Abstract
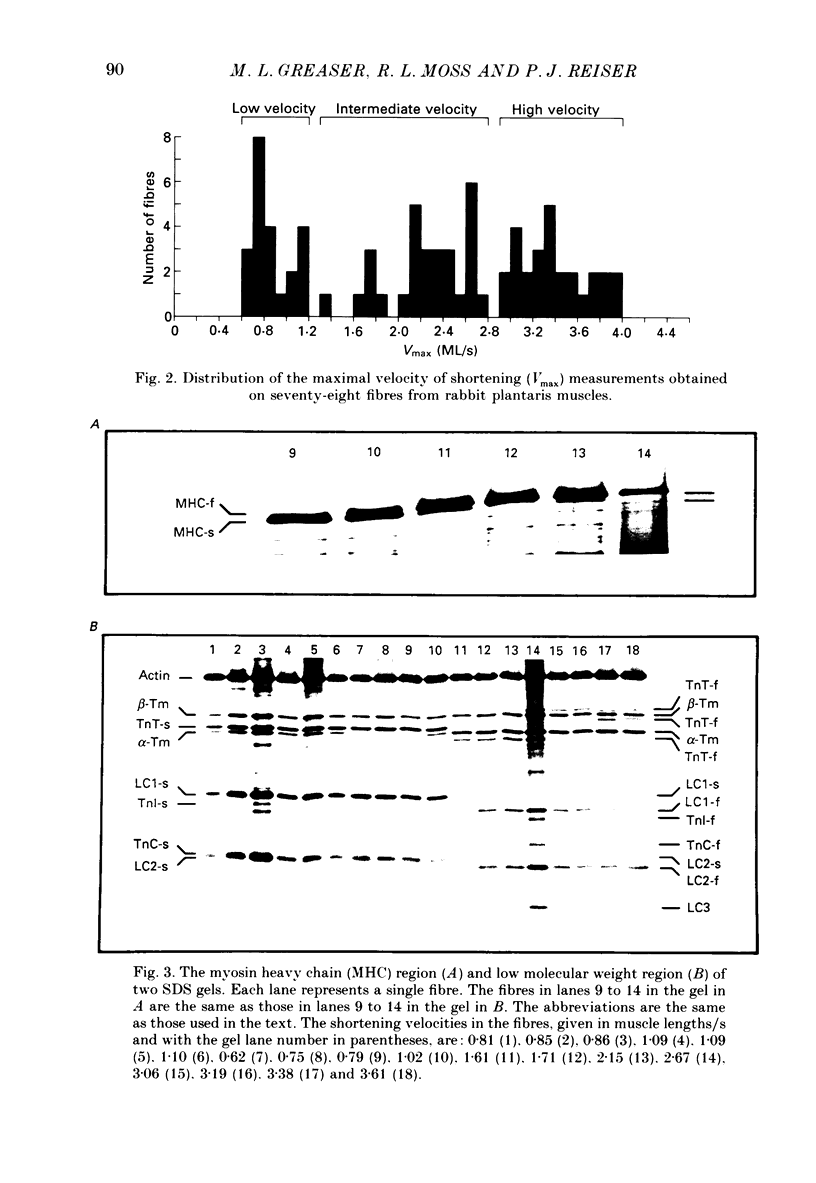
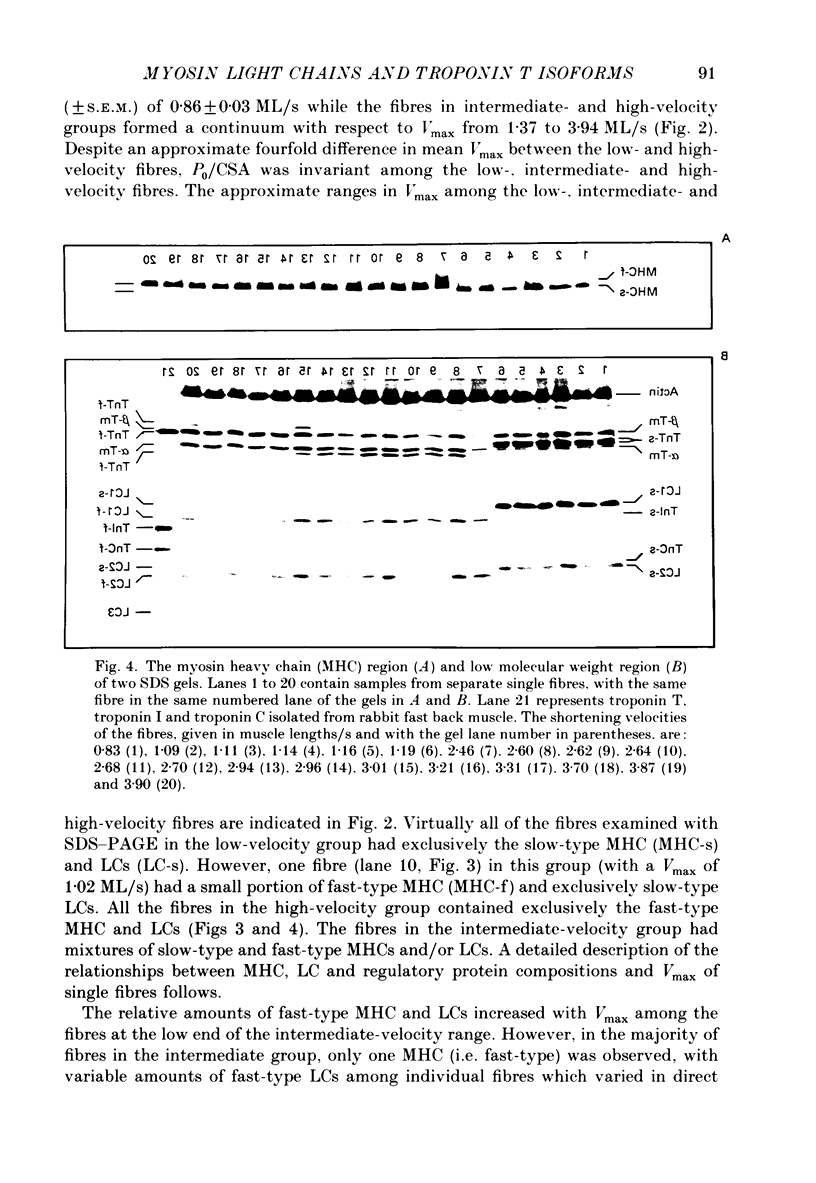
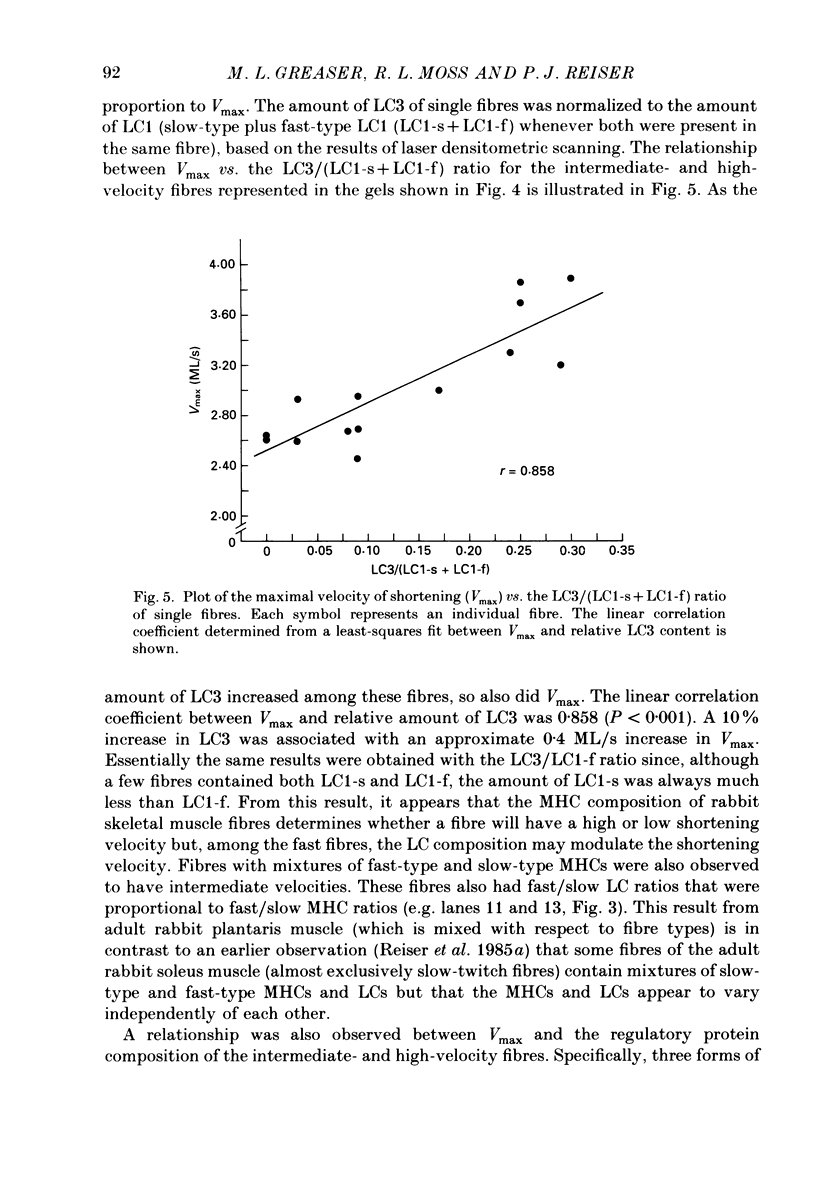
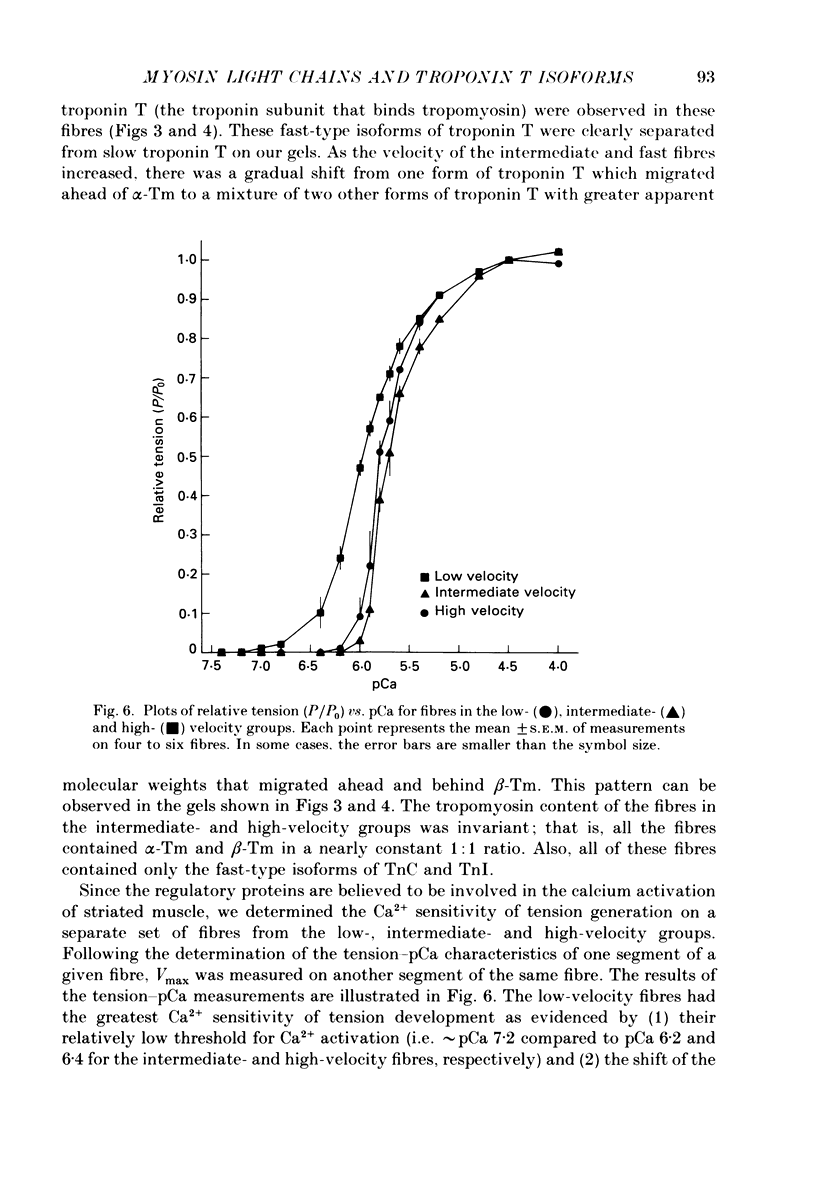
1. The maximal velocity of shortening (Vmax), tension-pCa relationships and the contractile and regulatory protein composition were determined in single, chemically skinned fibres from adult rabbit plantaris muscles. 2. Three groups of fibres were identified based on their protein compositions. One group had exclusively the slow-type myosin heavy chain (MHC) and myosin light chains (LC) and had low velocities. Another group of fibres had mixtures of fast-type and slow-type MHCs and LCs and had intermediate shortening velocities. The third group of fibres had fast-type myosin heavy and light chains and high velocities. 3. The low-velocity fibres had a mean velocity (+/- S.E.M.) of 0.86 +/- 0.03 muscle lengths/s (ML/s) at 15 degrees C. The remaining fibres formed a continuum with respect to Vmax from 1.37 to 3.94 ML/s. These results indicate that a much greater diversity exists among single fibres from adult mammalian skeletal muscle than previously recognized. The intermediate- and high-velocity fibres formed a continuum (from slow to fast) with respect to the amount of myosin light chain 3 (LC3). That is, Vmax increased with the relative LC3 content in single fibres in the intermediate- and high-velocity groups in a quantitative, statistically significant manner. 4. Three isoforms of fast-type troponin T were identified among the intermediate- and high-velocity fibres. These fibres also contained fast-type troponin C and troponin I. As was the case with the relative LC3 content, these fibres also formed a continuum with respect to the relative proportions of the three isoforms of fast-type troponin T. It appears that different isoforms of troponin T are responsible for a slightly higher Ca2+ sensitivity of tension development in the high-velocity fibres compared to the intermediate fibres. The continuum in troponin T isoform composition paralleled an increase in Vmax among these fibres. 5. The low-velocity fibres had the highest Ca2+ sensitivity of the three groups and had exclusively the slow-type isoforms of the regulatory proteins in the troponin complex. 6. The co-ordinated variations in troponin T and LC3 compositions among the intermediate- and high-velocity fibres are discussed as a possible means for the further differentiation of the contractile properties of the fibres in these two groups, beyond that provided by myosin heavy chain isoforms alone.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Phelps R. O. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984 Nov;171(3):259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Babu A., Scordilis S. P., Sonnenblick E. H., Gulati J. The control of myocardial contraction with skeletal fast muscle troponin C. J Biol Chem. 1987 Apr 25;262(12):5815–5822. [PubMed] [Google Scholar]
- Bandman E. Myosin isoenzyme transitions in muscle development, maturation, and disease. Int Rev Cytol. 1985;97:97–131. doi: 10.1016/s0074-7696(08)62349-9. [DOI] [PubMed] [Google Scholar]
- Barnard R. J., Edgerton V. R., Furukawa T., Peter J. B. Histochemical, biochemical, and contractile properties of red, white, and intermediate fibers. Am J Physiol. 1971 Feb;220(2):410–414. doi: 10.1152/ajplegacy.1971.220.2.410. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Briggs M. M., Lin J. J., Schachat F. H. The extent of amino-terminal heterogeneity in rabbit fast skeletal muscle troponin T. J Muscle Res Cell Motil. 1987 Feb;8(1):1–12. doi: 10.1007/BF01767259. [DOI] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Flicker P. F., Phillips G. N., Jr, Cohen C. Troponin and its interactions with tropomyosin. An electron microscope study. J Mol Biol. 1982 Dec 5;162(2):495–501. doi: 10.1016/0022-2836(82)90540-x. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981 Oct;391(4):334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Waller G. S. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol. 1981 Feb;311:201–218. doi: 10.1113/jphysiol.1981.sp013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi K., Pinter K., Mabuchi Y., Sreter F., Gergely J. Characterization of rabbit masseter muscle fibers. Muscle Nerve. 1984 Jul-Aug;7(6):431–438. doi: 10.1002/mus.880070603. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Destree A. T., Summers E., Nadal-Ginard B. A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene. Cell. 1984 Sep;38(2):409–421. doi: 10.1016/0092-8674(84)90496-3. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Takeda S., Shimizu T., Kitaura T. Gene expression of myofibrillar proteins in single muscle fibers of adult chicken: micro two dimensional gel electrophoretic analysis. J Biochem. 1981 Jun;89(6):1951–1962. doi: 10.1093/oxfordjournals.jbchem.a133397. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. E., Briggs M. M., Schachat F. H. Patterns of troponin T expression in mammalian fast, slow and promiscuous muscle fibres. J Muscle Res Cell Motil. 1987 Feb;8(1):13–22. doi: 10.1007/BF01767260. [DOI] [PubMed] [Google Scholar]
- Moore G. E., Schachat F. H. Molecular heterogeneity of histochemical fibre types: a comparison of fast fibres. J Muscle Res Cell Motil. 1985 Aug;6(4):513–524. doi: 10.1007/BF00712587. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Lauer M. R., Giulian G. G., Greaser M. L. Altered Ca2+ dependence of tension development in skinned skeletal muscle fibers following modification of troponin by partial substitution with cardiac troponin C. J Biol Chem. 1986 May 5;261(13):6096–6099. [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Swinford A. E., Greaser M. L. Alterations in the Ca2+ sensitivity of tension development by single skeletal muscle fibers at stretched lengths. Biophys J. 1983 Jul;43(1):115–119. doi: 10.1016/S0006-3495(83)84329-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki I., Maruyama K., Ebashi S. Regulatory and cytoskeletal proteins of vertebrate skeletal muscle. Adv Protein Chem. 1986;38:1–67. doi: 10.1016/s0065-3233(08)60525-2. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity and myosin heavy chains of developing rabbit muscle fibers. J Biol Chem. 1985 Nov 25;260(27):14403–14405. [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Risnik V. V., Verin A. D., Gusev N. B. Comparison of the structure of two cardiac troponin T isoforms. Biochem J. 1985 Jan 15;225(2):549–552. doi: 10.1042/bj2250549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat F. H., Bronson D. D., McDonald O. B. Heterogeneity of contractile proteins. A continuum of troponin-tropomyosin expression in mammalian skeletal muscle. J Biol Chem. 1985 Jan 25;260(2):1108–1113. [PubMed] [Google Scholar]
- Schachat F. H., Diamond M. S., Brandt P. W. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987 Dec 5;198(3):551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit tibialis anterior muscle. Biochem J. 1987 May 1;243(3):695–699. doi: 10.1042/bj2430695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Gergely J., Sréter F. A. Velocity of shortening and myosin isozymes in two types of rabbit fast-twitch muscle fibers. Am J Physiol. 1986 Sep;251(3 Pt 1):C431–C434. doi: 10.1152/ajpcell.1986.251.3.C431. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Syrový I. Isoforms of contractile proteins. Prog Biophys Mol Biol. 1987;49(1):1–27. doi: 10.1016/0079-6107(87)90007-1. [DOI] [PubMed] [Google Scholar]
- Tobacman L. S., Lee R. Isolation and functional comparison of bovine cardiac troponin T isoforms. J Biol Chem. 1987 Mar 25;262(9):4059–4064. [PubMed] [Google Scholar]
- White S. P., Cohen C., Phillips G. N., Jr Structure of co-crystals of tropomyosin and troponin. 1987 Feb 26-Mar 4Nature. 325(6107):826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]