Abstract
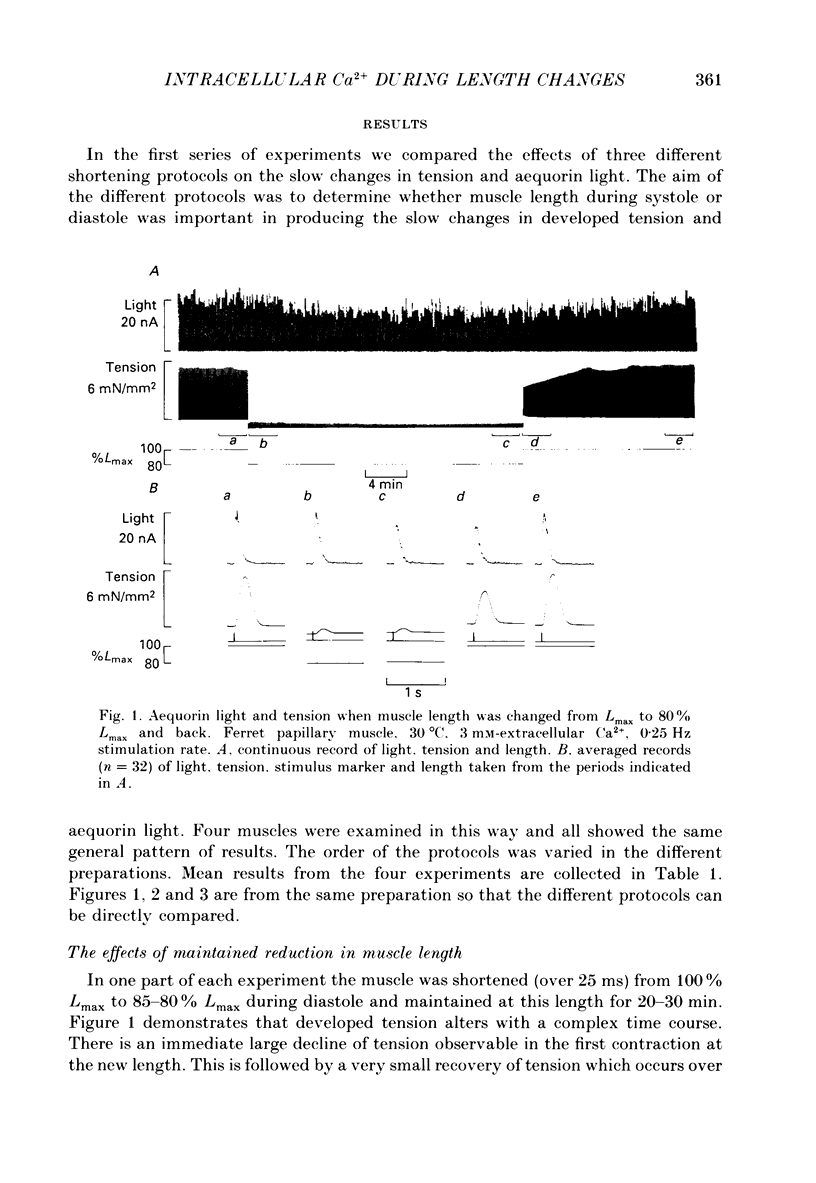
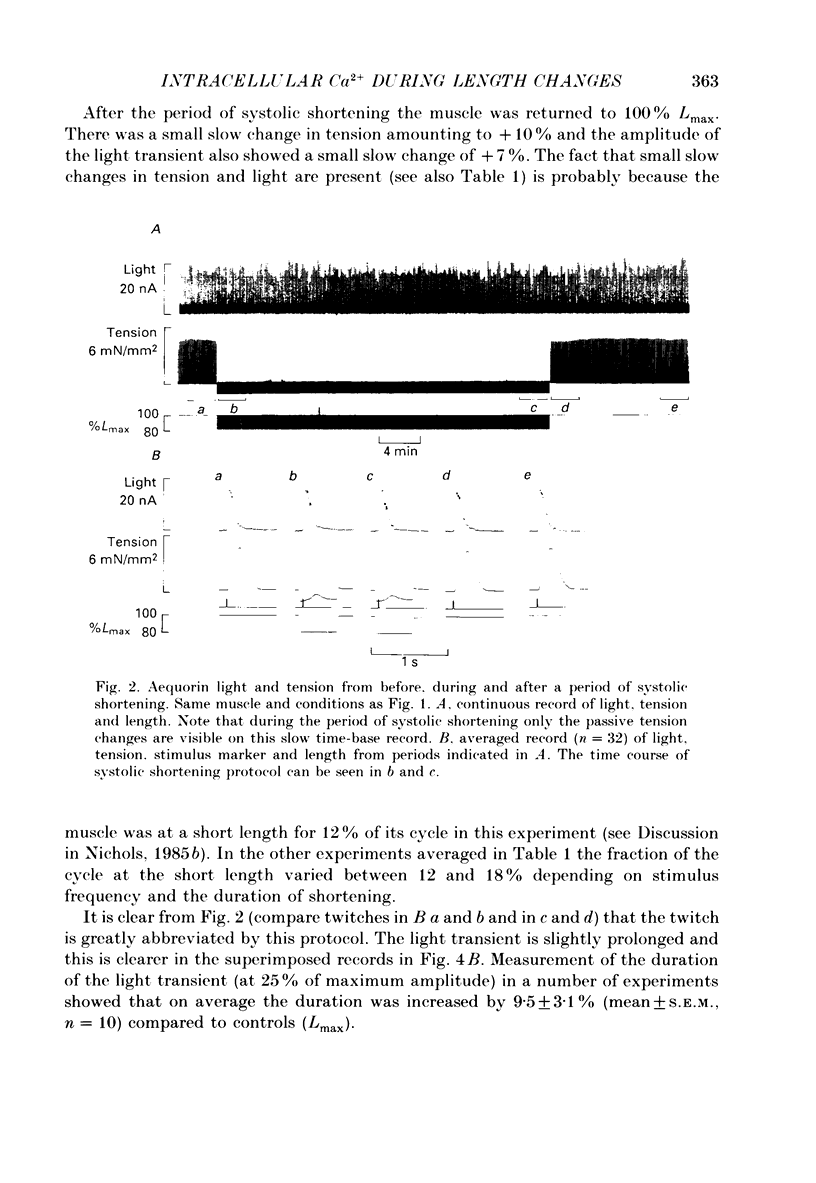
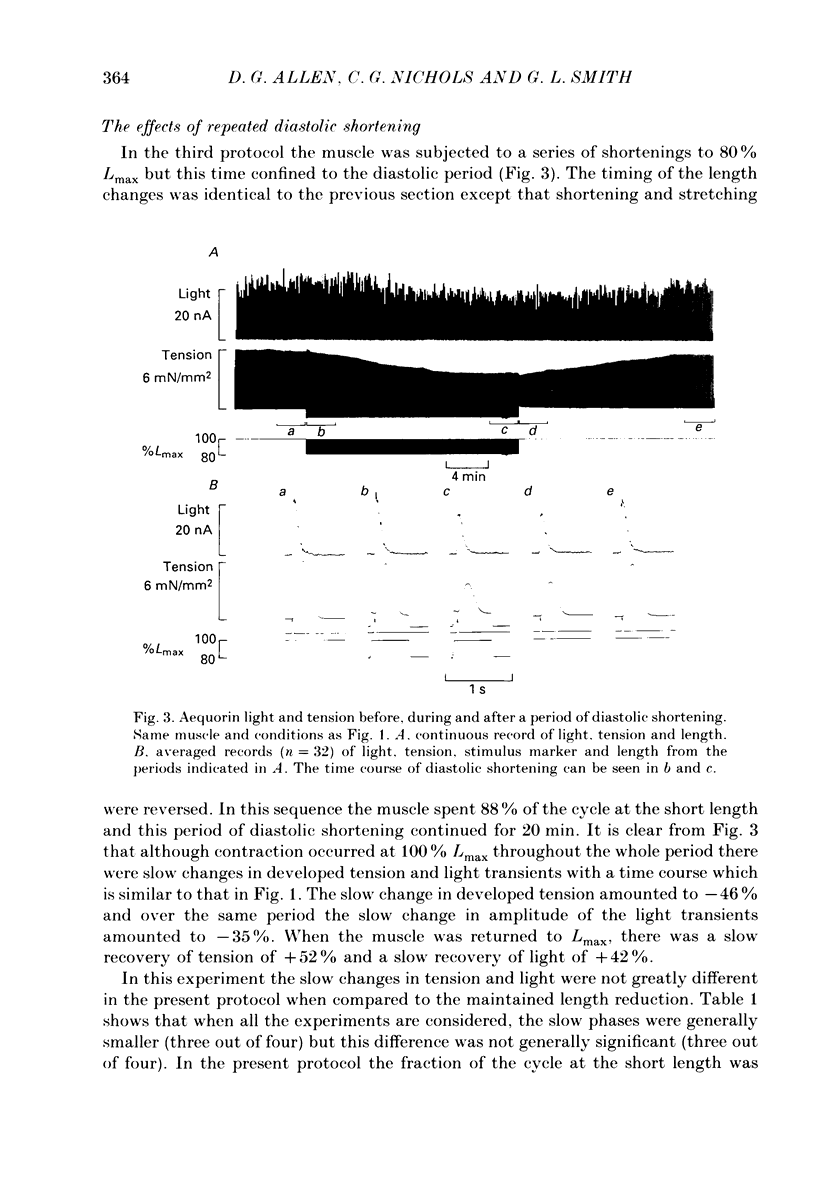
1. Ferret papillary muscles were isolated and injected with aequorin to measure intracellular Ca2+ concentration [( Ca2+]i). Developed tension and [Ca2+]i were measured in response to length changes. 2. A maintained reduction in muscle length produced an immediate decrease in developed tension followed by slow decline over 10-20 min. This slow decline in tension was accompanied by a slow decline in the amplitude of the systolic [Ca2+]i rise (the Ca2+ transient). The immediate decrease in tension was accompanied by a prolongation of the Ca2+ transient and an abbreviation of the twitch. 3. Repeated reductions in muscle length timed to occur only during the period of contraction (systolic shortening) produced an immediate decrease of developed tension but the subsequent slow decline was substantially smaller. The slow decline in the amplitude of the Ca2+ transients was also smaller. The prolongation of the Ca2+ transient and abbreviation of the twitch were similar to those observed with a maintained reduction of length. 4. Repeated reductions in muscle length during the period between contractions (diastolic shortening) did not produce the immediate decrease of tension but the slow decline of tension was present. The slow decline in the amplitude of the Ca2+ transients was also present. However no change in the duration of the Ca2+ transient or the twitch was present under these conditions. 5. These results suggest that diastolic muscle length can influence the amplitude of the Ca2+ transients achieved during systole. This conclusion was confirmed by experiments in which the recovery of tension and Ca2+ transients was observed after periods of rest. Both developed tension and Ca2+ transients on recovery from a rest were reduced when the rest occurred at a short length in comparison with a long length. 6. We suggest that muscle length influences resting [Ca2+]i and this in turn affects the Ca2+ transients and developed tension.
Full text
PDF


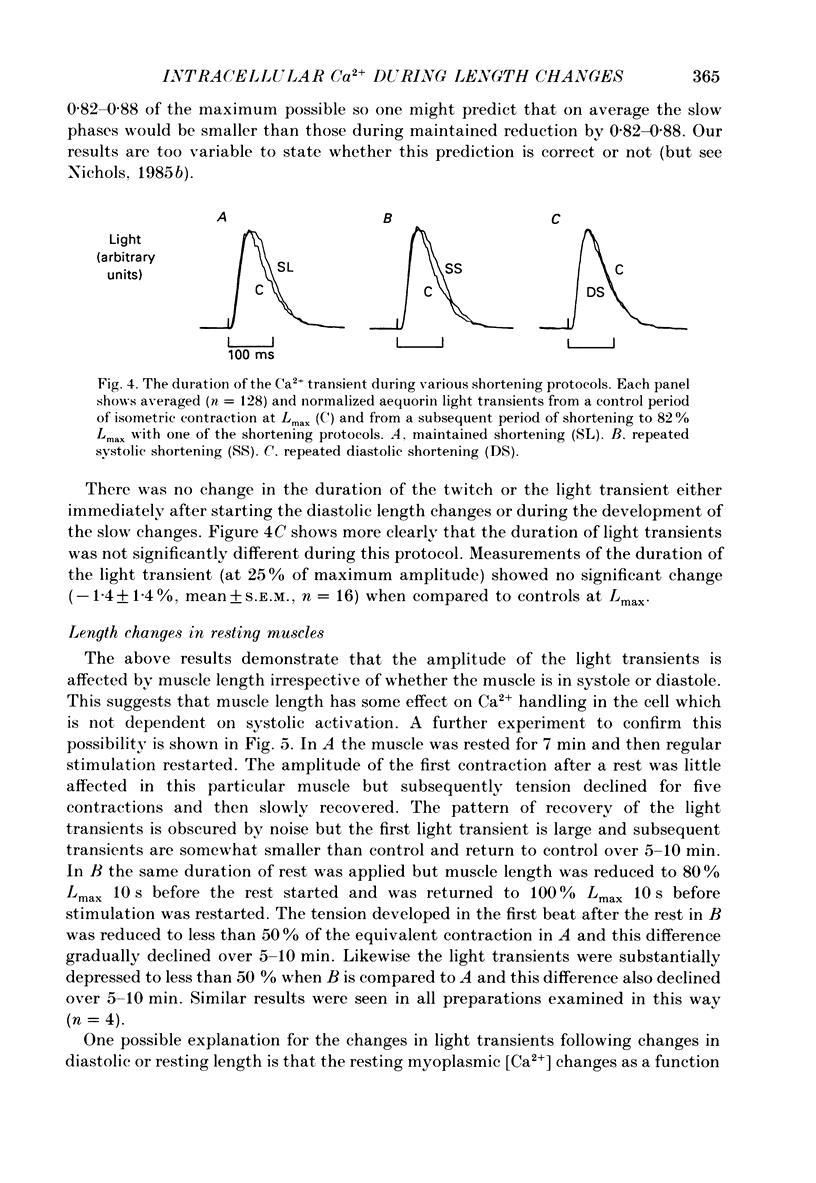
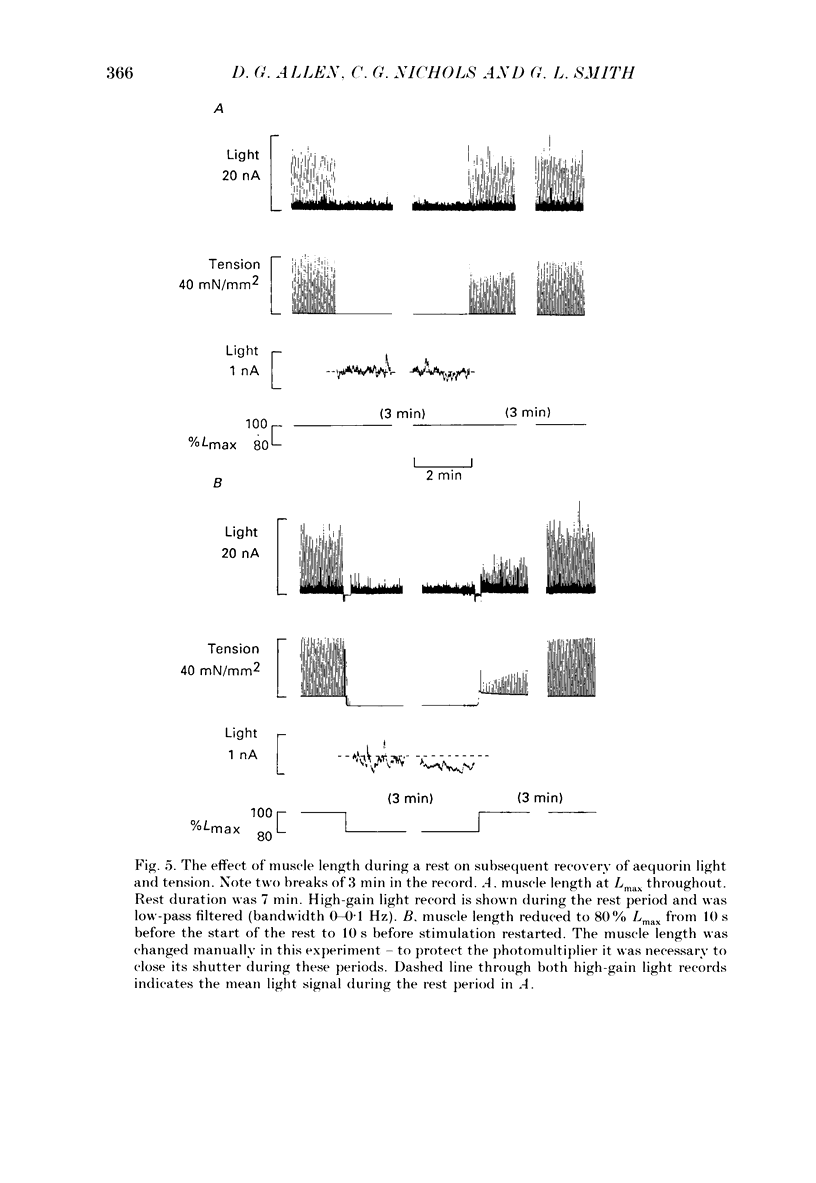
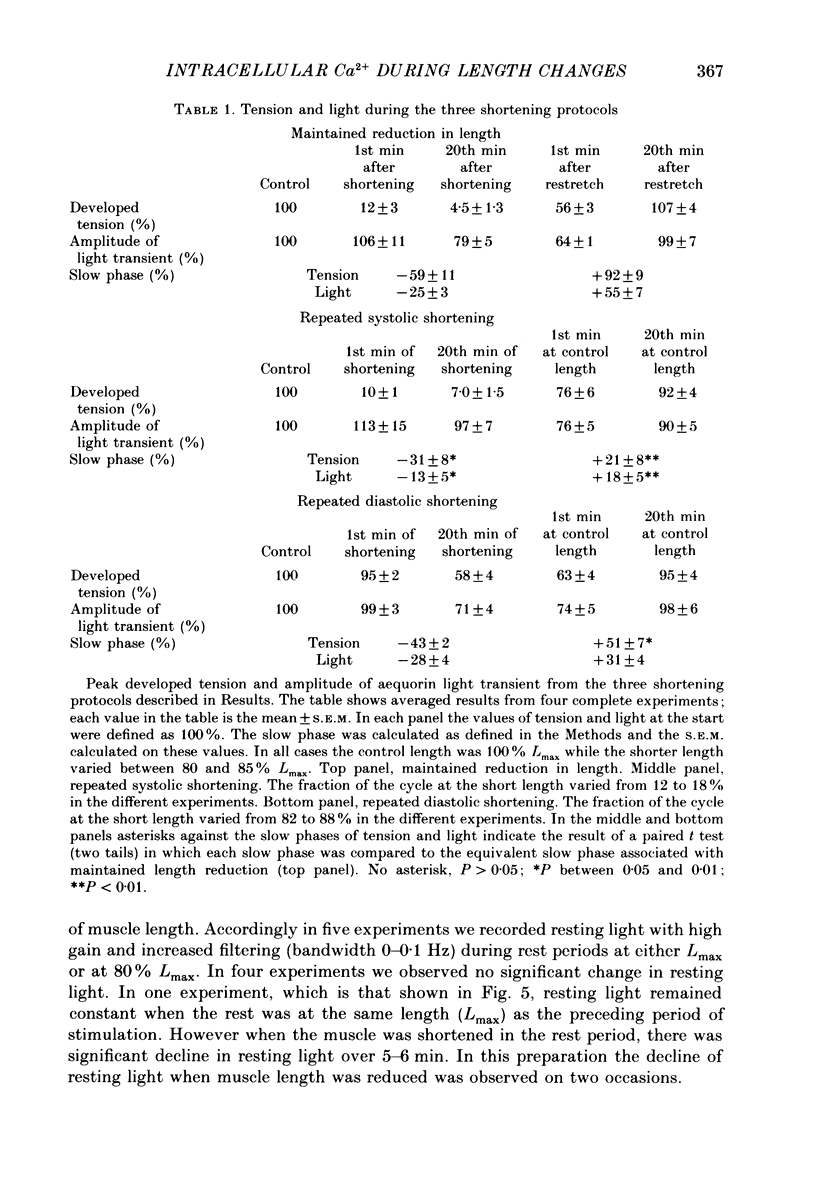



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol. 1984 May;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanck D. A., Jewell B. R. Effects of physiological beating on the contractility of cat ventricular muscle. Am J Physiol. 1985 Jun;248(6 Pt 2):H894–H900. doi: 10.1152/ajpheart.1985.248.6.H894. [DOI] [PubMed] [Google Scholar]
- Housmans P. R., Lee N. K., Blinks J. R. Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle. Science. 1983 Jul 8;221(4606):159–161. doi: 10.1126/science.6857274. [DOI] [PubMed] [Google Scholar]
- Jewell B. R. A reexamination of the influence of muscle length on myocardial performance. Circ Res. 1977 Mar;40(3):221–230. doi: 10.1161/01.res.40.3.221. [DOI] [PubMed] [Google Scholar]
- Lakatta E. G., Jewell B. R. Length-dependent activation: its effect on the length-tension relation in cat ventricular muscle. Circ Res. 1977 Mar;40(3):251–257. doi: 10.1161/01.res.40.3.251. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Nichols C. G. The influence of 'diastolic' length on the contractility of isolated cat papillary muscle. J Physiol. 1985 Apr;361:269–279. doi: 10.1113/jphysiol.1985.sp015645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley W. W., Chuck L. Length-dependent changes in myocardial contractile state. Am J Physiol. 1973 May;224(5):1195–1199. doi: 10.1152/ajplegacy.1973.224.5.1195. [DOI] [PubMed] [Google Scholar]
- Patterson S. W., Starling E. H. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914 Sep 8;48(5):357–379. doi: 10.1113/jphysiol.1914.sp001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci P. J., Bregagnollo E. A., Spadaro J., Cicogna A. C., Ribeiro M. C. Length dependence of activation studied in the isovolumic blood-perfused dog heart. Circ Res. 1984 Jul;55(1):59–66. doi: 10.1161/01.res.55.1.59. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Yue D. T. Intracellular calcium transients underlying the short-term force-interval relationship in ferret ventricular myocardium. J Physiol. 1986 Jul;376:507–530. doi: 10.1113/jphysiol.1986.sp016167. [DOI] [PMC free article] [PubMed] [Google Scholar]