Abstract
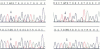
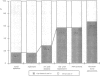
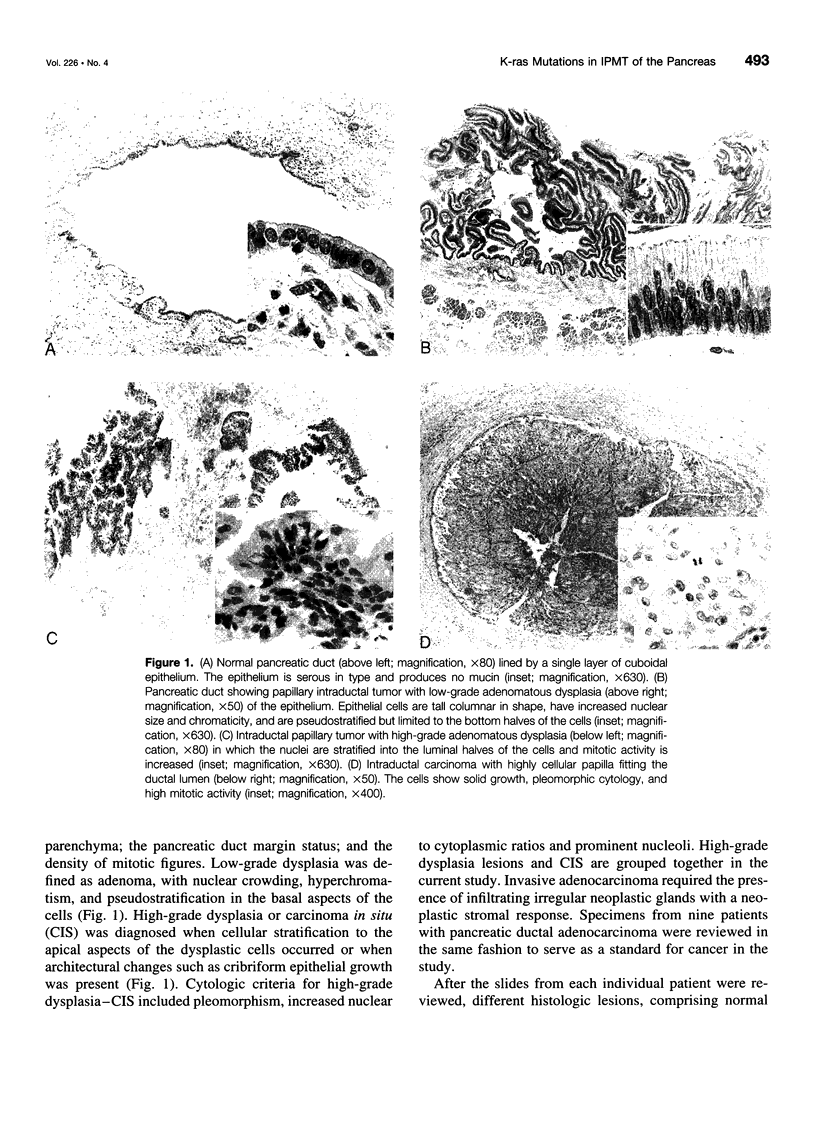
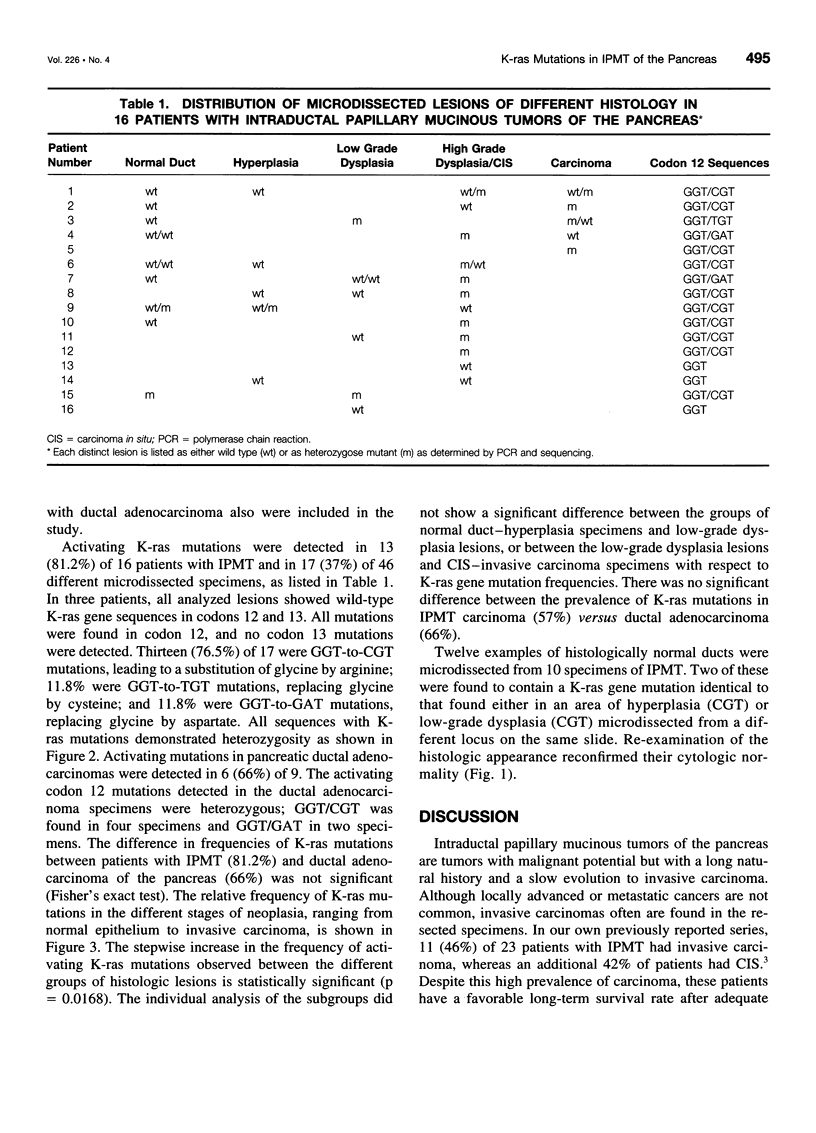
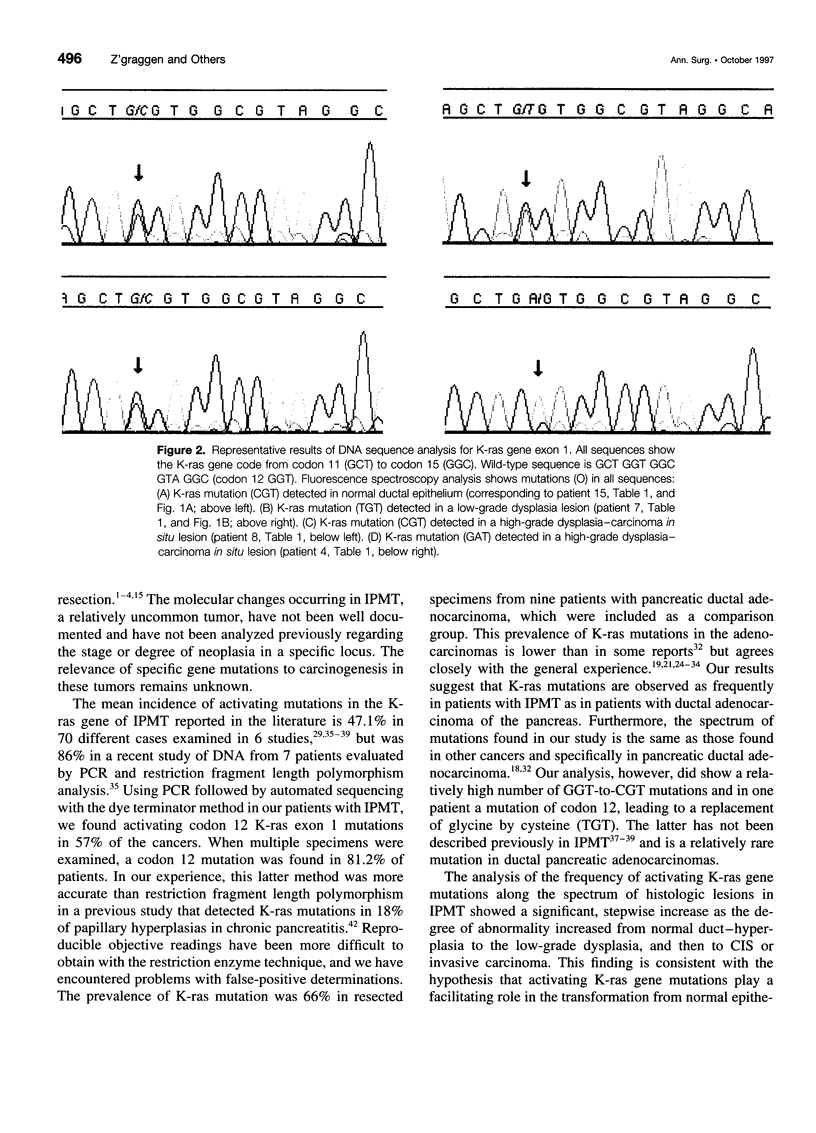
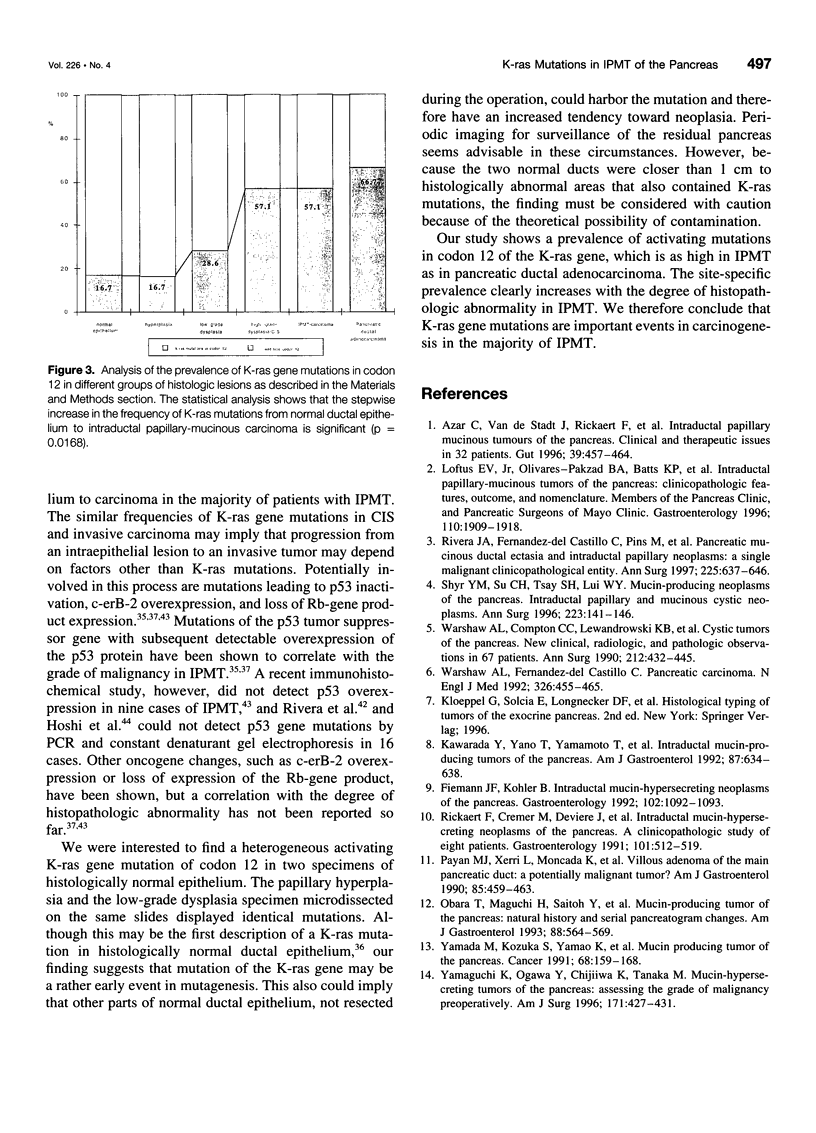
OBJECTIVE: The purpose of the study was to determine the prevalence of activating K-ras mutations in the pancreas of patients with intraductal papillary mucinous tumors (IPMT) and to analyze their relation to the degree of site-specific histopathologic abnormality. BACKGROUND: Intraductal papillary mucinous tumors of the pancreas have a biologic behavior that is significantly different from pancreatic ductal adenocarcinoma. Activating K-ras mutations, which may be important events in a multistage process of carcinogenesis, have been reported in IPMT. METHODS: Forty-six different histologic specimens (comprising normal pancreatic ducts, hyperplasia, low-grade dysplasia, high-grade dysplasia-carcinoma in situ, and carcinoma) from 16 patients with IPMT and 9 specimens from patients with pancreatic ductal adenocarcinomas were designated by a pathologist. Genomic DNA was extracted from paraffin-embedded tissue sections after microdissection. The K-ras gene was amplified by polymerase chain reaction and subjected to DNA sequencing. RESULTS: The K-ras mutations were detected in at least one specimen in 13 (81.2%) of 16 patients with IPMT. All mutations were found in codon 12. No codon 13 mutations were detected. The relative frequency of K-ras mutations in the different stages of IPMT was 16.7% in normal epithelium and papillary hyperplasia, 28.6% in low-grade dysplasia, and 57.1% in high-grade dysplasia-carcinoma in situ and invasive carcinoma. The K-ras mutations were detected in 6 (66%) of 9 pancreatic ductal adenocarcinomas. CONCLUSIONS: The K-ras codon 12 point mutations are as frequent in IPMT as in ductal adenocarcinoma. A stepwise increase in the frequency of codon 12 mutations correlated with the stage of neoplastic evolution to cancer. This finding is consistent with an important role of K-ras gene mutations in the transformation from normal epithelium to invasive carcinoma in the majority of patients with IPMT.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Azar C., Van de Stadt J., Rickaert F., Devière M., Baize M., Klöppel G., Gelin M., Cremer M. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996 Sep;39(3):457–464. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Burmer G. C., Rabinovitch P. S., Loeb L. A. Frequency and spectrum of c-Ki-ras mutations in human sporadic colon carcinoma, carcinomas arising in ulcerative colitis, and pancreatic adenocarcinoma. Environ Health Perspect. 1991 Jun;93:27–31. doi: 10.1289/ehp.919327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella G., Cronauer-Mitra S., Pienado M. A., Perucho M. Frequency and spectrum of mutations at codons 12 and 13 of the c-K-ras gene in human tumors. Environ Health Perspect. 1991 Jun;93:125–131. doi: 10.1289/ehp.9193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess H., Berberat P., Schilling M., Kunz J., Korc M., Büchler M. W. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med (Berl) 1996 Jan;74(1):35–42. doi: 10.1007/BF00202070. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cadavid N. F., Zhou D., Battifora H., Bar-Eli M., Cline M. J. Direct sequencing analysis of exon 1 of the c-K-ras gene shows a low frequency of mutations in human pancreatic adenocarcinomas. Oncogene. 1989 Sep;4(9):1137–1140. [PubMed] [Google Scholar]
- Grünewald K., Lyons J., Fröhlich A., Feichtinger H., Weger R. A., Schwab G., Janssen J. W., Bartram C. R. High frequency of Ki-ras codon 12 mutations in pancreatic adenocarcinomas. Int J Cancer. 1989 Jun 15;43(6):1037–1041. doi: 10.1002/ijc.2910430614. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Imai M., Ogawa K. Frequent K-ras mutations and absence of p53 mutations in mucin-producing tumors of the pancreas. J Surg Oncol. 1994 Feb;55(2):84–91. doi: 10.1002/jso.2930550205. [DOI] [PubMed] [Google Scholar]
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Kawarada Y., Yano T., Yamamoto T., Yokoi H., Imai T., Ogura Y., Mizumoto R. Intraductal mucin-producing tumors of the pancreas. Am J Gastroenterol. 1992 May;87(5):634–638. [PubMed] [Google Scholar]
- Lemoine N. R., Jain S., Hughes C. M., Staddon S. L., Maillet B., Hall P. A., Klöppel G. Ki-ras oncogene activation in preinvasive pancreatic cancer. Gastroenterology. 1992 Jan;102(1):230–236. doi: 10.1016/0016-5085(92)91805-e. [DOI] [PubMed] [Google Scholar]
- Loftus E. V., Jr, Olivares-Pakzad B. A., Batts K. P., Adkins M. C., Stephens D. H., Sarr M. G., DiMagno E. P. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996 Jun;110(6):1909–1918. doi: 10.1053/gast.1996.v110.pm8964418. [DOI] [PubMed] [Google Scholar]
- Mariyama M., Kishi K., Nakamura K., Obata H., Nishimura S. Frequency and types of point mutation at the 12th codon of the c-Ki-ras gene found in pancreatic cancers from Japanese patients. Jpn J Cancer Res. 1989 Jul;80(7):622–626. doi: 10.1111/j.1349-7006.1989.tb01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima K., Tsunoda T., Kanematsu T., Nagata Y., Urano T., Shiku H. Distinguishing pancreatic carcinoma from other periampullary carcinomas by analysis of mutations in the Kirsten-ras oncogene. Ann Surg. 1991 Dec;214(6):657–662. doi: 10.1097/00000658-199112000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Abe M., Motoshima K., Nakayama E., Shiku H. Frequent glycine-to-aspartic acid mutations at codon 12 of c-Ki-ras gene in human pancreatic cancer in Japanese. Jpn J Cancer Res. 1990 Feb;81(2):135–140. doi: 10.1111/j.1349-7006.1990.tb02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman W. L., Wasylyshyn M. L., Jacoby R., Erroi F., Angriman I., Montag A., Brasitus T., Michelassi F., Westbrook C. A. Evidence for a common molecular pathogenesis in colorectal, gastric, and pancreatic cancer. Genes Chromosomes Cancer. 1991 Nov;3(6):468–473. doi: 10.1002/gcc.2870030609. [DOI] [PubMed] [Google Scholar]
- Obara T., Maguchi H., Saitoh Y., Itoh A., Arisato S., Ashida T., Nishino N., Ura H., Namiki M. Mucin-producing tumor of the pancreas: natural history and serial pancreatogram changes. Am J Gastroenterol. 1993 Apr;88(4):564–569. [PubMed] [Google Scholar]
- Obara T., Maguchi H., Saitoh Y., Ura H., Koike Y., Kitazawa S., Namiki M. Mucin-producing tumor of the pancreas: a unique clinical entity. Am J Gastroenterol. 1991 Nov;86(11):1619–1625. [PubMed] [Google Scholar]
- Obara T., Saitoh Y., Maguchi H., Ura H., Yokota K., Okamura K., Namiki M. Papillary adenoma of the pancreas with excessive mucin secretion. Pancreas. 1992;7(1):114–117. doi: 10.1097/00006676-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Payan M. J., Xerri L., Moncada K., Bastid C., Agostini S., Sastre B., Sahel J., Choux R. Villous adenoma of the main pancreatic duct: a potentially malignant tumor? Am J Gastroenterol. 1990 Apr;85(4):459–463. [PubMed] [Google Scholar]
- Rall C. J., Yan Y. X., Graeme-Cook F., Beauchamp R., Yandell D. W., Povoski S. P., Rustgi A. K. Ki-ras and p53 mutations in pancreatic ductal adenocarcinoma. Pancreas. 1996 Jan;12(1):10–17. doi: 10.1097/00006676-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Rickaert F., Cremer M., Devière J., Tavares L., Lambilliotte J. P., Schröder S., Wurbs D., Klöppel G. Intraductal mucin-hypersecreting neoplasms of the pancreas. A clinicopathologic study of eight patients. Gastroenterology. 1991 Aug;101(2):512–519. doi: 10.1016/0016-5085(91)90032-g. [DOI] [PubMed] [Google Scholar]
- Riemann J. F., Kohler B. Intraductal mucin-hypersecreting neoplasms of the pancreas. Gastroenterology. 1992 Mar;102(3):1092–1093. doi: 10.1016/0016-5085(92)90220-s. [DOI] [PubMed] [Google Scholar]
- Rivera J. A., Fernández-del Castillo C., Pins M., Compton C. C., Lewandrowski K. B., Rattner D. W., Warshaw A. L. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms. A single malignant clinicopathologic entity. Ann Surg. 1997 Jun;225(6):637–646. doi: 10.1097/00000658-199706000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J. A., Rall C. J., Graeme-Cook F., Fernández-del Castillo C., Shu P., Lakey N., Tepper R., Rattner D. W., Warshaw A. L., Rustgi A. K. Analysis of K-ras oncogene mutations in chronic pancreatitis with ductal hyperplasia. Surgery. 1997 Jan;121(1):42–49. doi: 10.1016/s0039-6060(97)90181-1. [DOI] [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Satoh K., Sawai T., Shimosegawa T., Koizumi M., Yamazaki T., Mochizuki F., Toyota T. The point mutation of c-Ki-ras at codon 12 in carcinoma of the pancreatic head region and in intraductal mucin-hypersecreting neoplasm of the pancreas. Int J Pancreatol. 1993 Oct;14(2):135–143. doi: 10.1007/BF02786119. [DOI] [PubMed] [Google Scholar]
- Satoh K., Shimosegawa T., Moriizumi S., Koizumi M., Toyota T. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas. 1996 May;12(4):362–368. doi: 10.1097/00006676-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Sessa F., Solcia E., Capella C., Bonato M., Scarpa A., Zamboni G., Pellegata N. S., Ranzani G. N., Rickaert F., Klöppel G. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425(4):357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- Shibata D., Capella G., Perucho M. Mutational activation of the c-K-ras gene in human pancreatic carcinoma. Baillieres Clin Gastroenterol. 1990 Mar;4(1):151–169. doi: 10.1016/0950-3528(90)90044-h. [DOI] [PubMed] [Google Scholar]
- Shyr Y. M., Su C. H., Tsay S. H., Lui W. Y. Mucin-producing neoplasms of the pancreas. Intraductal papillary and mucinous cystic neoplasms. Ann Surg. 1996 Feb;223(2):141–146. doi: 10.1097/00000658-199602000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit V. T., Boot A. J., Smits A. M., Fleuren G. J., Cornelisse C. J., Bos J. L. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Omata M., Ohto M. Ras gene mutations in intraductal papillary neoplasms of the pancreas. Analysis in five cases. Cancer. 1991 Feb 1;67(3):634–637. doi: 10.1002/1097-0142(19910201)67:3<634::aid-cncr2820670318>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Terada T., Ohta T., Nakanuma Y. Expression of oncogene products, anti-oncogene products and oncofetal antigens in intraductal papillary-mucinous neoplasm of the pancreas. Histopathology. 1996 Oct;29(4):355–361. doi: 10.1111/j.1365-2559.1996.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Compton C. C., Lewandrowski K., Cardenosa G., Mueller P. R. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990 Oct;212(4):432–445. doi: 10.1097/00000658-199010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw A. L., Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992 Feb 13;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Yamada M., Kozuka S., Yamao K., Nakazawa S., Naitoh Y., Tsukamoto Y. Mucin-producing tumor of the pancreas. Cancer. 1991 Jul 1;68(1):159–168. doi: 10.1002/1097-0142(19910701)68:1<159::aid-cncr2820680129>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Ogawa Y., Chijiiwa K., Tanaka M. Mucin-hypersecreting tumors of the pancreas: assessing the grade of malignancy preoperatively. Am J Surg. 1996 Apr;171(4):427–431. doi: 10.1016/S0002-9610(97)89624-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Tanaka M. Mucin-hypersecreting tumor of the pancreas with mucin extrusion through an enlarged papilla. Am J Gastroenterol. 1991 Jul;86(7):835–839. [PubMed] [Google Scholar]
- Yanagisawa A., Kato Y., Ohtake K., Kitagawa T., Ohashi K., Hori M., Takagi K., Sugano H. c-Ki-ras point mutations in ductectatic-type mucinous cystic neoplasms of the pancreas. Jpn J Cancer Res. 1991 Oct;82(10):1057–1060. doi: 10.1111/j.1349-7006.1991.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]