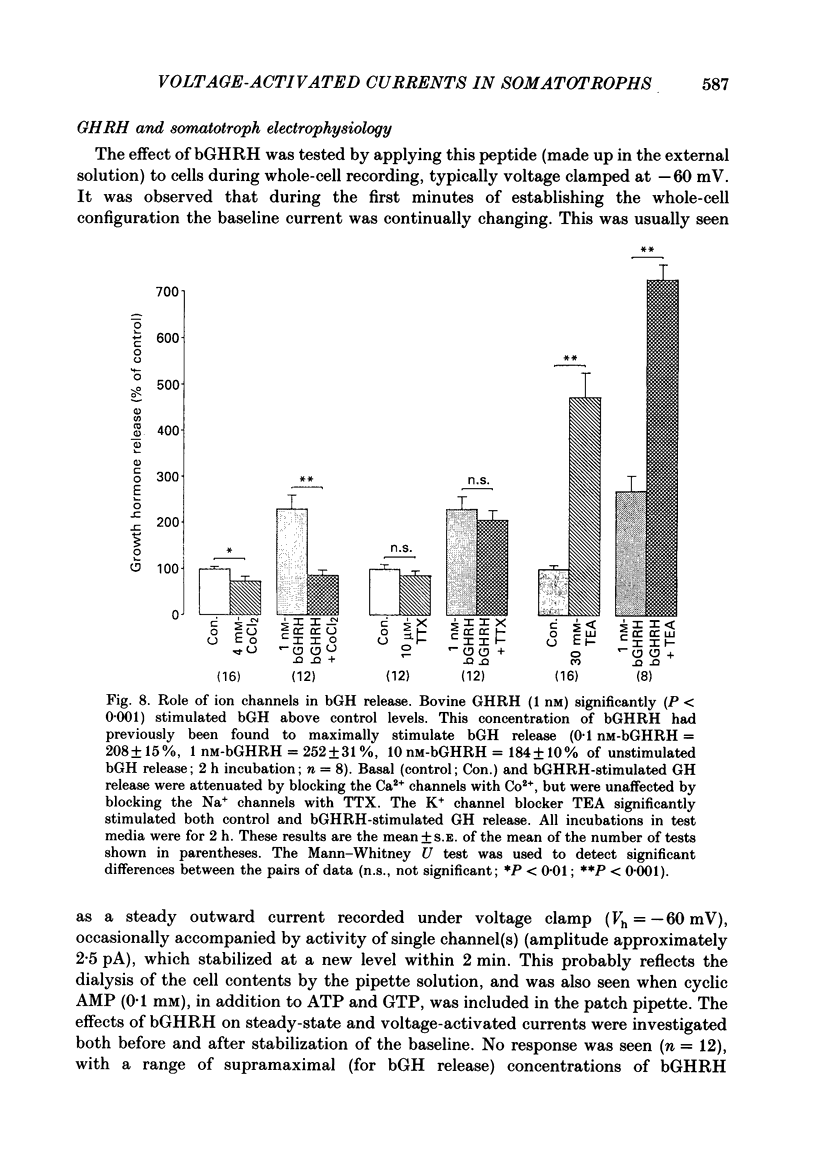
- Barros F., Katz G. M., Kaczorowski G. J., Vandlen R. L., Reuben J. P. Calcium currents in GH3 cultured pituitary cells under whole-cell voltage-clamp: inhibition by voltage-dependent potassium currents. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1108–1112. doi: 10.1073/pnas.82.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Ingram C. D., Mason W. T. Sodium and potassium currents involved in action potential propagation in normal bovine lactotrophs. J Physiol. 1987 Nov;392:273–299. doi: 10.1113/jphysiol.1987.sp016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Ingram C. D., Mason W. T. Voltage-activated currents through calcium channels in normal bovine lactotrophs. Neuroscience. 1987 Nov;23(2):661–677. doi: 10.1016/0306-4522(87)90084-4. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C. Gonadotropin release from pituitary cultures following activation of endogenous ion channels. Endocrinology. 1980 Dec;107(6):2133–2134. doi: 10.1210/endo-107-6-2133. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Dual modulation of K channels by thyrotropin-releasing hormone in clonal pituitary cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4282–4286. doi: 10.1073/pnas.82.12.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B., Jaken S., Barker J. L. Intracellular Ca2+-dependent protein kinase C activation mimics delayed effects of thyrotropin-releasing hormone on clonal pituitary cell excitability. Endocrinology. 1987 Aug;121(2):793–802. doi: 10.1210/endo-121-2-793. [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Denef C., Vincent J. D. Electrophysiological properties of normal somatotrophs in culture. An intracellular study. Neuroendocrinology. 1983 Sep;37(3):193–199. doi: 10.1159/000123542. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Lingle C. J., Sombati S., Freeman M. E. Membrane currents in identified lactotrophs of rat anterior pituitary. J Neurosci. 1986 Oct;6(10):2995–3005. doi: 10.1523/JNEUROSCI.06-10-02995.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C., Childs G. V., Brown A. M. Membrane currents of identified isolated rat corticotropes and gonadotropes. Am J Physiol. 1987 Mar;252(3 Pt 1):E340–E346. doi: 10.1152/ajpendo.1987.252.3.E340. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Ingram C. D. Techniques for studying the role of electrical activity in control of secretion by normal anterior pituitary cells. Methods Enzymol. 1986;124:207–242. doi: 10.1016/0076-6879(86)24017-3. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Sikdar S. K. Characterization of voltage-gated sodium channels in ovine gonadotrophs: relationship to hormone secretion. J Physiol. 1988 May;399:493–517. doi: 10.1113/jphysiol.1988.sp017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Electrophysiological recordings from gonadotrophs. Evidence for Ca2+ channels mediated by gonadotrophin-releasing hormone. Neuroendocrinology. 1985 Sep;41(3):258–268. doi: 10.1159/000124186. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinovitch I. Growth hormone releasing factor evokes rhythmic hyperpolarizing currents in rat anterior pituitary cells. J Physiol. 1988 Jan;395:303–318. doi: 10.1113/jphysiol.1988.sp016920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. K. Thyrotropin-releasing hormone stimulates a calcium-activated potassium current in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:611–625. doi: 10.1113/jphysiol.1987.sp016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. K. Two distinct calcium-activated potassium currents in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:591–609. doi: 10.1113/jphysiol.1987.sp016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand O., Haug E., Gautvik K. M. Effects of thyroliberin and 4-aminopyridine on action potentials and prolactin release and synthesis in rat pituitary cells in culture. Acta Physiol Scand. 1980 Mar;108(3):247–252. doi: 10.1111/j.1748-1716.1980.tb06530.x. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Catecholamines of supposed inhibitory hypophysiotrophic function suppress action potentials in prolactin cells. Nature. 1978 Dec 21;276(5690):832–834. doi: 10.1038/276832a0. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Electrical behaviour in a line of anterior pituitary cells (GH cells) and the influence of the hypothalamic peptide, thyrotrophin releasing factor. Neuroscience. 1980;5(2):421–431. doi: 10.1016/0306-4522(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Thorner M. O., Hackett J. T., Murad F., MacLeod R. M. Calcium rather than cyclic AMP as the physiological intracellular regulator of prolactin release. Neuroendocrinology. 1980 Dec;31(6):390–402. doi: 10.1159/000123109. [DOI] [PubMed] [Google Scholar]
- Zeytin F. N., Gick G. G., Brazeau P., Ling N., McLaughlin M., Bancroft C. Growth hormone (GH)-releasing factor does not regulate GH release or GH mRNA levels in GH3 cells. Endocrinology. 1984 Jun;114(6):2054–2059. doi: 10.1210/endo-114-6-2054. [DOI] [PubMed] [Google Scholar]