Abstract
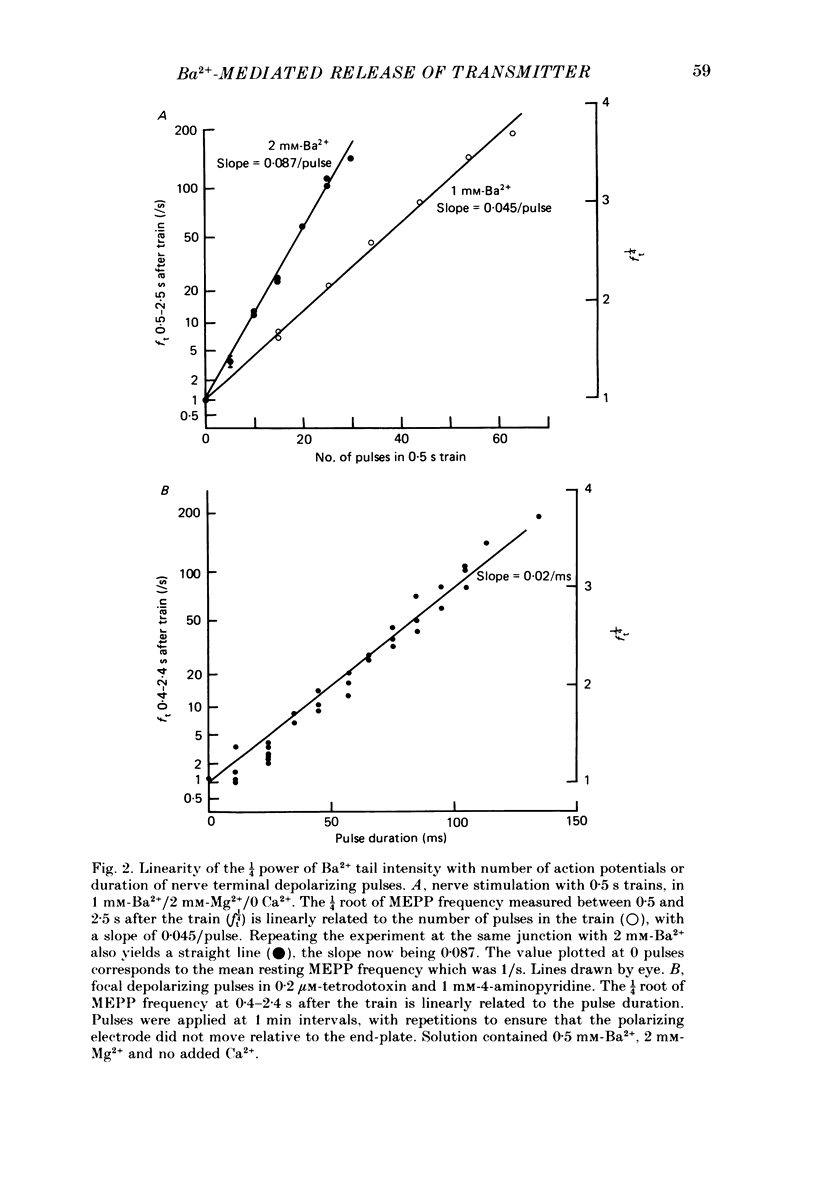
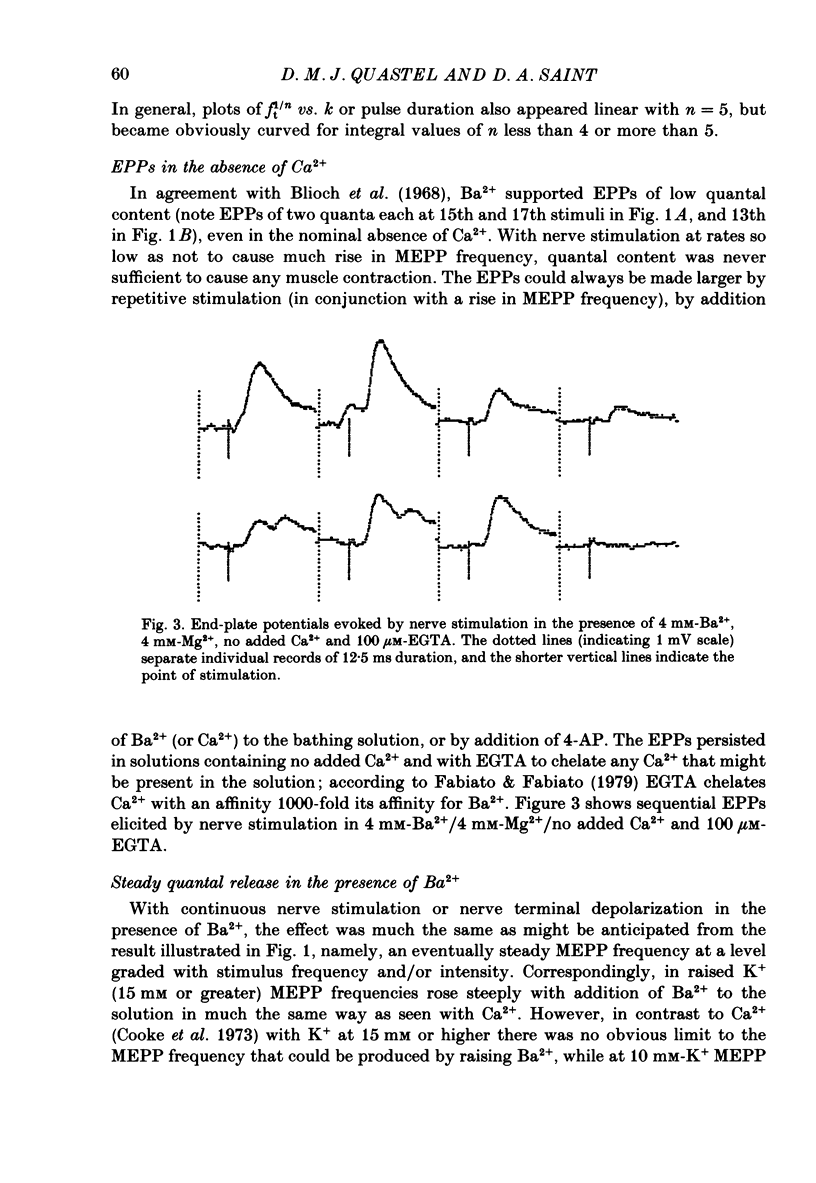
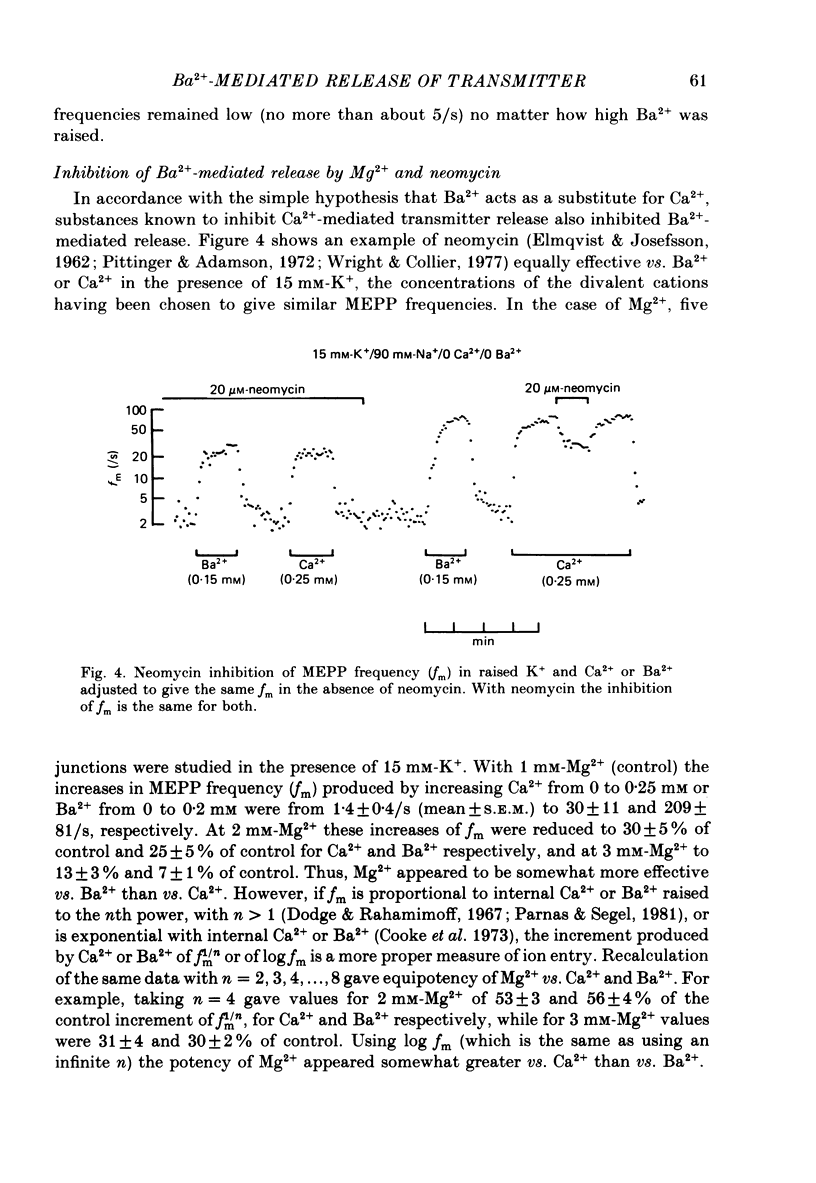
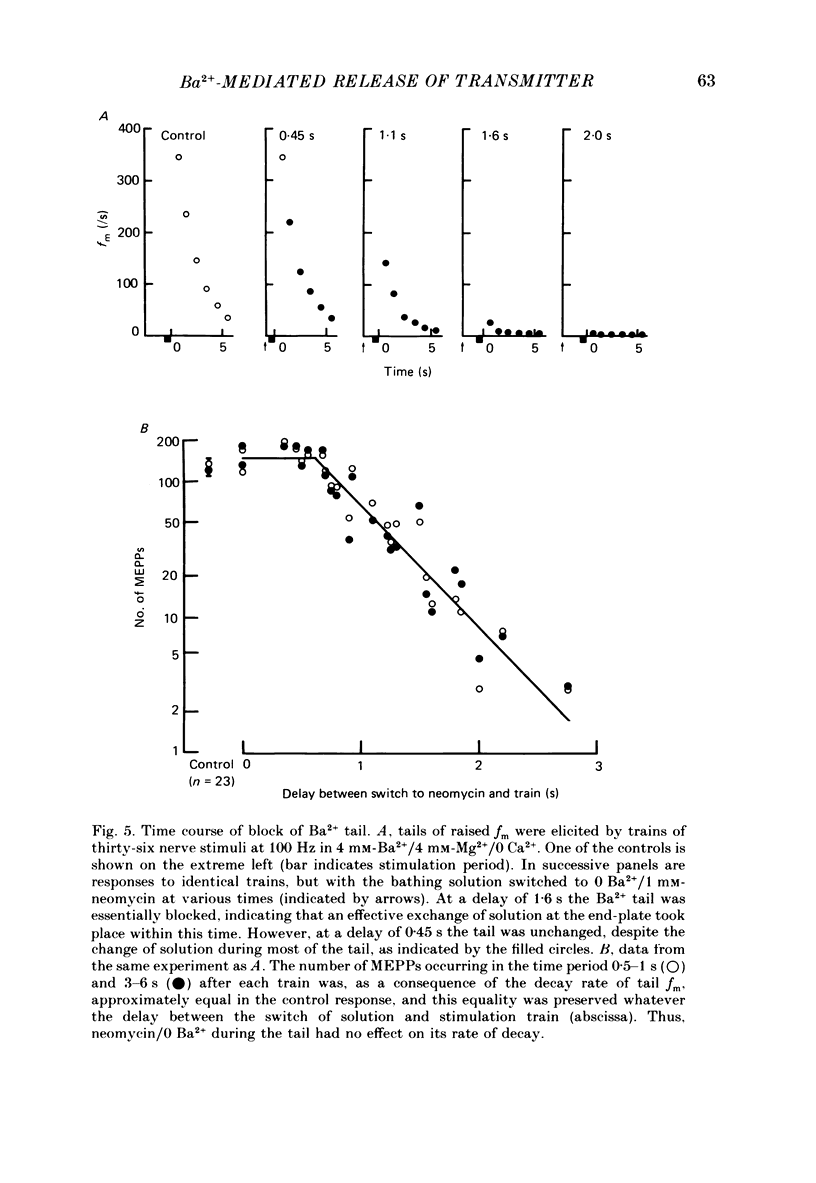
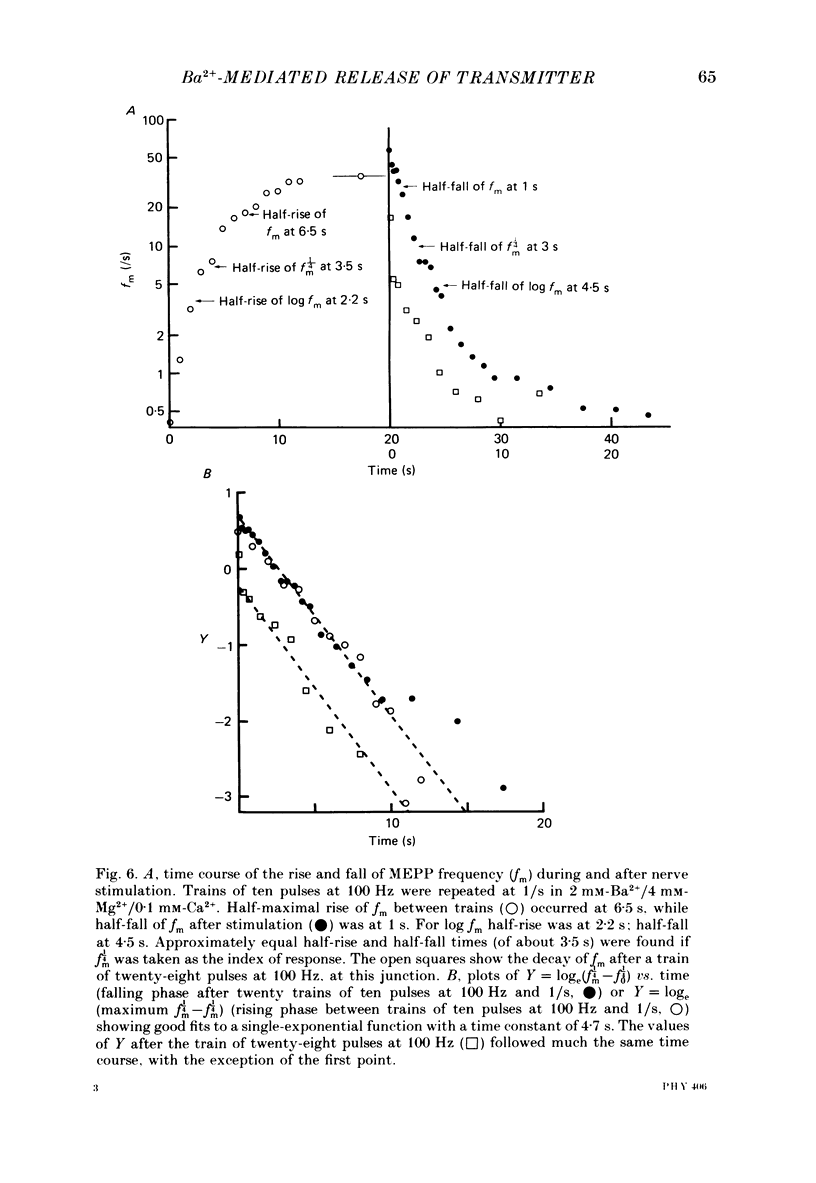
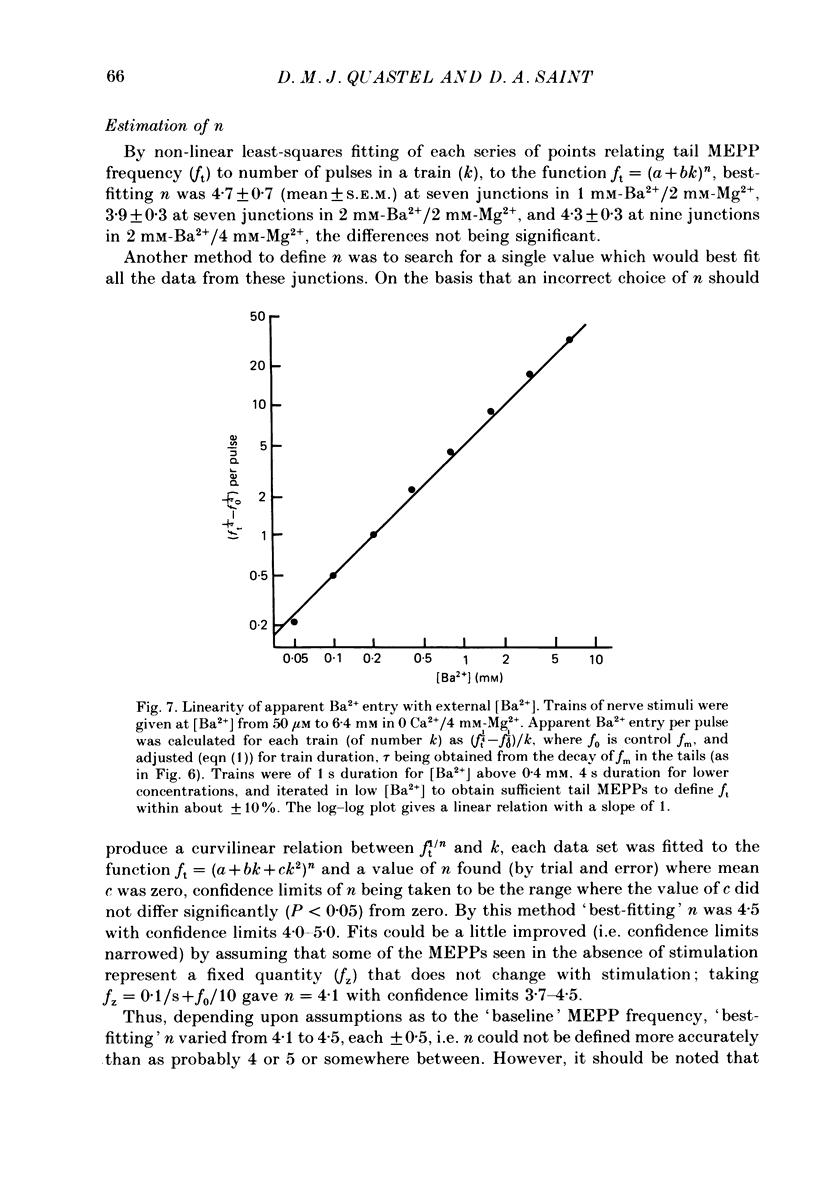
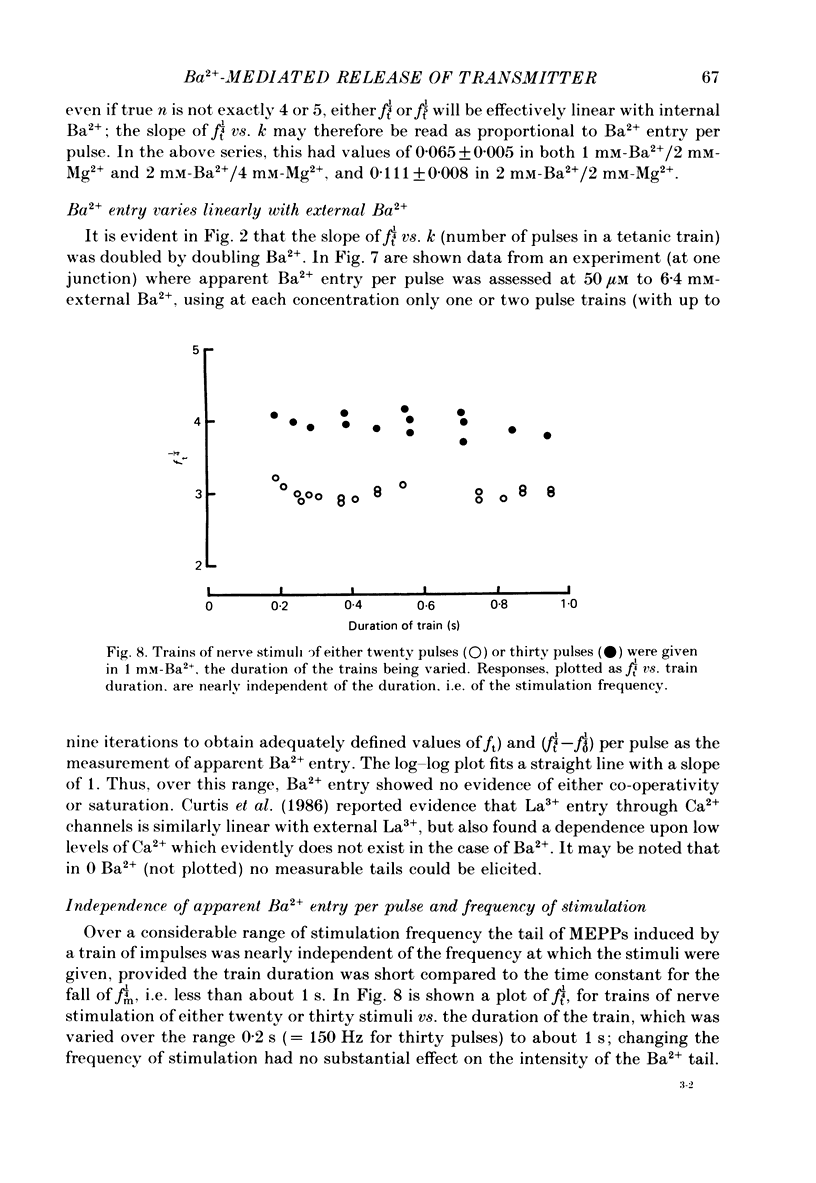
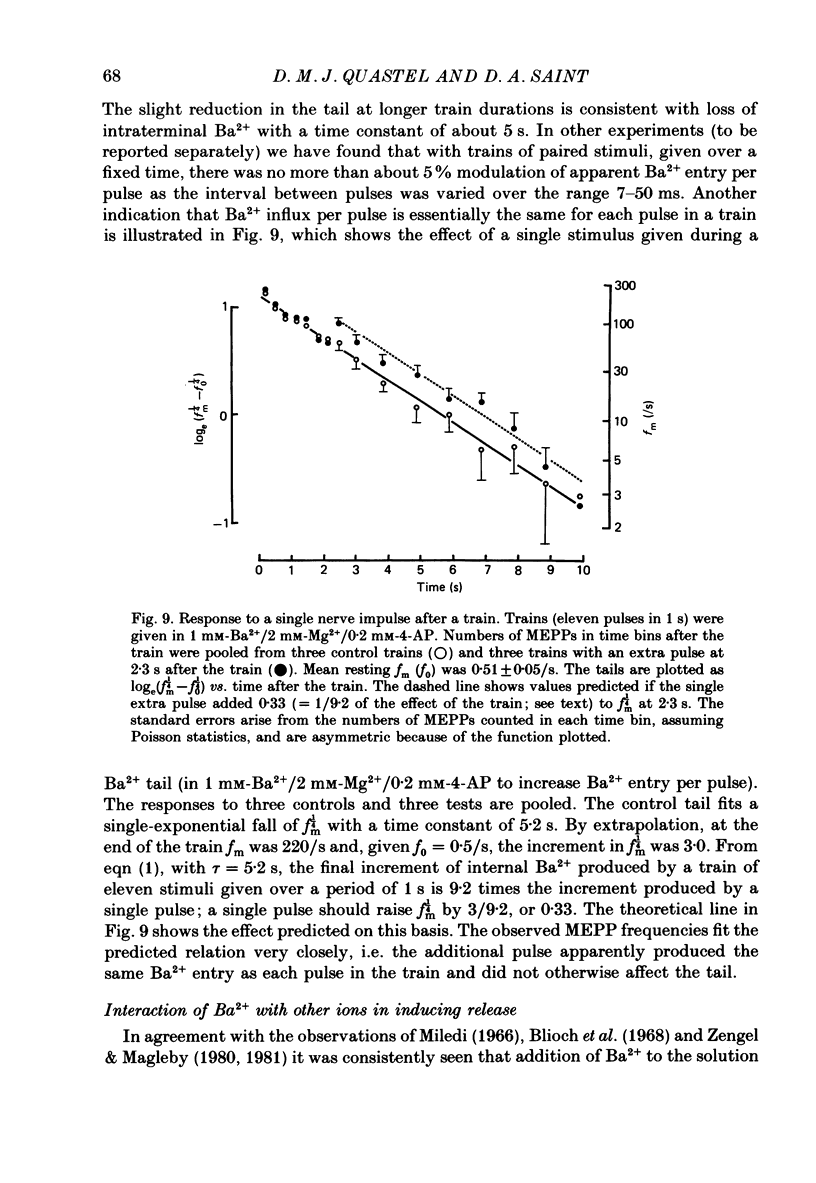
1. In isolated mouse diaphragm, tetanic nerve stimulation in the presence of Ba2+ causes an increase in frequency of MEPPs which continues as an after-discharge or 'tail' of raised MEPP frequency that subsides over a period of seconds, in addition to EPPs of low quantal content. 'Ba2+ tails' are also seen with focal depolarization of nerve terminals in the presence of tetrodotoxin. 2. The development of 'Ba2+ tails' could be inhibited or blocked by neomycin, raised Mg2+, or Cd2+ present at the time of stimulation; the presence of the blocking substance during the tail itself had no effect. 3. The time course of MEPP frequency changes during and after stimulation could be expressed as a simple exponential process, with the same time constant for both the rise and the fall, by taking as the time-dependent variable the nth root of MEPP frequency, n being 4 or 5. The time constant (tau) derived from the rate of fall of the 1/4 power of MEPP frequency during the tail was at most junctions between 3 and 6 s, and apparently unaffected by concentration of Ba2+, or of Ca2+, or by tonic depolarization of the nerve terminal. 4. The intensity of 'Ca2+ tails' was graded steeply with the number of stimuli applied, but was nearly independent of stimulus frequency, when train duration was kept brief compared to tau, i.e. about a second or less. The nth root of the number of MEPPs at a given time period in the tail was linearly related to the number of stimuli, when n was chosen to be 4 or 5. 5. The above data are consistent with a model in which (a) with each nerve impulse in a train there occurs the same entry of Ba2+ into the terminal, (b) transmitter release (MEPP frequency) is proportional to the fourth or fifth power of [Ba2+] at critical sites within the nerve terminal, (c) the Ba2+ leaves these sites as a first-order process with a time constant of a few seconds. Compared to Ca2+, Ba2+ persists longer but has lower potency. 6. With variation of external [Ba2+] over the range 50 microM to 6.4 mM, apparent Ba2+ entry per nerve impulse grew linearly with concentration. 7. Evidence is presented indicating that intraterminal Ba2+ can 'co-operate' with Ca2+ or La3+ in promoting transmitter release.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Eckert R. Divalent cations differentially support transmitter release at the squid giant synapse. J Physiol. 1984 Jan;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M. J., Quastel D. M., Saint D. A. Lanthanum as a surrogate for calcium in transmitter release at mouse motor nerve terminals. J Physiol. 1986 Apr;373:243–260. doi: 10.1113/jphysiol.1986.sp016045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., JOSEFSSON J. O. The nature of the neuromuscular block produced by neomycine. Acta Physiol Scand. 1962 Feb;54:105–110. doi: 10.1111/j.1748-1716.1962.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A., Rahamimoff R. A study of tetanic and post-tetanic potentiation of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1980 Dec;309:247–273. doi: 10.1113/jphysiol.1980.sp013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Lechat P. Effects of 4-aminopyridine at the frog neuromuscular junction. J Pharmacol Exp Ther. 1977 Dec;203(3):653–663. [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Influx of calcium, strontium, and barium in presynaptic nerve endings. J Gen Physiol. 1982 Jun;79(6):1065–1087. doi: 10.1085/jgp.79.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. A theoretical study of calcium entry in nerve terminals, with application to neurotransmitter release. J Theor Biol. 1981 Jul 7;91(1):125–169. doi: 10.1016/0022-5193(81)90378-7. [DOI] [PubMed] [Google Scholar]
- Pittinger C., Adamson R. Antibiotic blockade of neuromuscular function. Annu Rev Pharmacol. 1972;12:169–184. doi: 10.1146/annurev.pa.12.040172.001125. [DOI] [PubMed] [Google Scholar]
- Saint D. A., McLarnon J. G., Quastel D. M. Anion permeability of motor nerve terminals. Pflugers Arch. 1987 Jul;409(3):258–264. doi: 10.1007/BF00583474. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Woll K. H. The effect of internal barium on the K current of the node of Ranvier. Pflugers Arch. 1982 Jun;393(4):318–321. doi: 10.1007/BF00581417. [DOI] [PubMed] [Google Scholar]
- Wright J. M., Collier B. The effects of neomycin upon transmitter release and action. J Pharmacol Exp Ther. 1977 Mar;200(3):576–587. [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Augmentation and facilitation of transmitter release. A quantitative description at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):583–611. doi: 10.1085/jgp.80.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Differential effects of Ba2+, Sr2+, and Ca2+ on stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1980 Aug;76(2):175–211. doi: 10.1085/jgp.76.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


