Abstract
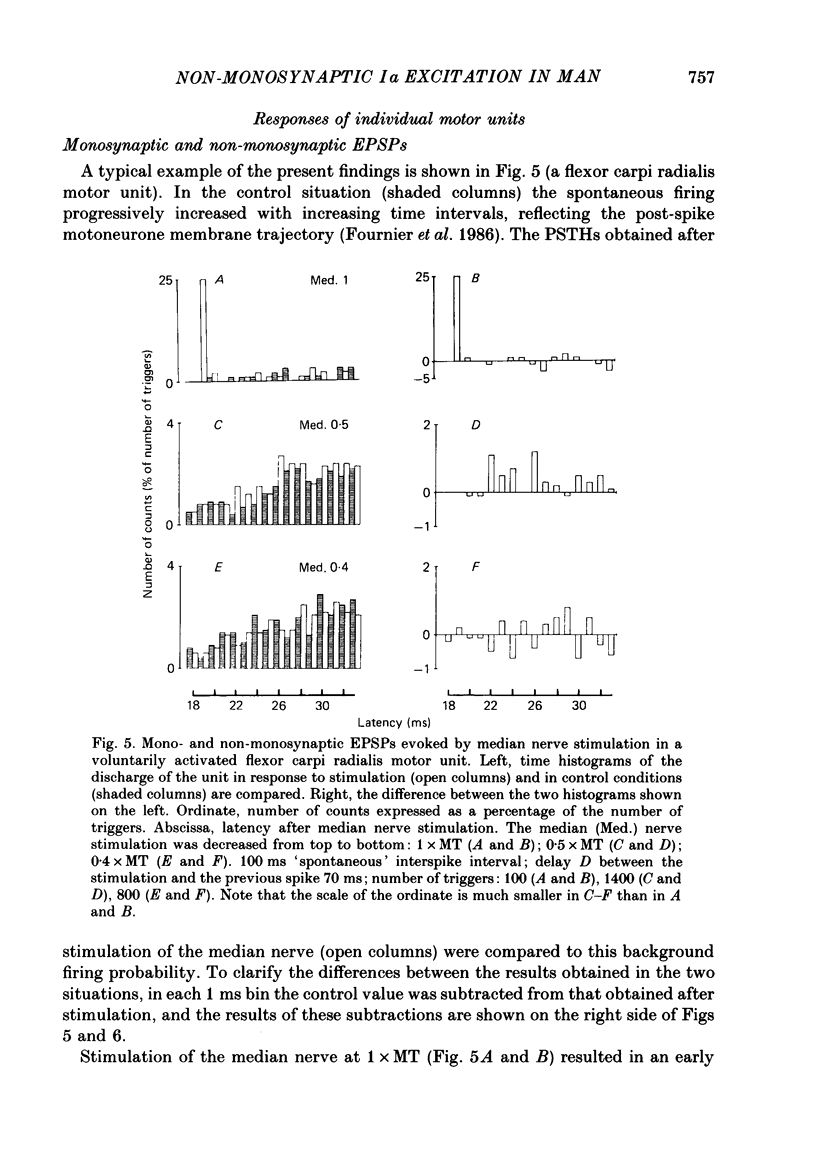
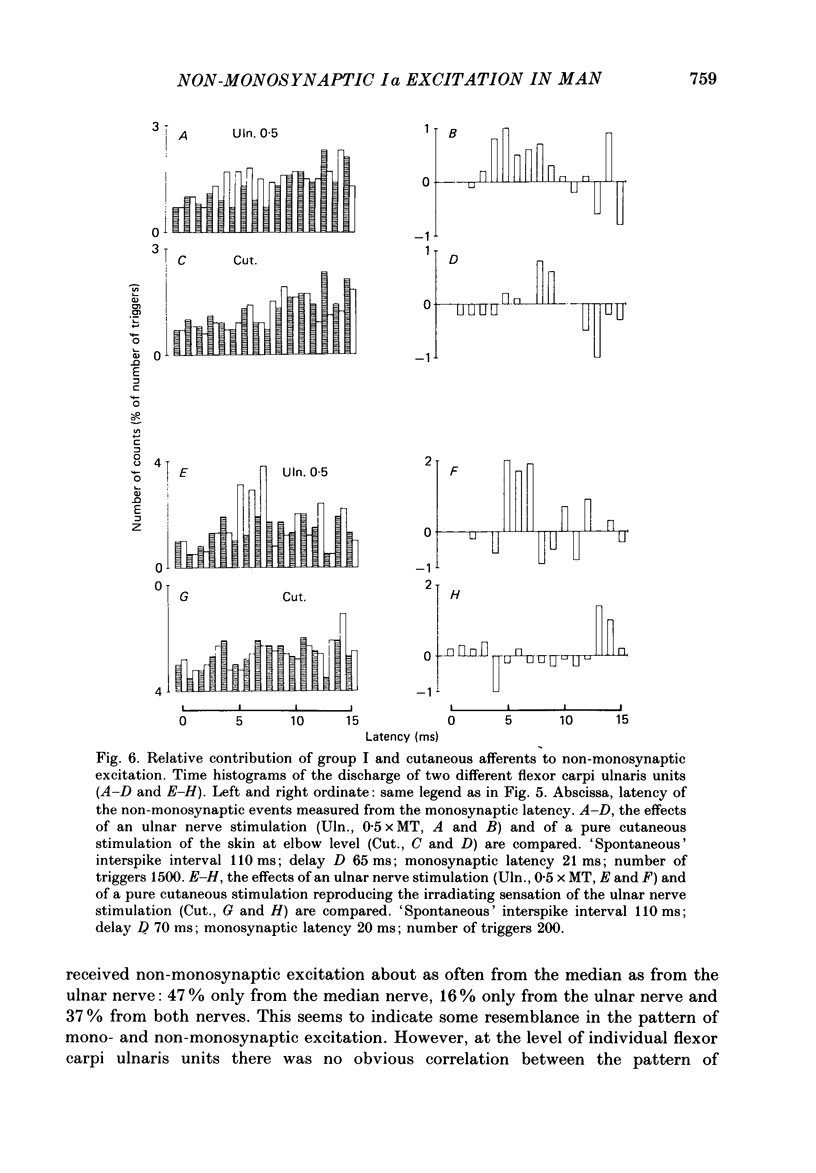
1. The possibility that activation of low-threshold afferents in the median and ulnar nerves in man evokes an interneuronally mediated excitation of wrist flexor motoneurones was investigated. Two independent techniques were used: (i) the indirect technique of spatial and temporal facilitation of the flexor carpi radialis H reflex; (ii) the post-stimulus time histogram (PSTH) method for measurement of the firing probability of voluntarily activated motor units following different stimuli. 2. Conditioning volleys were evoked by weak electrical stimuli (to the median nerve, the ulnar nerve, the skin and cutaneous nerve branches) and by a tap to the tendon of the flexor carpi radialis muscle. 3. In the H reflex experiments the comparison was drawn between the effects of two conditioning stimuli applied separately or together. In some experimental conditions the facilitation of the reflex evoked when combining two conditioning stimuli was larger than the algebraic sum of the effects from separate stimuli. The central latency of this additional facilitation, which is denoted 'extra facilitation', was 3 ms. It is argued that the extra facilitation reflects summation at a premotoneuronal level. 4. PSTHs of voluntarily activated flexor carpi radialis and flexor carpi ulnaris motor units were computed following stimulation of the median or the ulnar nerve. In 60% of the recordings the afferent volley evoked a peak of increased firing probability with a latency which was 3-6 ms longer than the monosynaptic Ia latency. 5. Both the extra facilitation of the reflex and the late peak in the PSTH were evoked from very low-threshold afferents. A contribution from Ia afferents was demonstrated in reflex experiments. Group I afferents alone are responsible for the onset of the non-monosynaptic excitation but it is probable that cutaneous afferents give a later contribution. 6. On the basis of the characteristics of the non-monosynaptic excitation studied with the two methods (same very low-threshold, comparable central latencies), it is argued that both the extra facilitation of the H reflex on combined stimulation and the late peak in the PSTHs from individual motor units are mediated through the same pre-motoneuronal pathway. 7. The possibility that the non-monosynaptic excitation may be mediated through propriospinal neurones is discussed.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B., Lundberg A., Norrsell U., Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981;42(3-4):299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Day B. L., Marsden C. D., Obeso J. A., Rothwell J. C. Reciprocal inhibition between the muscles of the human forearm. J Physiol. 1984 Apr;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Meunier S., Pierrot-Deseilligny E., Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986 Aug;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Yamashita N., Shimada Y. Facilitation of H-reflex by homonymous Ia-afferent fibers in man. J Neurophysiol. 1982 Nov;48(5):1079–1088. doi: 10.1152/jn.1982.48.5.1079. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol. 1980 Jun;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Pierrot-Deseilligny E., Shindo M. Changes in polysynaptic Ia excitation to quadriceps motoneurones during voluntary contraction in man. Exp Brain Res. 1986;63(2):436–438. doi: 10.1007/BF00236863. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Padel Y., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3--C4 propriospinal neurones. Exp Brain Res. 1978 Sep 15;33(1):101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriospinal neurones transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp Brain Res. 1977 Sep 28;29(3-4):323–346. doi: 10.1007/BF00236174. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Evidence that low threshold afferents both evoke and depress polysynaptic excitation of wrist flexor motoneurones in man. Exp Brain Res. 1987;67(2):429–432. doi: 10.1007/BF00248563. [DOI] [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Inhibition of neurones transmitting non-monosynaptic Ia excitation to human wrist flexor motoneurones. J Physiol. 1988 Nov;405:765–783. doi: 10.1113/jphysiol.1988.sp017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C. C., Ashby P., Wang M., McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56(2):341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P., Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976 Sep 23;263(5575):343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]



