Abstract
Background
Surrogate insulin resistance (IR) indices are simpler and more practical alternatives to insulin-based IR indicators for clinical use. This study explored the association between surrogate IR indices, including triglyceride-glucose index (TyG), triglyceride glucose-body mass index (TyG-BMI), triglyceride glucose-waist circumference (TyG-WC), triglyceride glucose-waist to height ratio (TyG-WHtR), metabolic score for insulin resistance (METS-IR), and the triglycerides/high-density lipoprotein cholesterol (TG/HDL-C) ratio, and coronary artery disease (CAD) in patients with type 2 diabetes (T2D).
Methods
Patients with T2D were enrolled in this study and divided into two groups, matched for age and diabetes duration: those with CAD and those without CAD. The association between surrogate IR indices and CAD was evaluated using restricted cubic spline (RCS) and multivariable logistic regression and their discriminative ability was assessed via Receiver operating characteristic (ROC) curve analysis. Additionally, machine learning models, including Logistic Regression, Random Forest, eXtreme Gradient Boosting (XGBoost), Light Gradient Boosting Machine (LightGBM), and Support Vector Machine (SVM), were employed to predict CAD presence using multiple surrogate IR indices and their components.
Results
All surrogate IR indices exhibited non-linear associations with CAD. TyG demonstrated a U-shaped relationship, where both extremely low and high levels were associated with higher odds of CAD compared to intermediate levels. The surrogate IR indices showed a relatively strong discriminative ability for CAD, with AUC values exceeding 0.708 across all indices. The TG/HDL-C ratio displayed the highest AUC (0.721), accuracy (68%), and sensitivity (71%), whereas TyG-WC showed the highest specificity (78%). Machine learning algorithms (except logistic regression) demonstrated greater discriminative power than individual IR indices. Random forest and XGBoost revealed the best performance when using either multiple surrogate IR indices or their components.
Conclusions
Surrogate IR indices could be used as valuable tools for evaluating cardiometabolic risk in patients with T2D, who are at high risk for CAD. Integrating machine learning models further improved CAD prediction, underscoring their potential for better risk stratification. The observed association between these indices and CAD in T2D may help clarify the complex pathophysiology of CAD and offer insights for future research.
Keywords: Insulin resistance, Triglyceride-glucose index, Coronary artery disease, Diabetes mellitus, Lipids, Machine learning
Introduction
Diabetes is often accompanied by several cardiovascular risk factors, including hypertension, dyslipidemia, and obesity [1–6]. Patients with diabetes have a 2 to 4 times higher risk of developing coronary artery disease (CAD) compared to non-diabetics [7]. In addition, the clinical presentation of CAD in people with diabetes is milder and atypical, with an estimated 20–60% of patients with diabetes having asymptomatic CAD [8, 9]. The high prevalence of silent CAD in diabetes highlights the need for early prediction and appropriate screening to improve prognosis [9, 10]. Various non-invasive tests, such as functional tests for myocardial ischemia, coronary artery calcium (CAC) scans, and coronary computed tomography angiography (CTA), are employed to assess the presence and severity of CAD [11]. However, using these screening tests in asymptomatic individuals requires careful evaluation, considering their prognostic value, impact on treatment and outcomes, cost-effectiveness, and risks such as high radiation exposure [10, 12].
Insulin resistance (IR) is associated with the presence of CAD in individuals with diabetes [13]. Various methods are used to assess IR, including direct measures such as the hyperinsulinemic euglycemic glucose clamp and insulin-suppression test (IST), indirect measures such as the minimal model analysis of the frequently sampled intravenous glucose tolerance test (FSIVGTT) and the oral glucose tolerance test (OGTT), and surrogate IR indices [14]. While direct and indirect methods are difficult, time-consuming, and require specialized expertise, surrogate indices provide a more practical and accessible alternative for clinical use [15]. Moreover, given that many patients with diabetes are prescribed insulin, using insulin-based indicators may not be suitable for this population [16].
Earlier studies have investigated the association between surrogate IR indices and CAD in the general population. A meta-analysis found that higher triglyceride-glucose (TyG) index levels are linked to an increased risk of CAD, and TyG can aid in managing patients with CAD [17]. Yang et al. reported a linear relationship between triglyceride glucose-body mass index (TyG-BMI) and CAD severity in female patients with acute coronary syndromes [18]. In addition, a longitudinal study showed that metabolic score for insulin resistance (METS-IR) predicts ischemic heart disease (IHD) in individuals without diabetes [19]. Furthermore, the triglycerides/high-density lipoprotein cholesterol (TG/HDL-C) ratio is significantly associated with CAD-related outcomes in both the general population and individuals with established CAD [20].
The association between surrogate IR indices and CAD in individuals with diabetes has been evaluated in a limited number of studies, with a primary focus on TyG [21, 22]. Additionally, the comparative advantage of these indices in predicting CAD in individuals with diabetes has yet to be determined. Clarifying these associations could provide valuable data for identifying a more accessible tool for screening CAD in diabetes. This study aimed to investigate the association of TyG, TyG-BMI, triglyceride glucose-waist to height ratio (TyG-WHtR), triglyceride glucose-waist circumference (TyG-WC), METS-IR, and TG/HDL-C ratio with CAD in patients with type 2 diabetes (T2D).
Methods
Study design
Individuals with T2D who were referred to the diabetes clinic of a tertiary hospital affiliated with Tehran University of Medical Sciences were enrolled in this study. Participants were divided into two groups based on CAD status, matched by age and duration of diabetes. Individuals were excluded if they had malignancies, thyroid disease, liver cirrhosis, pregnancy, smoked, or used antioxidant supplements or oral contraceptives. Participants were homogenous, had a middle to high school education level, and were mostly from the middle class of socio-economic status. The study population had access to medical care facilities and insurance coverage. The participants followed a regular diet and engaged in routine physical activity. The research ethics committee of Tehran University of Medical Sciences approved this study. All participants provided written informed consent before participating, and the study adhered to the declaration of Helsinki.
Data collection
Demographic characteristics of the study population, such as age, diabetes duration, and gender, were gathered. Additionally, a complete medical history, including acute or chronic diseases, diabetes complications, and medications, was taken. Well-trained nurses assessed weight, height, waist circumference (WC), and blood pressure. Weight was measured to the nearest 0.1 kg using a digital scale (Tefal PP1100), with participants in light clothing and barefoot. Height and WC were measured using non-elastic tapes with an accuracy of 0.1 cm. Height was measured with the participant standing upright with their feet together. The WC was considered at the midpoint between the lower rib cage and the iliac crest [23]. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m²). The waist-to-height ratio (WHtR) was determined by dividing waist circumference (cm) by height (cm). After resting for at least 3–5 min while seated without talking or moving around, participants were asked to place their right hand on a surface at the level of the fourth intercostal space with their palms facing upwards and their back supported [24]. Blood pressure (BP), including systolic and diastolic measurements, was taken twice, ten minutes apart, and the median value was recorded. Measurements were only conducted if participants had avoided caffeine, exercise, and smoking for at least 30 min before admission [24]. A calibrated digital sphygmomanometer (Omron M7, Hoofddorp, The Netherlands) was used to assess BP.
Venous blood samples were collected from each individual after 12 h of fasting (only water was allowed) to measure fasting blood sugar (FBS), serum creatinine, hemoglobin A1c (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). 2-hour postprandial blood glucose (2hpp) was analyzed from specimens collected two hours after the beginning of an adequate breakfast. FBS and 2hpp were measured using the glucose oxidase method with the Parsazmun auto-analyzer (BT-3000 (plus), Biotechnica). TC, TG, LDL-C, HDL-C, and creatinine were quantified using enzymatic techniques with the Parsazmun auto-analyzer (BT-3000 (plus), Biotechnica). HbA1c was measured via high-performance liquid chromatography (HPLC) using the DS5 analyzer (Drew, England). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [25]. Non-high-density lipoprotein cholesterol (non-HDL-C) was determined by subtracting HDL-C from TC.
Indices formulas
TyG = Ln(TG (mg/dL)×FBS (mg/dL))/2
TyG-BMI = TyG×BMI (kg/m2)
TyG-WC = TyG×WC (cm)
TyG-WHtR = TyG×WHtR
METS-IR = Ln [(2×FBS (mg/dL)) + TG (mg/dL)] × BMI (kg/m2))/(Ln [HDL-C (mg/dL)])
TG/HDL-C ratio = TG (mg/dL)/HDL-C (mg/dL)
Definitions
Diabetes was determined according to the American Diabetes Association (ADA) criteria as follows: FBS ≥ 126 mg/dL, 2hpp ≥ 200 mg/dL, or HbA1c ≥ 6.5% [26]. Hypertension was defined as BP ≥ 130/80 mmHg or being on antihypertensive therapy [27]. CAD was determined based on a positive medical history of myocardial infarction (MI), unstable angina, cardiac care unit admission, coronary revascularization procedures (percutaneous interventions or bypass graft surgery), or abnormal results of coronary angiography.
Medications
The study population was treated with oral anti-diabetic drugs (OADs), insulin, or a combination of both. Insulin glargine or NPH was used alongside mealtime insulin aspart or regular, respectively. Dyslipidemia medications included atorvastatin, rosuvastatin, and fibrates, with atorvastatin being the most commonly prescribed due to its availability and cost-effectiveness. The medication adherence among the study population was consistent. Several medications can impact surrogate IR indices by affecting glucose and lipid metabolism. For instance, insulin lowers blood glucose and suppresses lipolysis, leading to lower TG levels [28]. In addition, dyslipidemia drugs such as statins and fibrates reduce TG levels, a key component of the TyG [28]. To ensure reliable results, we have adjusted for these medications in the analysis.
Statistical analysis
Data analysis was performed using Python (version 3.12) along with libraries such as NumPy (1.26), Pandas (2.1.4), Matplotlib (3.8.1), Scikit-learn (1.4.0), SciPy (1.13.1), XGBoost (2.1.3), and Lightgbm (4.6.0), as well as SPSS (version 22.0, IBM Corporation, USA) [29–35]. To assess normality, the Kolmogorov-Smirnov test and visual inspections of P-P plots and histograms were used. Categorical variables were shown as frequencies and percentages. Continuous variables were presented as mean ± standard deviation (SD) or median (Q1–Q3). Student’s t-test and Mann-Whitney U test were used for continuous variables, and the chi-squared test for categorical variables.
Restricted cubic spline (RCS) analysis with four knots at the 5th, 35th, 65th, and 95th percentiles was utilized to examine non-linear associations between CAD and surrogate IR indices, with the median of each index as the reference (odds ratio = 1).
To further examine the relationship between surrogate IR indices and CAD, multivariable logistic regression was performed. Each index was divided into quartiles, and the odds of having CAD were compared across quartiles, with the first quartile as the reference. This analysis began with an unadjusted model and was followed by adjustments for confounding factors, including gender, hypertension, dyslipidemia drug, antihyperglycemic agents, LDL-C, systolic blood pressure, and BMI; variables that showed significant group differences and are well-established risk factors for CAD. The results were reported as odds ratios (ORs) with their 95% confidence intervals (CIs).
The predictive value of surrogate IR indices for CAD was assessed using a receiver operating characteristic (ROC) curve. The ROC curve analysis was adjusted for gender, hypertension, dyslipidemia drug, antihyperglycemic agents, LDL-C, systolic blood pressure, and BMI. Cut-off values were determined based on the maximum Youden index. Statistical significance was defined as a p-value of less than 0.05.
Machine learning analysis
For data preprocessing, all variables were standardized to facilitate comparability. The data was subsequently divided into training and testing groups following a 70–30% split, assigning 70% of the data to the training group and 30% to the testing group. Five machine learning models, including Logistic Regression, Random Forest, eXtreme Gradient Boosting (XGBoost), Light Gradient Boosting Machine (LightGBM), and Support Vector Machine (SVM), were trained using the training subset, and their performance was assessed on the testing subset. Model performance was evaluated using the area under the curve (AUC) derived from ROC curves, reflecting each model’s capability to distinguish between CAD and non-CAD cases. To evaluate potential multicollinearity among predictor variables, the variance inflation factor (VIF) was computed for each variable. Variables with a VIF exceeding 5 were eliminated from the analysis to reduce the risk of overfitting and enhance model stability.
To evaluate the robustness of the models, a 10-fold cross-validation was implemented. The dataset was partitioned into 10 subsets, with each subset acting as the test set one time, while the remaining 9 subsets were utilized for training. This procedure was repeated 10 times. In addition, hyperparameter tuning was conducted using the grid search embedded in the cross-validation process. A predefined set of hyperparameters was examined for each model to optimize performance. The AUC from the ROC curve was the primary performance metric. The mean and SD of AUC values across cross-validation folds were reported.
Results
Baseline characteristics
A total of 2702 patients with T2D were studied, comprising 1376 individuals with CAD and 1326 without CAD. Table 1 presents the baseline characteristics of the study population, both overall and stratified by CAD status. Participants with CAD included a higher proportion of men, were more often hypertensive, and had higher WC and TG levels (all p-values < 0.001). Additionally, individuals with CAD showed higher systolic blood pressure (SBP) (p-value = 0.007), WHtR (p-value = 0.012), and BMI (p-value = 0.002) compared to those without CAD. TC, LDL-C, HDL-C, and non-HDL-C levels were higher among patients without CAD (all p-values < 0.001). Diastolic blood pressure (DBP), FBS, 2hpp, HbA1c, and eGFR did not significantly differ between the two groups (Table 1).
Table 1.
Baseline characteristics of the study population: patients with and without CAD
| Variable | Total (N = 2702) |
Patients without CAD (N = 1326) |
Patients with CAD (N = 1376) |
P-value | ||
|---|---|---|---|---|---|---|
| Age (years) | 62.7 ± 9.3 | 62.6 ± 9.2 | 62.8 ± 9.4 | - | ||
| Duration of Diabetes (years) | 12 (6–20) | 11 (6–18) | 12 (6–20) | - | ||
| Gender | Men (N, %) | 1380 (51.1%) | 608 (45.9%) | 772 (56.1%) | < 0.001 | |
| Women (N, %) | 1322 (48.9%) | 718 (54.1%) | 604 (43.9%) | |||
| Hypertension (N, %) | 1399 (51.8%) | 573 (43.2%) | 826 (60.0%) | < 0.001 | ||
| SBP (mmHg) | 132.4 ± 18.6 | 131.4 ± 17.9 | 133.3 ± 19.1 | 0.007 | ||
| DBP (mmHg) | 78.5 ± 9.7 | 78.7 ± 9.4 | 78.3 ± 10.0 | 0.186 | ||
| WC (cm) | 97.4 ± 10.2 | 96.4 ± 10.5 | 98.4 ± 9.8 | < 0.001 | ||
| WHtR | 0.60 ± 0.07 | 0.59 ± 0.07 | 0.60 ± 0.06 | 0.012 | ||
| BMI (kg/m2) | 28.3 ± 4.7 | 28.1 ± 4.7 | 28.6 ± 4.6 | 0.002 | ||
| FBS (mg/dL) | 170.9 ± 64.1 | 169.9 ± 63.0 | 171.9 ± 65.2 | 0.418 | ||
| 2hpp (mg/dL) | 239.5 ± 91.9 | 237.9 ± 93.5 | 241.1 ± 90.4 | 0.359 | ||
| HbA1c (%) | 8.05 ± 1.72 | 8.01 ± 1.73 | 8.08 ± 1.70 | 0.252 | ||
| eGFR (ml/min/1.73m2) | 73.9 ± 17.6 | 74.5 ± 17.2 | 73.4 ± 18.1 | 0.094 | ||
| TG (mg/dL) | 136 (100–192) | 128 (96–180) | 144 (106–206) | < 0.001 | ||
| TC (mg/dL) | 173.3 ± 44.3 | 181.2 ± 42.6 | 165.7 ± 44.6 | < 0.001 | ||
| LDL-C (mg/dL) | 97.1 ± 34.3 | 103.3 ± 33.8 | 91.0 ± 33.6 | < 0.001 | ||
| HDL-C (mg/dL) | 45.3 ± 11.7 | 47.3 ± 12.3 | 43.3 ± 10.7 | < 0.001 | ||
| Non-HDL-C (mg/dL) | 128.0 ± 42.2 | 133.9 ± 40.6 | 122.4 ± 42.9 | < 0.001 | ||
| Medication | ||||||
| Dyslipidemia drug N (%) | Atorvastatin | 2359 (87.3%) | 1227 (92.5%) | 1132 (82.3%) | < 0.001 | |
| Rosuvastatin | 313 (11.6%) | 92 (6.9%) | 221 (16.1%) | |||
| Fibrates | 30 (1.1%) | 7 (0.5%) | 23 (1.7%) | |||
|
Antihyperglycemic agents N (%) |
Multiple Drug Therapy | 1066 (39.5%) | 431 (32.5%) | 635 (46.1%) | < 0.001 | |
| Insulin | 16 (0.6%) | 7 (0.5%) | 9 (0.7%) | |||
| Metformin monotherapy | 1436 (53.1%) | 794 (59.9%) | 642 (46.7%) | |||
| Any sulfonylurea | 184 (6.8%) | 94 (7.1%) | 90 (6.5%) | |||
Data are presented as mean ± SD, median (Q1, Q3), or number (%)
CAD: coronary artery disease; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; WHtR: waist-to-height ratio; BMI: body mass index; FBS: fasting blood sugar; 2hpp: 2-hour postprandial glucose; HbA1c: hemoglobin A1c; eGFR: estimated glomerular filtration rate; TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; non-HDL-C: non-high-density lipoprotein cholesterol
All surrogate IR indices, including TyG (4.98 vs. 5.05), TyG-BMI (139.98 vs. 144.73), TyG-WC (480.83 vs. 497.07), TyG-WHtR (2.98 vs. 3.05), METS-IR (45.29 vs. 47.60), and TG/HDL-C (2.79 vs. 3.44), were higher in patients with CAD compared to those without CAD (all p-values < 0.001) (Table 2).
Table 2.
Comparison of surrogate insulin resistance indices by CAD status
| Insulin resistance indices | Total | Patients without CAD | Patients with CAD | P-value |
|---|---|---|---|---|
| TyG | 5.01 ± 0.33 | 4.98 ± 0.32 | 5.05 ± 0.34 | < 0.001 |
| TyG-BMI | 142.40 ± 26.56 | 139.98 ± 25.86 | 144.73 ± 27.01 | < 0.001 |
| TyG-WC | 489.10 ± 63.85 | 480.83 ± 63.32 | 497.07 ± 63.37 | < 0.001 |
| TyG-WHtR | 3.02 ± 0.43 | 2.98 ± 0.42 | 3.05 ± 0.43 | < 0.001 |
| METS-IR | 46.47 ± 8.77 | 45.29 ± 8.61 | 47.60 ± 8.78 | < 0.001 |
| TG/HDL-C | 3.13 (2.16–4.71) | 2.79 (1.98–4.17) | 3.44 (2.41–5.27) | < 0.001 |
Data are presented as mean ± SD or median (Q1, Q3)
CAD: coronary artery disease; TyG: triglyceride–glucose index; TyG-BMI: triglyceride glucose-body mass index; TyG-WC: triglyceride glucose-waist circumference index; TyG-WHtR: triglyceride glucose-waist to height ratio index; METS-IR: metabolic score for insulin resistance; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol
Association between surrogate insulin resistance indices and CAD
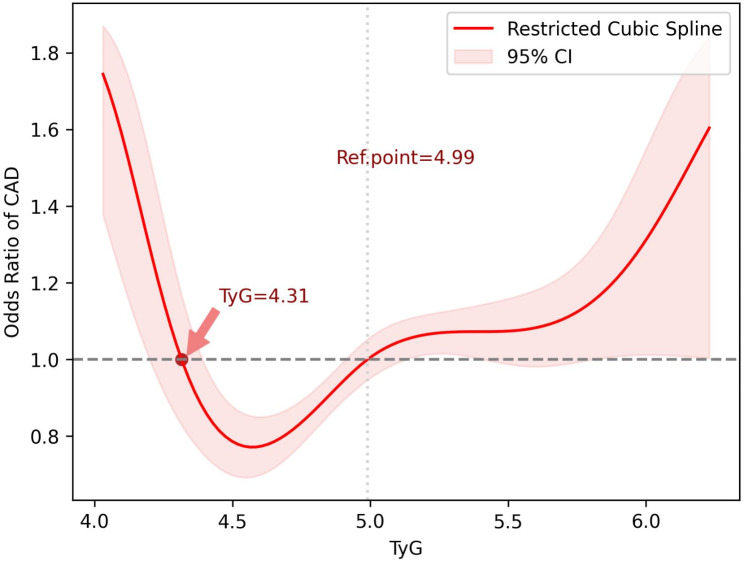
In the RCS analysis, the association between surrogate IR indices and CAD in patients with T2D was assessed (Fig. 1). Four knots were applied at the 5th,35th, 65th, and 95th percentile for each index distribution. The median of each distribution was used as the reference point, with its OR set to 1. The reference points for TyG, TyG-BMI, TyG-WC, TyG-WHtR, METS-IR, and TG/HDL-C were 4.99, 138.9, 485.9, 2.98, 45.50, and 3.13, respectively. Non-linear associations were observed between surrogate IR indices and CAD. The ORs with 95% CIs for the selected knots are provided in Table 3.
Fig. 1.
Association between surrogate insulin resistance indices and CAD in patients with T2D: The RCS function employed four knots based on the distribution of each index (5th, 35th, 65th, 95th percentiles). The ORs for CAD were calculated relative to the median value of each index. CAD: coronary artery disease; T2D: type 2 diabetes; RCS: restricted cubic spline; OR: odds ratio; TyG: triglyceride-glucose index; TyG-BMI: triglyceride glucose-body mass; TyG-WC: triglyceride glucose-waist circumference; TyG-WHtR: triglyceride glucose-waist-to-height ratio; METS-IR: metabolic score for insulin resistance; TG/HDL: triglycerides/high-density lipoprotein cholesterol ratio
Table 3.
Odds ratios for CAD at selected knots (5th, 35th, 65th, 95th percentiles) in the RCS analysis
| Insulin resistance indices | Knots | |||||||
|---|---|---|---|---|---|---|---|---|
| 5th percentile | 35th percentile | 65th percentile | 95th percentile | |||||
| Value | OR (95% CI) | Value | OR (95% CI) | Value | OR (95% CI) | Value | OR (95% CI) | |
| TyG | 4.49 | 0.72 (0.62, 0.83) | 4.88 | 0.98 (0.90, 1.05) | 5.13 | 1.01 (0.93, 1.07) | 5.61 | 1.15 (1.03, 1.26) |
| TyG-BMI | 103.3 | 0.81 (0.70, 0.92) | 130.7 | 0.98 (0.91, 1.05) | 149.6 | 1.03 (0.96, 1.10) | 191.0 | 1.16 (1.03, 1.28) |
| TyG-WC | 389.1 | 0.78 (0.67, 0.90) | 463.7 | 0.97 (0.90, 1.04) | 508.1 | 1.05 (0.98, 1.12) | 596.3 | 1.19 (1.07, 1.30) |
| TyG-WHtR | 2.35 | 0.79 (0.68, 0.91) | 2.83 | 0.97 (0.90, 1.04) | 3.14 | 1.02 (0.95, 1.09) | 3.79 | 1.19 (1.04, 1.29) |
| METS-IR | 33.69 | 0.78 (0.68, 0.89) | 42.59 | 0.92 (0.85, 0.99) | 48.76 | 1.11 (1.04, 1.17) | 62.68 | 1.19 (1.06, 1.31) |
| TG/HDL-C | 1.26 | 0.70 (0.59, 0.81) | 2.50 | 0.91 (0.85, 0.97) | 3.92 | 1.10 (1.04, 1.15) | 8.89 | 1.21 (1.08, 1.33) |
CAD: coronary artery disease; RCS: restricted cubic spline; OR: odds ratio; CI: confidence interval; TyG: triglyceride–glucose index; TyG-BMI: triglyceride glucose-body mass index; TyG-WC: triglyceride glucose-waist circumference index; TyG-WHtR: triglyceride glucose-waist to height ratio index; METS-IR: metabolic score for insulin resistance; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol
The findings indicate that as surrogate IR indices increased across percentiles, the odds of having CAD increased (Fig. 1). For TyG, the odds of CAD exhibited a U-shaped pattern. The ORs were higher than the reference point (TyG = 4.99) for values < 4.31. In the range of 4.31 < TyG < 4.99, the ORs of CAD were lower than the reference point, with the minimum OR observed at TyG = 4.56 (OR = 0.77, 95% CI:0.69–0.85). Beyond the reference point (TyG = 4.99), the ORs followed an increasing trend (Fig. 2).
Fig. 2.
U-shaped association between TyG and the odds of CAD in patients with T2D. TyG: triglyceride-glucose index; CAD: coronary artery disease; T2D: type 2 diabetes
Table 4 presents the odds ratios for CAD in patients with T2D across quartiles of each surrogate IR index, with the first quartile regarded as the reference. Results were adjusted for gender, hypertension, use of dyslipidemia drugs, antihyperglycemic agents, LDL-C, systolic blood pressure, and BMI. For all indices, the odds of CAD in patients with T2D increased significantly in the higher quartiles compared to the first quartile (Table 4).
Table 4.
Odds ratios for CAD in patients with T2D across quartiles of surrogate insulin resistance indices
| Insulin resistance indices quartiles | OR (95% CI) a | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| TyG | Q1 | Ref | - | |
| Q2 | 1.38 (1.12–1.71) | 1.49 (1.19–1.88) | ||
| Q3 | 1.40 (1.13–1.73) | 1.52 (1.20–1.91) | ||
| Q4 | 1.77 (1.43–2.20) | 2.06 (1.62–2.61) | ||
| TyG-BMI | Q1 | Ref | - | |
| Q2 | 1.33 (1.08–1.65) | 1.53 (1.11–2.11) | ||
| Q3 | 1.36 (1.10–1.68) | 1.78 (1.18–2.68) | ||
| Q4 | 1.68 (1.35–2.08) | 3.04 (1.83–5.06) | ||
| TyG-WC | Q1 | Ref | - | |
| Q2 | 1.13 (0.91–1.40) | 1.13 (0.88–1.44) | ||
| Q3 | 1.56 (1.26–1.93) | 1.51 (1.16–1.96) | ||
| Q4 | 1.94 (1.56–2.41) | 1.93 (1.43–2.62) | ||
| TyG-WHtR | Q1 | Ref | - | |
| Q2 | 1.18 (0.96–1.47) | 1.13 (0.87–1.45) | ||
| Q3 | 1.02 (0.83–1.27) | 1.06 (0.80–1.40) | ||
| Q4 | 1.60 (1.29–1.98) | 1.77 (1.28–2.45) | ||
| METS-IR | Q1 | Ref | - | |
| Q2 | 1.24 (0.99–1.53) | 1.43 (1.07–1.91) | ||
| Q3 | 1.63 (1.31–2.02) | 2.27 (1.61–3.20) | ||
| Q4 | 2.13 (1.71–2.65) | 3.78 (2.48–5.76) | ||
| TG/HDL-C | Q1 | Ref | - | |
| Q2 | 1.21 (0.97–1.50) | 1.25 (0.99–1.57) | ||
| Q3 | 1.88 (1.51–2.33) | 1.95 (1.54–2.45) | ||
| Q4 | 2.29 (1.84–2.85) | 2.44 (1.93–3.08) | ||
a: adjusted for gender, hypertension, dyslipidemia drug, antihyperglycemic agents, low-density lipoprotein cholesterol, systolic blood pressure, and body mass index
CAD: coronary artery disease; T2D: type 2 diabetes; OR: odds ratio; CI: confidence interval; TyG: triglyceride–glucose index; TyG-BMI: triglyceride glucose-body mass index; TyG-WC: triglyceride glucose-waist circumference index; TyG-WHtR: triglyceride glucose-waist to height ratio index; METS-IR: metabolic score for insulin resistance; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol
Predictive performance of surrogate insulin resistance indices for CAD
The discriminative ability of surrogate IR indices for CAD in patients with T2D is presented in Fig. 3; Table 5. Using the maximum Youden index, optimal cut-off values for TyG, TyG-BMI, TyG-WC, TyG-WHtR, METS-IR, and TG/HDL-C were identified as 4.65, 113.94, 694.78, 2.78, 45.18, and 2.33, respectively. The AUC values for all indices ranged from 0.708 to 0.721 (all p-values < 0.001). Among these indices, TG/HDL-C demonstrated the highest AUC (0.721), accuracy (68%), and sensitivity (71%), while TyG-WC exhibited the highest specificity (78%). Detailed values for sensitivity, specificity, and AUC for each index are presented in Table 5.
Fig. 3.
ROC curve showing the AUC, sensitivity, and specificity of surrogate insulin resistance indices for discriminating CAD in patients with T2D; adjusted for gender, hypertension, dyslipidemia drug, antihyperglycemic agents, low-density lipoprotein cholesterol, systolic blood pressure, and body mass index. ROC: Receiver operating characteristic; AUC: area under curve; CAD: coronary artery disease; T2D: type 2 diabetes; TyG: triglyceride-glucose index; TyG-BMI: triglyceride glucose-body mass; TyG-WC: triglyceride glucose-waist circumference; TyG-WHtR: triglyceride glucose-waist-to-height ratio; METS-IR: metabolic score for insulin resistance; TG/HDL: triglycerides/high-density lipoprotein cholesterol ratio
Table 5.
ROC analysis of surrogate insulin resistance indices for discriminating CAD status in patients with T2D
| Insulin resistance indices | Cutoff | AUC (95% CI) a | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|
| TyG | 4.65 | 0.709 (0.674–0.744) | 58% | 76% | 67% |
| TyG-BMI | 113.94 | 0.711 (0.676–0.745) | 58% | 76% | 67% |
| TyG-WC | 694.78 | 0.710 (0.675–0.747) | 55% | 78% | 67% |
| TyG-WHtR | 2.78 | 0.708 (0.673–0.743) | 65% | 67% | 66% |
| METS-IR | 45.18 | 0.713 (0.677–0.747) | 67% | 67% | 67% |
| TG/HDL-C | 2.33 | 0.721 (0.687–0.757) | 71% | 64% | 68% |
a: adjusted for gender, hypertension, dyslipidemia drug, antihyperglycemic agents, low-density lipoprotein cholesterol, systolic blood pressure, and body mass index
ROC: receiver operating characteristic; CAD: coronary artery disease; T2D: type 2 diabetes; AUC: area under the curve; CI: confidence interval; TyG: triglyceride–glucose index; TyG-BMI: triglyceride glucose-body mass index; TyG-WC: triglyceride glucose-waist circumference index; TyG-WHtR: triglyceride glucose-waist to height ratio index; METS-IR: metabolic score for insulin resistance; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol
Performance of machine learning algorithms in discriminating CAD using surrogate insulin resistance indices
Five machine learning models, including logistic regression, random forest, XGBoost, LightGBM, and SVM, were employed to predict the presence of CAD using multiple surrogate IR indices such as TyG, TyG-WHtR, and the TG/HDL-C ratio (Fig. 4). Other IR indices (TyG-BMI, TyG-WC, and METS-IR) were excluded due to high multicollinearity. AUCs across the models ranged from 0.716 to 0.792. The ROC curves (Fig. 4) show that XGBoost had the highest AUC (0.792; 95% CI: 0.763–0.822), along with the highest sensitivity (79%), specificity (67%), and accuracy (73%). All models, except for logistic regression, demonstrated higher AUCs and greater potential for discriminating CAD compared to each individual IR index (Table 6). To evaluate the robustness of predictive models, a 10-fold cross-validation was conducted. The mean AUC values (± SD) across the folds were as follows: logistic regression: 0.699 ± 0.028, random forest: 0.790 ± 0.014, XGBoost: 0.787 ± 0.014, LightGBM: 0.783 ± 0.018, and SVM: 0.716 ± 0.018. Random forest exhibited the highest mean AUC, followed by XGBoost, affirming their superior predictive capabilities among the evaluated models.
Fig. 4.
ROC curves for various machine learning models predicting CAD in patients with T2D using the combination of multiple surrogate insulin resistance indices (TyG, TyG-WHtR, and TG/HDL-C). ROC: Receiver operating characteristic; CAD: coronary artery disease; T2D: type 2 diabetes; TyG: triglyceride-glucose index; TyG-WHtR: triglyceride glucose-waist-to-height ratio; TG/HDL: triglycerides/high-density lipoprotein cholesterol ratio; AUC: area under the curve; SVM: support vector machine
Table 6.
Comparison of machine learning models for discriminating CAD status using surrogate insulin resistance indices and their components
| Machine learning models | AUC (95% CI) a | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Combination of IR indices a | ||||
| Logistic Regression | 0.716 (0.681–0.751) | 70% | 63% | 67% |
| Random Forest | 0.791 (0.760–0.820) | 78% | 64% | 71% |
| XGBoost | 0.792 (0.763–0.822) | 79% | 67% | 73% |
| LightGBM | 0.766 (0.733–0.798) | 74% | 64% | 69% |
| SVM | 0.737 (0.704–0.773) | 74% | 61% | 68% |
| Components of IR indices b | ||||
| Logistic Regression | 0.715 (0.677–0.749) | 70% | 62% | 66% |
| Random Forest | 0.791 (0.763–0.819) | 78% | 67% | 73% |
| XGBoost | 0.766 (0.733–0.797) | 74% | 64% | 69% |
| LightGBM | 0.758 (0.727–0.791) | 71% | 66% | 68% |
| SVM | 0.734 (0.698–0.768) | 76% | 59% | 67% |
a: adjusted for gender, hypertension, dyslipidemia drug, antihyperglycemic agents, low-density lipoprotein cholesterol, systolic blood pressure, and body mass index
b: adjusted for gender, hypertension, dyslipidemia drug, antihyperglycemic agents, low-density lipoprotein cholesterol, and systolic blood pressure
CAD: coronary artery disease; AUC: area under the curve; CI: confidence interval; IR: insulin resistance; SVM: support vector machine
Furthermore, we incorporated the components used to calculate the surrogate IR indices, including TG, FBS, HDL-C, BMI, and WHtR, into the machine learning models to predict CAD (Fig. 5). Other components, such as WC, were excluded due to high multicollinearity. Similar to the models based on the combination of surrogate IR indices, AUCs were higher than those for each individual surrogate IR index (except logistic regression), ranging from 0.734 to 0.791 (Table 6). The random forest model demonstrated the highest AUC (0.791; 95% CI: 0.763–0.819), along with the highest sensitivity (78%), specificity (67%), and accuracy (73%) (Fig. 5). To assess the robustness of our predictive models, we performed 10-fold cross-validation. The mean (± SD) AUC values across folds were as follows: logistic regression: 0.703 ± 0.028, random forest: 0.788 ± 0.015, XGBoost: 0.787 ± 0.023, LightGBM: 0.785 ± 0.018, and SVM: 0.716 ± 0.017. Of all the models, random forest displayed the highest mean AUC, followed by XGBoost.
Fig. 5.
ROC curves illustrate the performance of different machine learning models in predicting CAD in patients with T2D based on components of surrogate insulin resistance indices (TG, FBS, HDL-C, BMI, and WHtR). ROC: Receiver operating characteristic; CAD: coronary artery disease; T2D: type 2 diabetes; TG: triglycerides; FBS: fasting blood sugar; HDL-C: high-density lipoprotein cholesterol; BMI: body mass index; WHtR: waist-to-height ratio; AUC: area under the curve; SVM: support vector machine
Discussion
The current study found a non-linear association between CAD in patients with T2D and surrogate IR indices including, TyG, TyG-BMI, TyG-WC, TyG-WHtR, METS-IR, and TG/HDL-C ratio. The TyG exhibited a U-shaped association with CAD. The surrogate IR indices demonstrated a relatively strong discriminative ability for CAD, with AUC values exceeding 0.708 for all indices. Among them, the TG/HDL-C ratio showed the highest AUC (0.721) and sensitivity (71%), followed by METS-IR (AUC = 0.713). In addition, TyG-WC exhibited the highest specificity (78%). Machine learning algorithms (except logistic regression) outperformed individual IR indices in discriminative power. Random forest and XGBoost revealed the best performance when using either multiple surrogate IR indices or their individual components.
IR has been found to promote atherosclerosis and the formation of lipid lesions which has been considered an important risk factor for CAD in patients with diabetes [36, 37]. The current study identified a non-linear association between surrogate IR indices and CAD. The pathophysiology behind this correlation can be explained by several factors. IR induces the secretion of non-esterified fatty acids and pro-inflammatory cytokines, such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and leptin, which exacerbate inflammation [38]. Hyperglycemia linked to IR leads to metabolic disturbances, including dyslipidemia, hypertension, oxidative stress, endothelial dysfunction, and myocardial fibrosis, impairing cardiac function [39–43]. Additionally, chronic hyperglycemia induces the generation of advanced glycation end products (AGEs), which disrupt the extracellular matrix, damage endothelium, and inhibit nitric oxide (NO) activity [44–47]. Enhanced glycosylation initiated by IR increases the secretion of pro-coagulant factors, facilitating platelet aggregation and further impairing endothelial function [48, 49]. Moreover, IR-associated hyperinsulinemia further accelerates lipid plaque progression and promotes arterial wall remodeling, worsening CAD outcomes [50].
This study identified a U-shaped association between TyG levels and odds of CAD in patients with T2D, suggesting that both extremely high and low TyG levels may negatively impact health outcomes. In addition, a TyG cut-off of 4.65 demonstrated predictive value for CAD, with an AUC of 0.709, sensitivity of 58%, and specificity of 76%. Elevated TyG levels likely increase CAD risk due to hyperglycemia and hyperlipidemia, well-known CAD risk factors [51]. Conversely, low TyG levels may elevate CAD risk through hypoglycemic conditions, which can trigger sympathetic activation, increased adrenaline, and vasoconstriction [51]. Nonetheless, the current evidence regarding the association between TyG and CAD remains inconsistent. A prior study on National Health and Nutrition Examination Survey (NHANES) data between 2001 and 2018, reported an inverse relationship between TyG levels below 8.84 and CVD-related death and a positive correlation when TyG exceeded this threshold [52]. Another study using NHANES between 1999 and 2018, found that an intermediate TyG range (8.72–9.15) was associated with reduced CVD mortality risk [53]. Similar to our findings, a study that used data from NHANES between 2003 and 2018, observed a U-shaped association between TyG and CVD risk in the general population, although without an obvious non-linear association [54]. However, Da Silva et al. reported a direct association between TyG and symptomatic CAD in patients receiving secondary CVD care in Brazil [55]. Additionally, a dose-response relationship between cumulative TyG and CAD risk was demonstrated in China between 2018 and 2021, particularly among men and older individuals [56]. Thai et al. identified a TyG threshold above 10 as a predictor of coronary artery stenosis of > 70% in patients with diabetes in Vietnam between 2017 and 2018, with a sensitivity of 57% and a specificity of 75% [21]. Moreover, TyG has been shown to predict cardiovascular events in patients with acute coronary syndrome and diabetes in China [57, 58]. These findings highlight the valuable role of the TyG as a comprehensive tool for cardiometabolic risk evaluation. However, further research is required to clarify the association between TyG and CAD, particularly in patients with T2D. On the other hand, Chen et al. found a U-shaped association between TyG-BMI, TyG-WC, and TyG-WHtR with all-cause mortality in Poland, while TyG and TyG-WHR showed a J-shaped pattern [59]. They suggested that low TyG-BMI or TyG-WC may indicate malnutrition and weakened immunity, increasing infection risk and mortality. Contrarily, high levels reflect greater IR, metabolic disorders, and fat accumulation, contributing to inflammation, oxidative stress, and atherosclerosis, ultimately increasing mortality risk [59].
A non-linear relationship was observed in this study between TyG-derived markers (TyG-BMI, TyG-WC, and TyG-WHtR) and the odds of having CAD in patients with T2D. Similar to TyG, evidence on the association of these markers with CAD remains inconclusive. Chen et al. analyzed the NHANES (2005–2018) dataset and reported a non-linear relationship between TyG-BMI and heart failure in patients with diabetes [22]. Park et al. showed a higher risk of CVD associated with TyG-BMI, TyG-WC, and TyG-WHtR in individuals without diabetes in Korea [60]. Wang et al. found a linear relationship between TyG-BMI and CAD risk in China between 2021 and 2024 [61]. Furthermore, a positive correlation between TyG-WC and myocardial infarction was reported based on the NHANES (2011–2018) [62]. In contrast, Xiao et al. identified a U-shaped relationship between TyG-BMI and CVD mortality in patients with diabetes using the NHANES (2001–2018) dataset [63]. Another study based on NHANES (2011–2018), highlighted a U-shaped association between TyG-WHtR and coronary heart disease [62]. These inconsistencies underscore the complex nature of the association between TyG-derived markers and CAD, necessitating further research to clarify their role.
Research on the association between METS-IR and CAD in patients with T2D is limited. Qian et al. found a positive correlation between METS-IR and CVD risk in middle-aged and elderly individuals in China between 2011 and 2018 [64]. Yoon et al. reported that elevated METS-IR predicts IHD in non-diabetic participants in Korea [19]. Furthermore, Wang et al. demonstrated a linear relationship between METS-IR and coronary artery calcification in Korea [65]. Zhang et al. highlighted METS-IR as a marker for risk assessment and prognosis in individuals with ischemic cardiomyopathy and diabetes in China from 2013 to 2019 [66]. Additionally, Zhang et al. showed that METS-IR is associated with CAD severity in China [67]. This study further identified a non-linear association between METS-IR and CAD in patients with T2D.
There is a controversy regarding the prognostic role of TG/HDL-C in cardiovascular events among patients with diabetes. Consistent with this study, Wu et al. showed that the TG/HDL-C ratio was an independent predictor of CAD presence in China from 2016 to 2017 [68]. In addition, Chen et al. analyzed the NHANES (2005–2018) dataset and reported a non-linear relationship between TG/HDL-C and heart failure in patients with diabetes [22]. Eeg Olofsson et al. found a positive association between TG/HDL-C and CVD risk in obese individuals with T2D in Sweden [69]. Additionally, TG/HDL-C has been identified as a predictor of major adverse cardiovascular events (MACEs) in patients with CAD and diabetes in China [70]. However, Tohidi et al. reported that the TG/HDL-C was not a significant independent predictor of cardiovascular events in individuals with diabetes without CVD in Iran [71]. These discrepancies may be due to the variations in participant characteristics, differences in follow-up durations, and the influence of unmeasured confounding factors across studies.
In the current analysis, TG/HDL-C demonstrated the highest predictive power for CAD among the indices studied, with a cut-off of 2.33 (AUC = 0.721, sensitivity = 71%, specificity = 64%), followed by METS-IR (cut-off = 45.18, AUC = 0.713, sensitivity = 67%, specificity = 67%). Wu et al. conducted a study in China from 2016 to 2017 and reported that METS-IR had the strongest predictive power for CAD compared to TyG and TG/HDL-C, with a cut-off of 42.1 (AUC = 0.636, sensitivity = 37.7%, specificity = 82.6%), followed by TG/HDL-C, which showed a cut-off of 2.9 (AUC = 0.567, sensitivity = 43.6%, specificity = 69.8%) [68]. In contrast, Wang et al. found that TyG-BMI was the most powerful predictive marker for CAD in patients with diabetes and hypertension in China, with an AUC of 0.699 (sensitivity = 70.8%, specificity = 62.4%) [61]. Additionally, Zhang et al. demonstrated that METS-IR had the highest predictive value for CAD severity in China (AUC = 0.726, sensitivity = 44.4%, specificity = 92.2%), followed by TyG-BMI (AUC = 0.704, sensitivity = 70%, specificity = 58.6%), and TG/HDL-C (AUC = 0.652, sensitivity = 52%, specificity = 75%) [67]. These findings indicate that surrogate IR indices, particularly TG/HDL-C and METS-IR, are valuable predictors for CAD. However, their relative performance may differ among various populations and study settings, highlighting the need for customized risk assessment approaches.
In this study, the machine learning models exhibited superior discriminative power compared to individual surrogate IR indices. Random forest and XGBoost demonstrated the highest performance when utilizing either various surrogate IR indices (TyG, TyG-WHtR, and TG/HDL-C) or their components (TG, FBS, HDL-C, BMI, and WHtR). Research on the integration of surrogate IR indices into machine learning models for predicting cardiovascular disease in patients with T2D is limited. However, our results are consistent with earlier research assessing machine learning methods for predicting cardiovascular disease in individuals with T2D using alternative predictors. Hossain et al., incorporating social network-based features (such as comorbidity prevalence, transition patterns, and clustering membership) and demographic characteristics in Australia, reported that all tested models, including logistic regression, SVM, decision tree, random forest, K-nearest neighbors, and naïve bayes, achieved AUCs above 0.70, with the random forest model demonstrating the highest performance (AUC = 0.83) for cardiovascular disease prediction [72]. Fan et al., included demographic characteristics, anthropometric features (such as BMI), biochemical data (including FBS and lipid profile), and insulin secretion indices in their study in China. Their random forest model achieved an AUC of 0.77 in the training dataset, 0.80 in the testing dataset, and 0.71 in an independent validation dataset for predicting coronary heart disease in patients with T2D [73]. Additionally, Dinh et al. utilized survey data and laboratory results from the NHANES dataset and showed that random forest and XGBoost models outperformed others in predicting cardiovascular diseases in the general population. The AUC values for these models ranged from 0.816 to 0.839 [74]. These results emphasize the capability of machine learning models, especially tree-based algorithms, to improve cardiovascular disease prediction in individuals with T2D, irrespective of the specific predictors used.
Strengths and limitations
To the best of our knowledge, this is one of the first studies to comprehensively evaluate the association between various surrogate indices of IR and CAD in a relatively large number of patients with T2D. The current study provided a detailed examination of these associations by employing diverse analytical approaches, including RCS, multivariable logistic regression, ROC curve analysis, and machine learning models. In addition, adjustments for potential confounders were performed to enhance the robustness of the results. Nonetheless, there were some limitations. This study was cross-sectional, which limits the establishment of causal relationships between the assessed indices and CAD. As this study was conducted at a single center, its findings may not be fully generalizable to larger populations. Additionally, the indices used were surrogate markers and may not accurately represent the actual IR. Despite adjustments for confounding variables, unmeasured factors such as diet preferences, hereditary factors, and social determinants of health could have impacted the results. While 10-fold cross-validation was used to improve the reliability of machine learning models, there is still a risk of overfitting. Furthermore, the models were developed using just one dataset, highlighting the need for external validation. In addition, due to the lack of longitudinal data, the ability of machine learning models to predict causal associations between surrogate IR indices and CAD was restricted. Future prospective cohort studies are needed to address these limitations.
Conclusion
This study highlights the non-linear association and predictive value of TyG, TyG-BMI, TyG-WHtR, TyG-WC, METS-IR, and the TG/HDL-C ratio as surrogate indices of insulin resistance for CAD in patients with T2D, with the TG/HDL-C ratio demonstrating the highest predictive accuracy. The findings emphasize the usefulness of these indices in assessing cardiometabolic risk, particularly in high-risk individuals. In addition, integrating machine learning models enhanced CAD prediction, emphasizing their capabilities for better risk stratification. Further longitudinal studies are essential to validate these indices and establish specific thresholds for optimal risk assessment and clinical use.
Acknowledgements
Not applicable.
Author contributions
Conceptualization: MN, AY, FM; Methodology: AY, FM, SR; Formal analysis: AY, FM, SY; Investigation: SY, SAS, EBH; Writing-original draft preparation: KS, KM, SY; Writing-review and editing: AY, FM, MN, AE; Supervision: MN, AE. All authors read and approved the final manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The research ethics committee of Tehran University of Medical Sciences approved this study. All participants provided written informed consent before participating, and the study adhered to the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Preis SR, Pencina MJ, Hwang S-J, D’Agostino Sr RB, Savage PJ, Levy D, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham heart study. Circulation. 2009;120(3):212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schramm TK, Gislason GH, Køber L, Rasmussen S, Rasmussen JN, Abildstrøm SZ, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–54. [DOI] [PubMed] [Google Scholar]
- 3.Yadegar A, Mohammadi F, Rabizadeh S, Qahremani R, Esteghamati A, Nakhjavani M. Prevalence of different patterns of dyslipidemia in patients with type 2 diabetes in an Iranian population. Translational Med Commun. 2022;7(1):23. [Google Scholar]
- 4.Yadegar A, Mohammadi F, Rabizadeh S, Meysamie A, Nabipoorashrafi SA, Seyedi SA, et al. Decreasing trend of blood lipid profile in type 2 diabetes: not a promising change in HDL-C, a serial cross-sectional study. PLoS ONE. 2023;18(10):e0293410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaemi F, Rabizadeh S, Yadegar A, Mohammadi F, Asadigandomani H, Bafrani MA, et al. ApoA1/HDL-C ratio as a predictor for coronary artery disease in patients with type 2 diabetes: a matched case-control study. BMC Cardiovasc Disord. 2024;24(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadi F, Yadegar A, Rabizadeh S, Ayati A, Seyedi SA, Nabipoorashrafi SA, et al. Correlates of normal and decreased HDL cholesterol levels in type 2 diabetes: a cohort-based cross-sectional study. Lipids Health Dis. 2024;23(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Nozha MM, Ismail HM, Al Nozha OM. Coronary artery disease and diabetes mellitus. J Taibah Univ Med Sci. 2016;11(4):330–8. [Google Scholar]
- 8.Krishnaswami A, Hardison R, Nesto RW, Sobel B, Investigators BD. Presentation in patients with angiographically documented coronary artery disease and type II diabetes mellitus (from the BARI 2D clinical trial). Am J Cardiol. 2012;109(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baweja PS, Sandesara PB, Ashraf MJ. Asymptomatic coronary artery disease in type II diabetes. Mo Med. 2014;111(1):73. [PMC free article] [PubMed] [Google Scholar]
- 10.Saraste A, Knuuti J, Bax J. Screening for coronary artery disease in patients with diabetes. Curr Cardiol Rep. 2023;25(12):1865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39(35):3322–30. [DOI] [PubMed] [Google Scholar]
- 12.Davidson ST. Any dose is too high. Environ Health Perspect. 2005;113(11):A735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. [DOI] [PubMed] [Google Scholar]
- 14.Muniyappa R, Madan R, Varghese RT. Assessing insulin sensitivity and resistance in humans. 2015.
- 15.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–48. [DOI] [PubMed] [Google Scholar]
- 17.Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Li K, Wen J, Yang C, Li Y, Xu G, et al. Association of the triglyceride glucose-body mass index with the extent of coronary artery disease in patients with acute coronary syndromes. Cardiovasc Diabetol. 2024;23(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon J, Jung D, Lee Y, Park B. The metabolic score for insulin resistance (METS-IR) as a predictor of incident ischemic heart disease: a longitudinal study among Korean without diabetes. J Personalized Med. 2021;11(8):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koide Y, Miyoshi T, Nishihara T, Nakashima M, Ichikawa K, Miki T, et al. The association of triglyceride to high-density lipoprotein cholesterol ratio with high-risk coronary plaque characteristics determined by CT angiography and its risk of coronary heart disease. J Cardiovasc Dev Disease. 2022;9(10):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Qian L, Liu Y. Association between different insulin resistance indices and heart failure in US adults with diabetes mellitus. Ann Noninvasive Electrocardiol. 2024;29(6):e70035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi W, Neubeck L, Gallagher R. Measurement matters: a systematic review of waist measurement sites for determining central adiposity. Collegian. 2017;24(5):513–23. [Google Scholar]
- 24.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. [DOI] [PubMed] [Google Scholar]
- 26.2. Diagnosis and classification of diabetes: standards of care in diabetes—2024. Diabetes Care. 2024;47(Supplement1):S20–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Association AD. Standards of care in diabetes—2023 abridged for primary care providers. Clin Diabetes. 2023;41(1):4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang C, Peng N, Cheng J, Zhang X, Gu W, Zhu Z, et al. The association between TyG index and cardiovascular mortality is modified by antidiabetic or lipid-lowering agent: a prospective cohort study. Cardiovasc Diabetol. 2025;24(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter JD, Matplotlib. A 2D graphics environment. Comput Sci Eng. 2007;9(03):90–5.
- 30.Harris CR, Millman KJ, Van Der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with numpy. Nature. 2020;585(7825):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in python. J Mach Learn Res. 2011;12:2825–30. [Google Scholar]
- 32.McKinney W, editor. Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference; 2010: Austin, TX.
- 33.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in python. Nat Methods. 2020;17(3):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T, Guestrin C, editors. Xgboost: a scalable tree boosting system. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining; 2016.
- 35.Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W et al. Lightgbm: a highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. 2017;30.
- 36.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17). [DOI] [PMC free article] [PubMed]
- 37.Howard G, O’Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis. The insulin resistance atherosclerosis study (IRAS) investigators. Circulation. 1996;93(10):1809–17. [DOI] [PubMed] [Google Scholar]
- 38.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metabol. 2011;14(5):575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson JA, Parkin CG. Is hyperglycemia a causal factor in cardiovascular disease? Does proving this relationship really matter? Yes. Diabetes Care. 2009;32(Suppl 2):S331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Reviews Endocrinol. 2014;10(5):293–302. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y, Wang Y, Yin R, Xu Y, Zhang L, Zhang Y et al. Central role of cardiac fibroblasts in myocardial fibrosis of diabetic cardiomyopathy. Front Endocrinol. 2023;14. [DOI] [PMC free article] [PubMed]
- 43.Hutchinson KR, Lord CK, West TA, Stewart JA Jr. Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS ONE. 2013;8(8):e72080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabizadeh S, Heidari F, Karimi R, Rajab A, Rahimi-Dehgolan S, Yadegar A, et al. Vitamin C supplementation lowers advanced glycation end products (AGEs) and malondialdehyde (MDA) in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Food Sci Nutr. 2023;11(10):5967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circul Res. 2010;106(5):842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larry M, Rabizadeh S, Mohammadi F, Yadegar A, Jalalpour A, Mirmiranpour H, et al. Relationship between advanced glycation end-products and advanced oxidation protein products in patients with type 2 diabetes with and without albuminuria: a cross-sectional survey. Health Sci Rep. 2024;7(10):e70057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pistrosch F, Natali A, Hanefeld M. Is hyperglycemia a cardiovascular risk factor? Diabetes Care. 2011;34(Suppl 2):S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tousoulis D, Simopoulou C, Papageorgiou N, Oikonomou E, Hatzis G, Siasos G, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel Therapeutic Approaches Pharmacol Ther. 2014;144(3):253–67. [DOI] [PubMed] [Google Scholar]
- 50.Min J, Weitian Z, Peng C, Yan P, Bo Z, Yan W, et al. Correlation between insulin-induced estrogen receptor methylation and atherosclerosis. Cardiovasc Diabetol. 2016;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galassetti P, Davis SN. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci (Lond). 2000;99(5):351–62. [PubMed] [Google Scholar]
- 52.Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao M, Xiao M, Tan Q, Lu F. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci Rep. 2023;13(1):16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, Liang D. The association between the triglyceride–glucose index and the risk of cardiovascular disease in US population aged ≤ 65 years with prediabetes or diabetes: a population-based study. Cardiovasc Diabetol. 2024;23(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Xu W, Song Q, Zhao Z, Meng X, Xia C, et al. Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. 2022;21(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Z, He J, Yuan S, Song C, Bian X, Yang M, et al. Glycemic control and cardiovascular outcomes in patients with diabetes and coronary artery disease according to triglyceride-glucose index: a large-scale cohort study. Cardiovasc Diabetol. 2024;23(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Zhong Z, Gue Y, Banach M, McDowell G, Mikhailidis DP et al. Impact of surrogates for insulin resistance on mortality and life expectancy in primary care: a nationwide cross-sectional study with registry linkage (LIPIDOGRAM2015). Lancet Reg Health – Europe. 2025;49. [DOI] [PMC free article] [PubMed]
- 60.Park H-M, Han T, Heo S-J, Kwon Y-J. Effectiveness of the triglyceride-glucose index and triglyceride-glucose-related indices in predicting cardiovascular disease in middle-aged and older adults: a prospective cohort study. J Clin Lipidol. 2024;18(1):e70–9. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Li Z, Qiu R, Luo L, Yan X. Triglyceride glucose index–body mass index as a predictor of coronary artery disease severity in patients with H-type hypertension across different glucose metabolic states. Diabetol Metab Syndr. 2025;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhuang Y, Wang Y, Sun P, Ke J, Chen F. Association between triglyceride glucose-waist to height ratio and coronary heart disease: a population-based study. Lipids Health Dis. 2024;23(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao S, Zhang Q, Yang H-Y, Tong J-Y, Yang R-Q. The association between triglyceride glucose-body mass index and all-cause and cardiovascular mortality in diabetes patients: a retrospective study from NHANES database. Sci Rep. 2024;14(1):13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qian T, Sheng X, Shen P, Fang Y, Deng Y, Zou G. Mets-IR as a predictor of cardiovascular events in the middle-aged and elderly population and mediator role of blood lipids. Front Endocrinol. 2023;14. [DOI] [PMC free article] [PubMed]
- 65.Wang Z, Hui X, Huang X, Li J, Liu N. Relationship between a novel non–insulin-based metabolic score for insulin resistance (METS-IR) and coronary artery calcification. BMC Endocr Disorders. 2022;22(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Liu F, Li W, Zhang J, Zhang T, Yu X, et al. Metabolic score for insulin resistance (METS-IR) predicts adverse cardiovascular events in patients with type 2 diabetes and ischemic cardiomyopathy. Diabetes Metab Syndr Obes. 2023;16:1283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Wang R, Fu X, Song H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery disease. Diabetol Metab Syndr. 2022;14(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Z, Cui H, Li W, Zhang Y, Liu L, Liu Z et al. Comparison of three non-insulin-based insulin resistance indexes in predicting the presence and severity of coronary artery disease. Front Cardiovasc Med. 2022;9. [DOI] [PMC free article] [PubMed]
- 69.Eeg-Olofsson K, Gudbjörnsdottir S, Eliasson B, Zethelius B, Cederholm J. The triglycerides-to-HDL-cholesterol ratio and cardiovascular disease risk in obese patients with type 2 diabetes: an observational study from the Swedish National diabetes register (NDR). Diabetes Res Clin Pract. 2014;106(1):136–44. [DOI] [PubMed] [Google Scholar]
- 70.Yang SH, Du Y, Li XL, Zhang Y, Li S, Xu RX, et al. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in diabetics with coronary artery disease. Am J Med Sci. 2017;354(2):117–24. [DOI] [PubMed] [Google Scholar]
- 71.Tohidi M, Hatami M, Hadaegh F, Safarkhani M, Harati H, Azizi F. Lipid measures for prediction of incident cardiovascular disease in diabetic and non-diabetic adults: results of the 8.6 years follow-up of a population based cohort study. Lipids Health Dis. 2010;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hossain ME, Uddin S, Khan A. Network analytics and machine learning for predictive risk modelling of cardiovascular disease in patients with type 2 diabetes. Expert Syst Appl. 2021;164:113918. [Google Scholar]
- 73.Fan R, Zhang N, Yang L, Ke J, Zhao D, Cui Q. AI-based prediction for the risk of coronary heart disease among patients with type 2 diabetes mellitus. Sci Rep. 2020;10(1):14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dinh A, Miertschin S, Young A, Mohanty SD. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inf Decis Mak. 2019;19(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.