Abstract
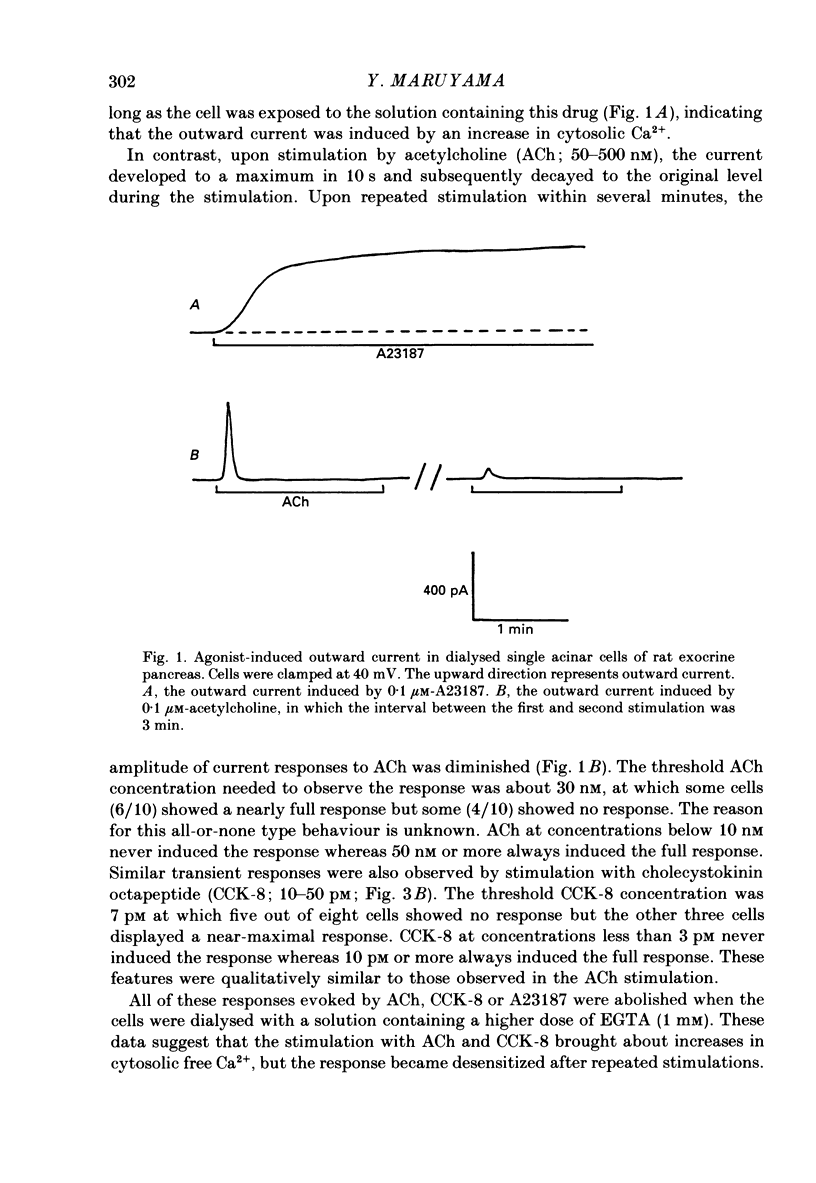
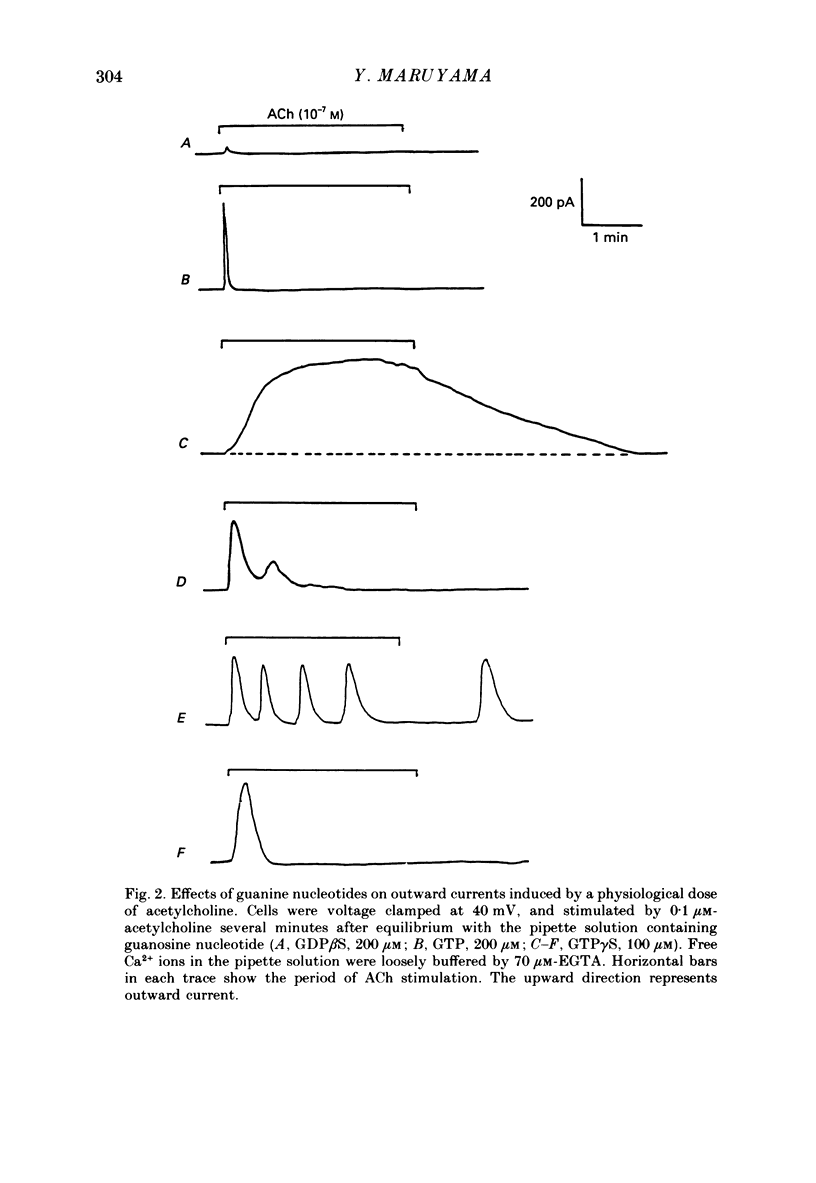
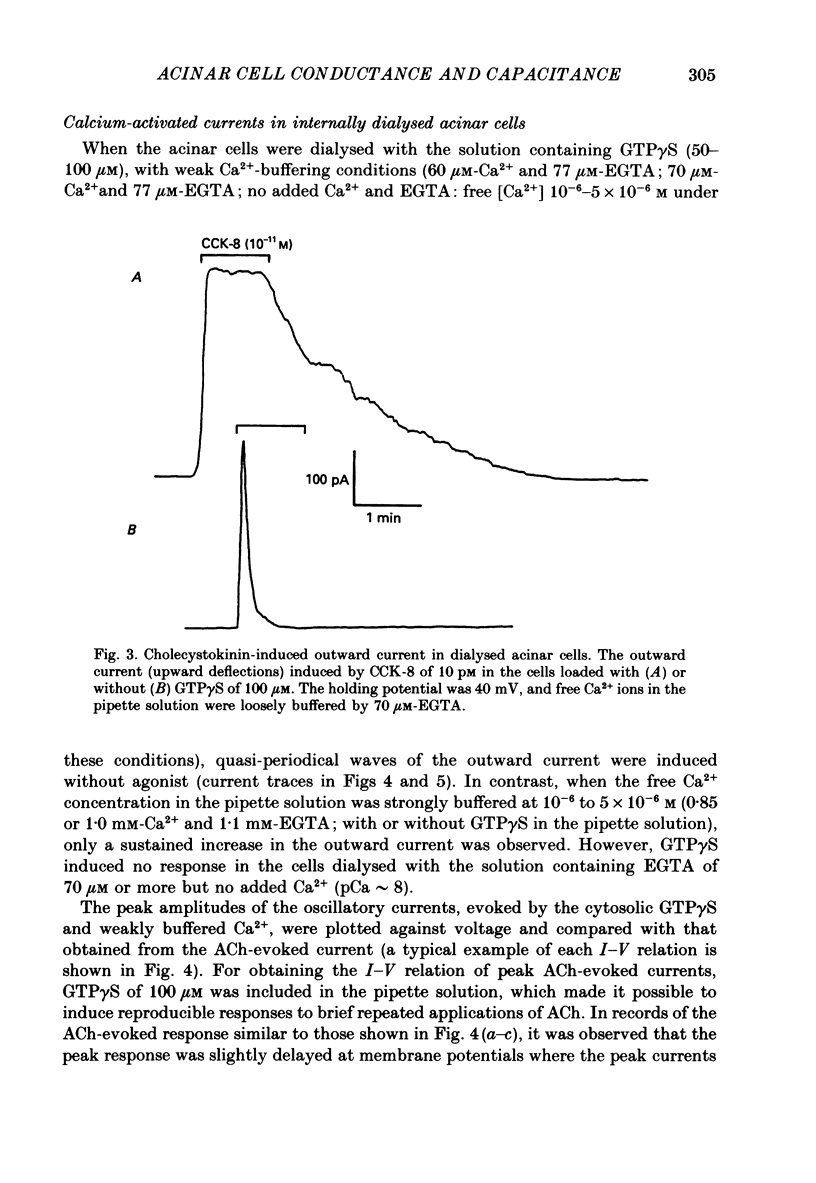
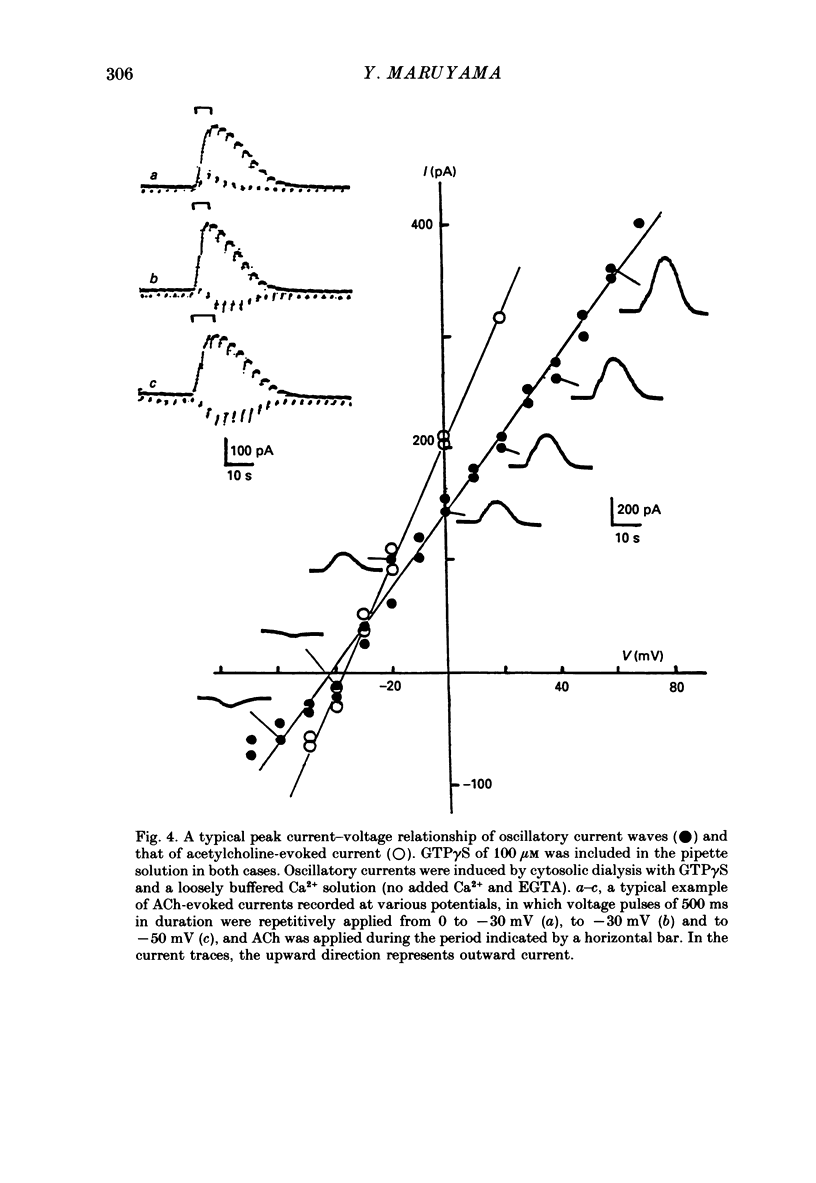
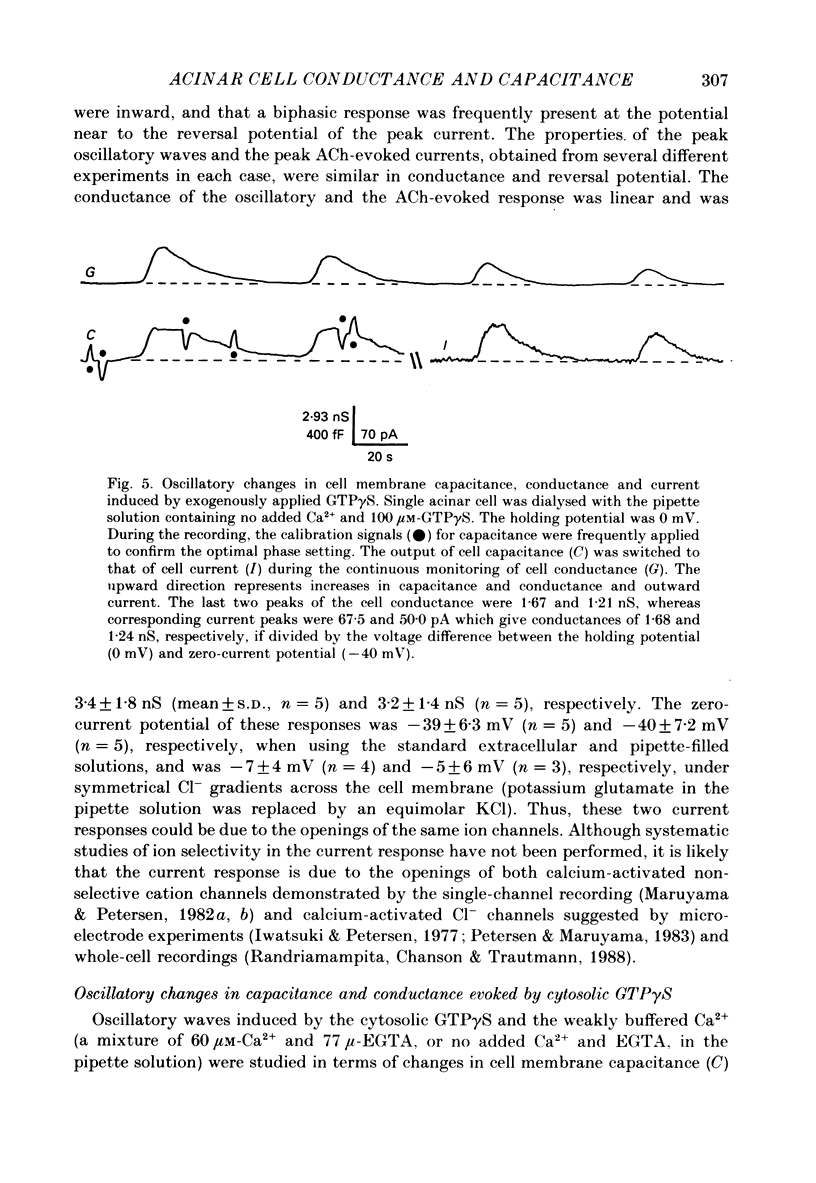
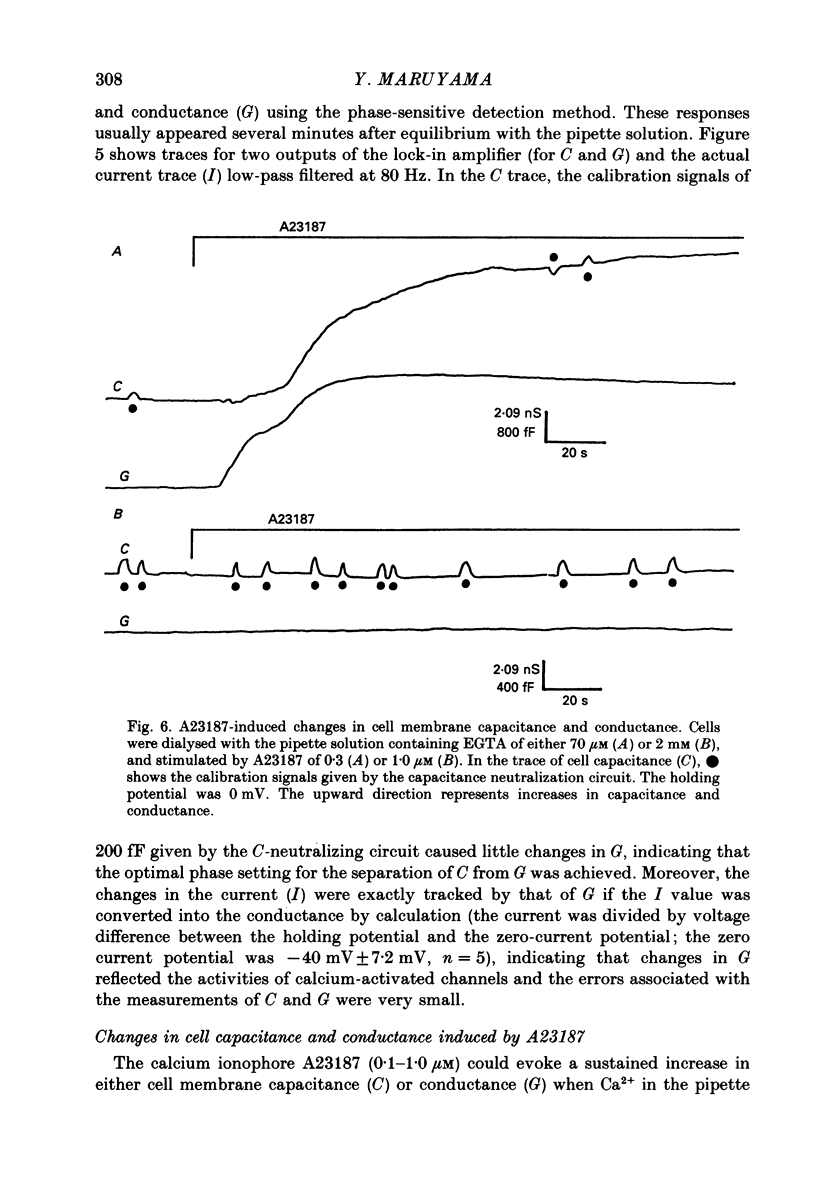
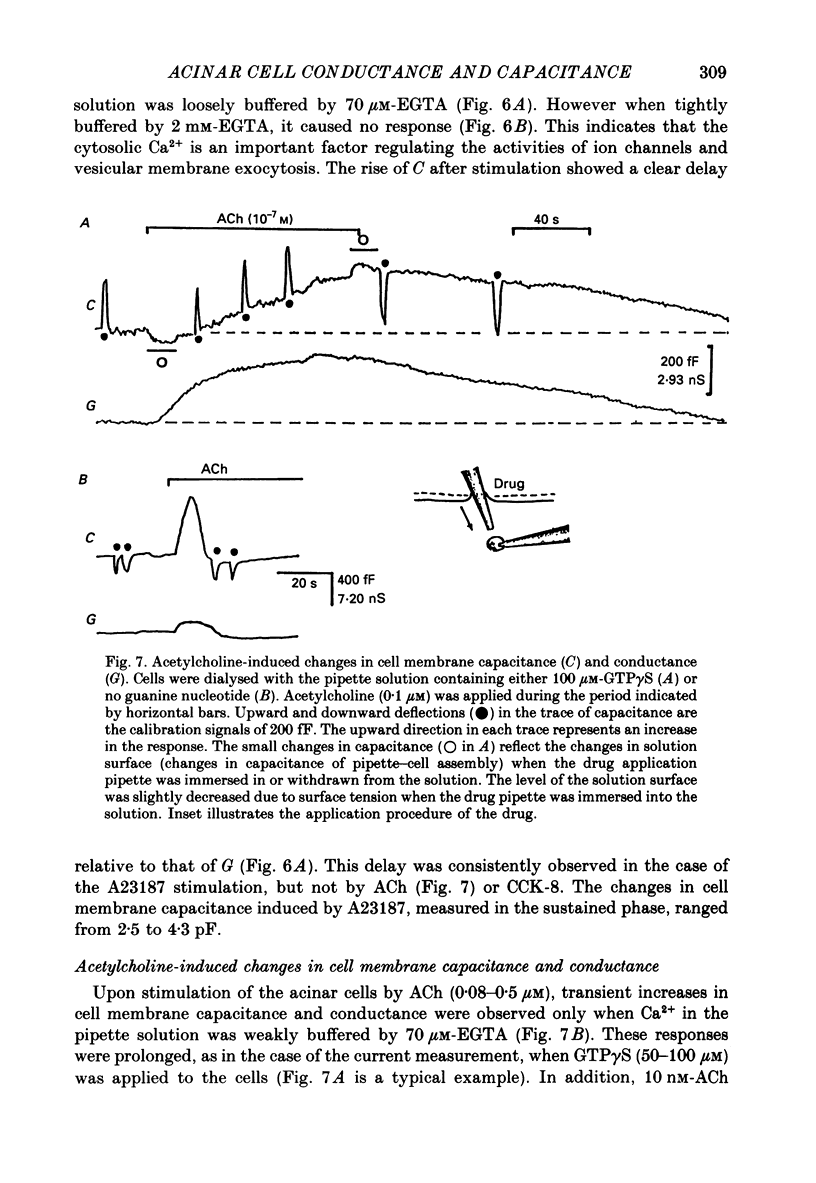
1. Single acinar cells enzymatically isolated from the rat pancreas were subjected to tight-seal whole-cell recordings. Changes in cell membrane capacitance and conductance were simultaneously recorded using a phase-sensitive detection method. 2. Acetylcholine (ACh, 0.05-0.5 microM) and cholecystokinin octapeptide (CCK-8, 10-50 pM) concomitantly induced transient increases in cell membrane current, capacitance and conductance only when cytosolic Ca2+ was weakly chelated by EGTA (70 microM). These responses were prolonged when the cells were dialysed with a solution containing GTP gamma S (a stable analogue of GTP, 50-100 microM), whereas they were inhibited by dialysing with that containing GDP beta S (a stable analogue of GDP). These results suggest that a type of guanine-nucleotide-binding protein (G-protein) could be involved in ACh- or CCK-receptor signalling. 3. The ACh- or CCK-induced responses (with or without GTP gamma S in the cytosol) were all abolished when a high dose of EGTA (1-2 mM) was injected into the acinar cells. In addition, A23187, a calcium ionophore, induced sustained responses when the cytosolic Ca2+ was weakly buffered by 70 microM-EGTA. These results suggest that the secretagogues regulate the changes in cell membrane capacitance and conductance via an increase and decrease of cytosolic Ca2+ concentration. 4. Oscillatory changes in cell membrane conductance and capacitance were consistently observed even without applying secretagogues when the cells were dialysed with a solution containing GTP gamma S (50-100 microM) and cytosolic free Ca2+ ions weakly buffered at about 10(-6) M with a low dose of EGTA and CaCl2. 5. The peak amplitude of changes in cell membrane capacitance induced by ACh or CCK-8, with or without GTP gamma S in the cytosol, varied between 200 and 1000 fF, thereby suggesting that 20-100 zymogen granules can fuse with the luminal cell membrane in response to these agonists in rat exocrine pancreatic acinar cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J Physiol. 1987 May;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. II. Functional characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1057–1073. doi: 10.1083/jcb.63.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolender R. P. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol. 1974 May;61(2):269–287. doi: 10.1083/jcb.61.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. The action of scretin, cholecystokinin-pancreozymin and caerulein on pancreatic secretion in the rat. J Physiol. 1972 Sep;225(3):679–692. doi: 10.1113/jphysiol.1972.sp009963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977 Aug;269(3):735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch P., Petersen O. H., Läuger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings. J Membr Biol. 1986;94(2):99–115. doi: 10.1007/BF01871191. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Maruyama Y. A patch-clamp study of mammalian platelets and their voltage-gated potassium current. J Physiol. 1987 Oct;391:467–485. doi: 10.1113/jphysiol.1987.sp016750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Ca2+-induced excess capacitance fluctuation studied by phase-sensitive detection method in exocrine pancreatic acinar cells. Pflugers Arch. 1986 Nov;407(5):561–563. doi: 10.1007/BF00657517. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Single calcium-dependent cation channels in mouse pancreatic acinar cells. J Membr Biol. 1984;81(1):83–87. doi: 10.1007/BF01868812. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinović S., Argent B. E., Schulz U., Sachs G. Studies on isolated subcellular components of cat pancreas. II. A Ca++-dependent interaction between membranes and zymogen granules of cat pancreas. J Membr Biol. 1977 Sep 14;36(2-3):281–295. doi: 10.1007/BF01868155. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman P. S., Mann G. E. Ionic dependence of amino-acid transport in the exocrine pancreatic epithelium: calcium dependence of insulin action. J Membr Biol. 1987;96(2):153–163. doi: 10.1007/BF01869241. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y., Graf J., Laugier R., Nishiyama A., Pearson G. T. Ionic currents across pancreatic acinar cell membranes and their role in fluid secretion. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):151–166. doi: 10.1098/rstb.1981.0179. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Chanson M., Trautmann A. Calcium and secretagogues-induced conductances in rat exocrine pancreas. Pflugers Arch. 1988 Jan;411(1):53–57. doi: 10.1007/BF00581646. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


