Abstract
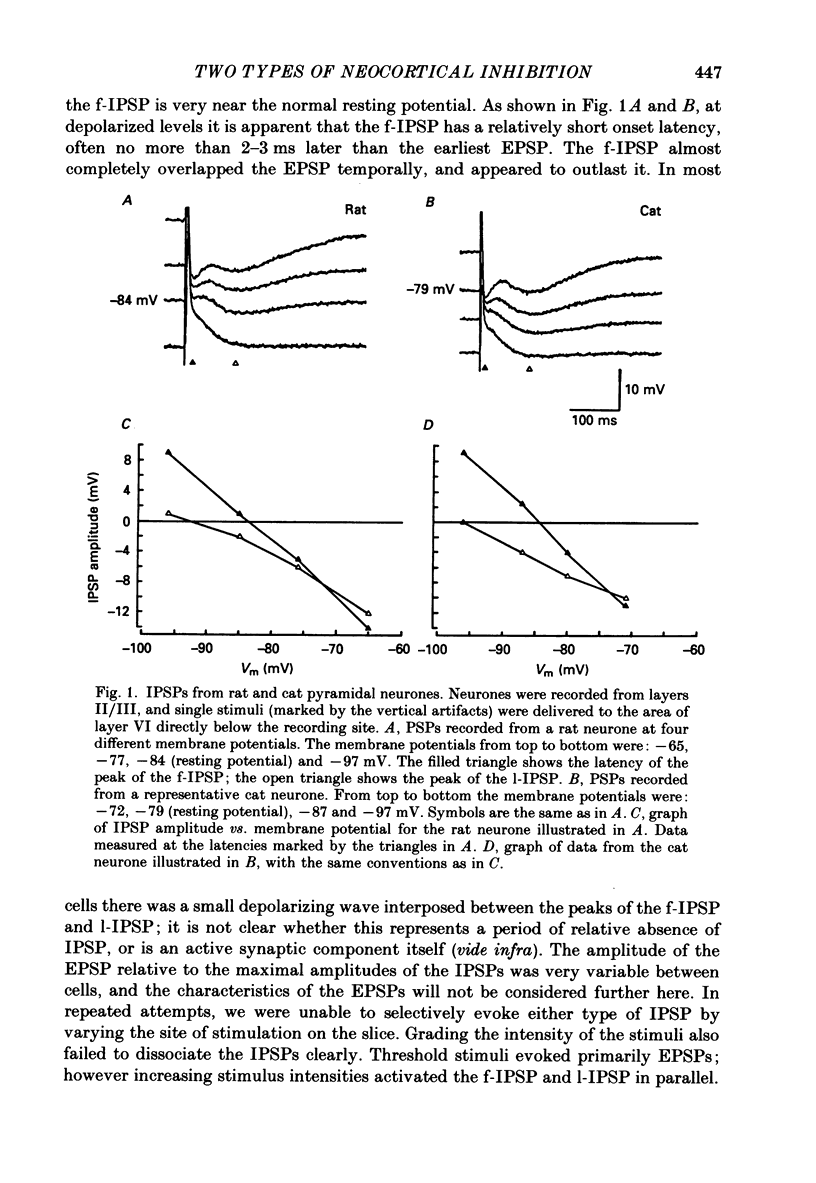
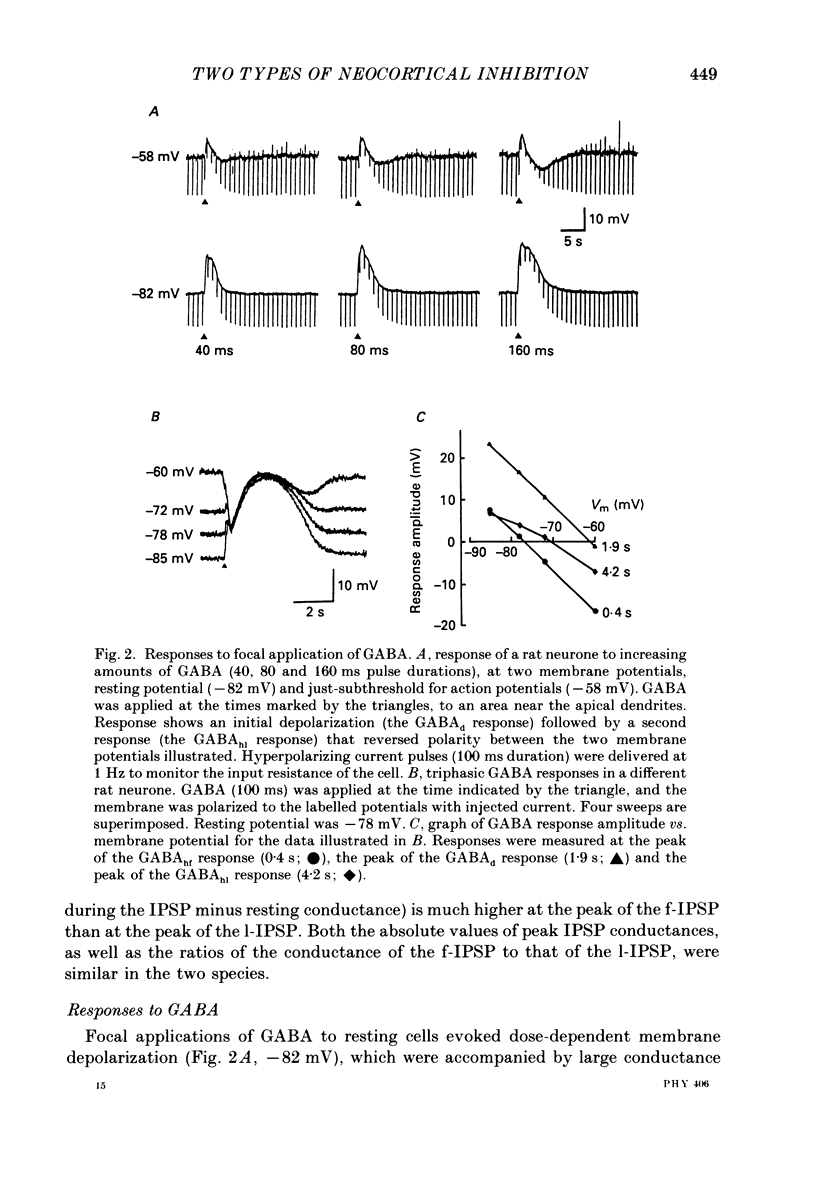
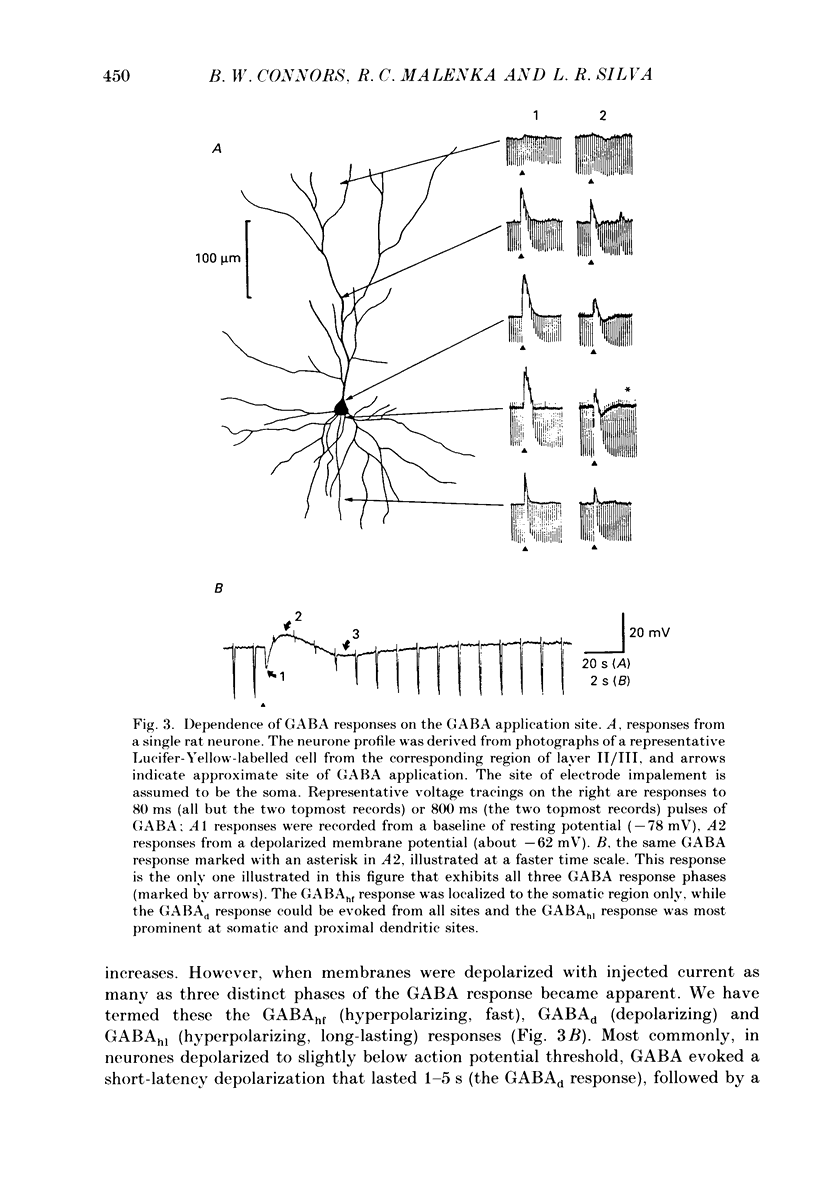
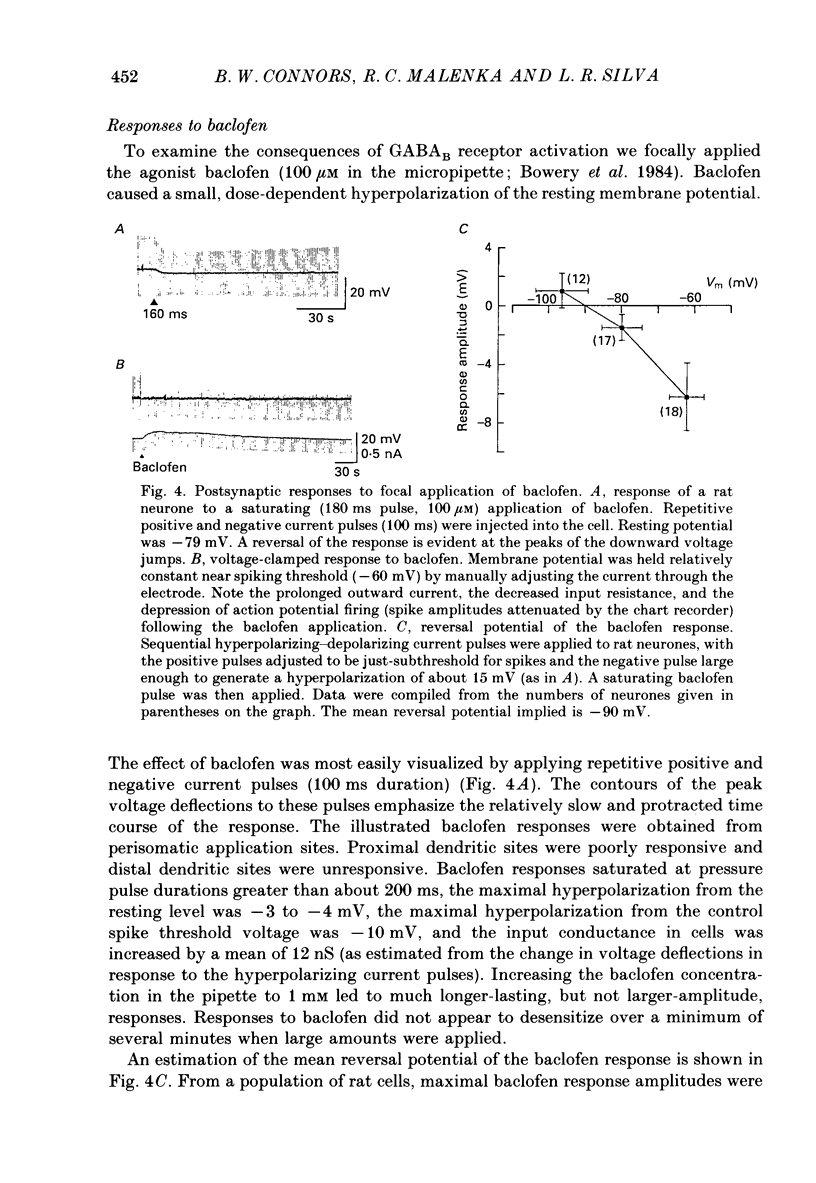
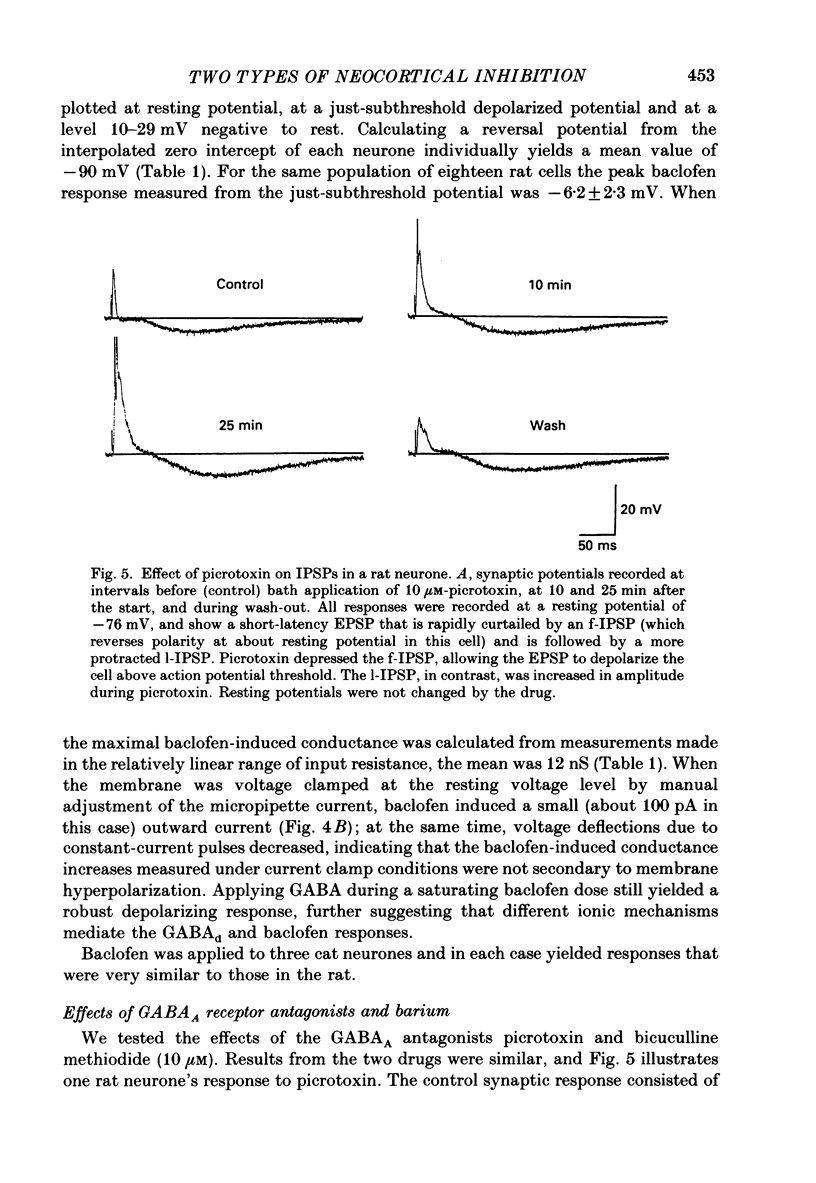
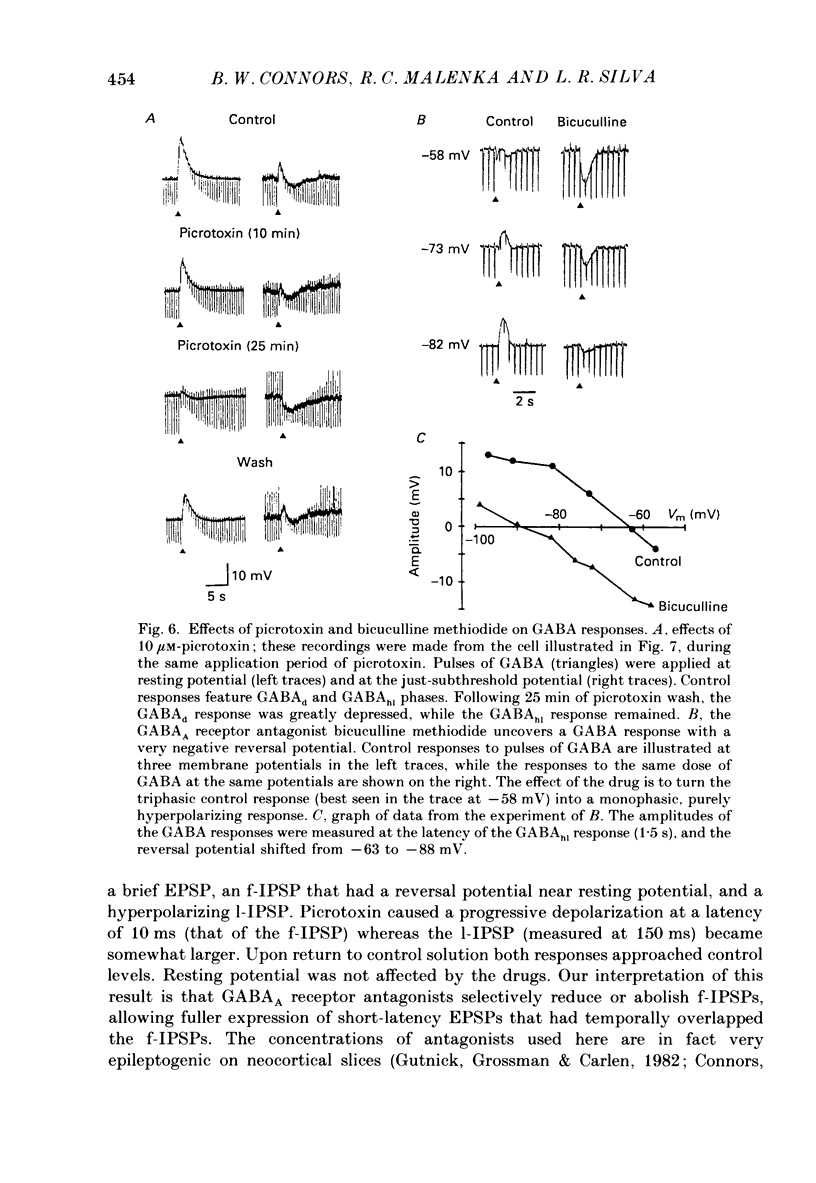
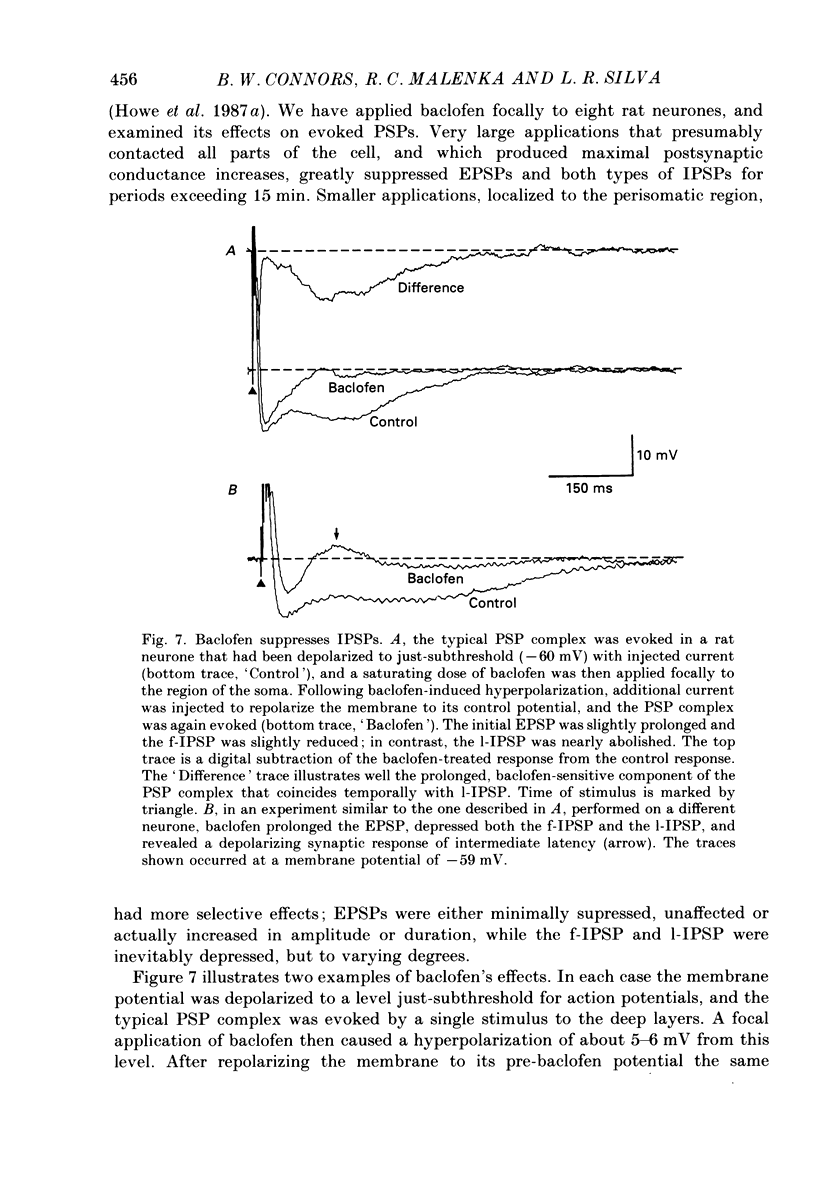
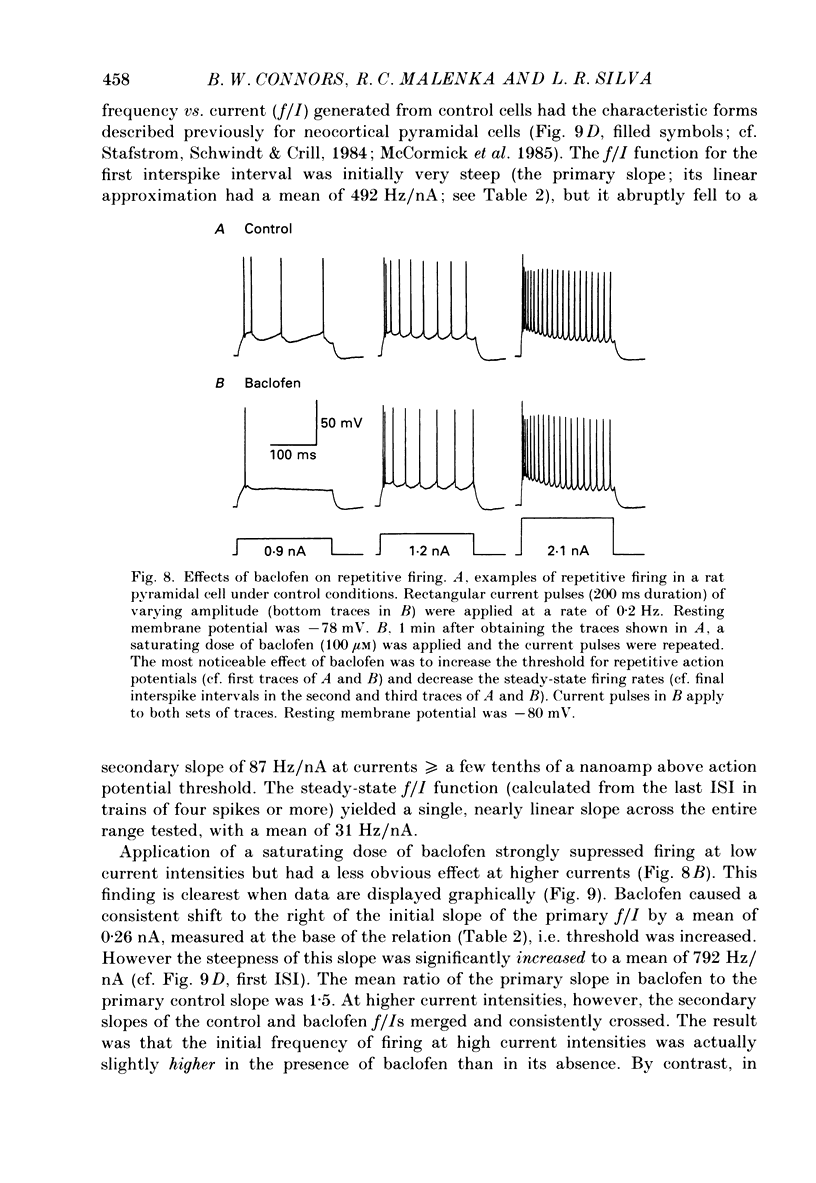
1. Pyramidal neurones from layers II and III of the rat primary somatosensory cortex and cat primary visual cortex were studied in vitro. Inhibitory postsynaptic potentials (IPSPs) and responses to exogenously applied gamma-aminobutyric acid (GABA) and its analogue baclofen were characterized. The results from rats and cats were very similar. 2. Single electrical stimuli to deep cortical layers evoked a sequence of PSPs in the resting neurone: (a) an initial, brief excitation (EPSP), (b) a short-latency, fast inhibition (the f-IPSP) and (c) a long-latency, more prolonged inhibition (the l-IPSP). The f-IPSP was accompanied by a large conductance increase (about 70-90 nS) and reversed polarity at -75 mV; the l-IPSP displayed a relatively small conductance increase (about 10-20 nS) and reversed at greater than -90 mV. 3. Focal application of GABA near the soma evoked a triphasic response when measured near the threshold voltage for action potentials: (a) the GABAhf (hyperpolarizing, fast) phase was very brief and was generated by a large conductance increase with a reversal potential of -78 mV, (b) the GABAd (depolarizing) phase also had a high conductance but reversed at -51 mV, (c) the GABAhl (hyperpolarizing, long-lasting) phase had a relatively low conductance and reversed at -70 mV. The GABAhf response was specifically localized to the soma, whereas the apical or basilar dendrites generated predominantly GABAd responses. 4. Baclofen, a selective GABAB receptor agonist, caused a small (about 2 mV), slow hyperpolarization of the resting potential, which reversed at -90 mV. Saturating baclofen doses increased membrane conductance by a maximum of about 12 nS. Baclofen depressed the amplitude and conductance of PSPs; when baclofen was focally applied near the soma. IPSPs were selectively depressed. 5. The GABAA receptor antagonists bicuculline methiodide or picrotoxin (10 microM) greatly depressed f-IPSPs, but either enhanced or did not affect l-IPSPs. Concomitantly, GABAhf and GABAd responses were antagonized, leaving a more prominent GABAhl response that reversed polarity at a more negative level of -87 mV. Baclofen responses were unaffected by bicuculline and picrotoxin. Extracellular barium abolished the baclofen response, and shifted the reversal potentials of the GABAd and GABAhl responses in the positive direction; the GABAhf response was unaffected. 6. Both focal GABA and f-IPSPs strongly depressed the intrinsic excitability of pyramidal neurones. Each greatly increased spike threshold and abolished or vastly reduced the capacity of the cells to fire repetitively during intense stimuli.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







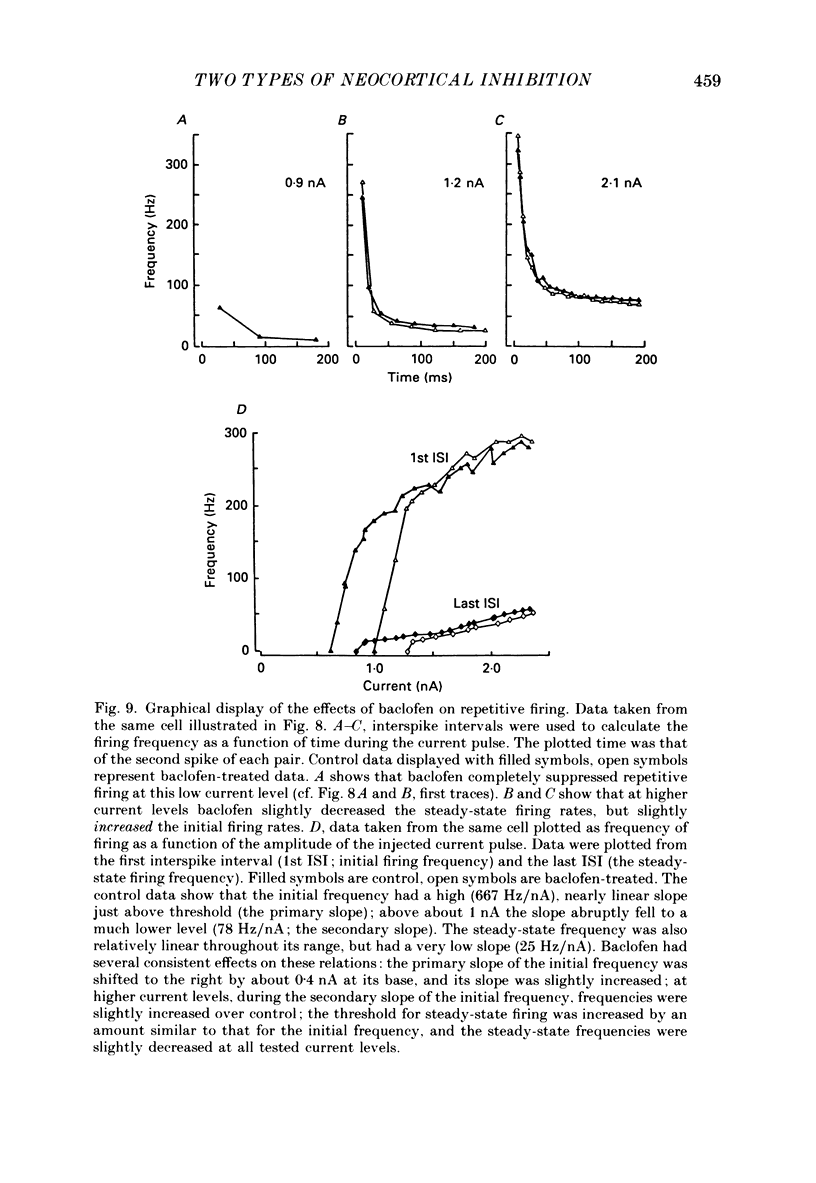

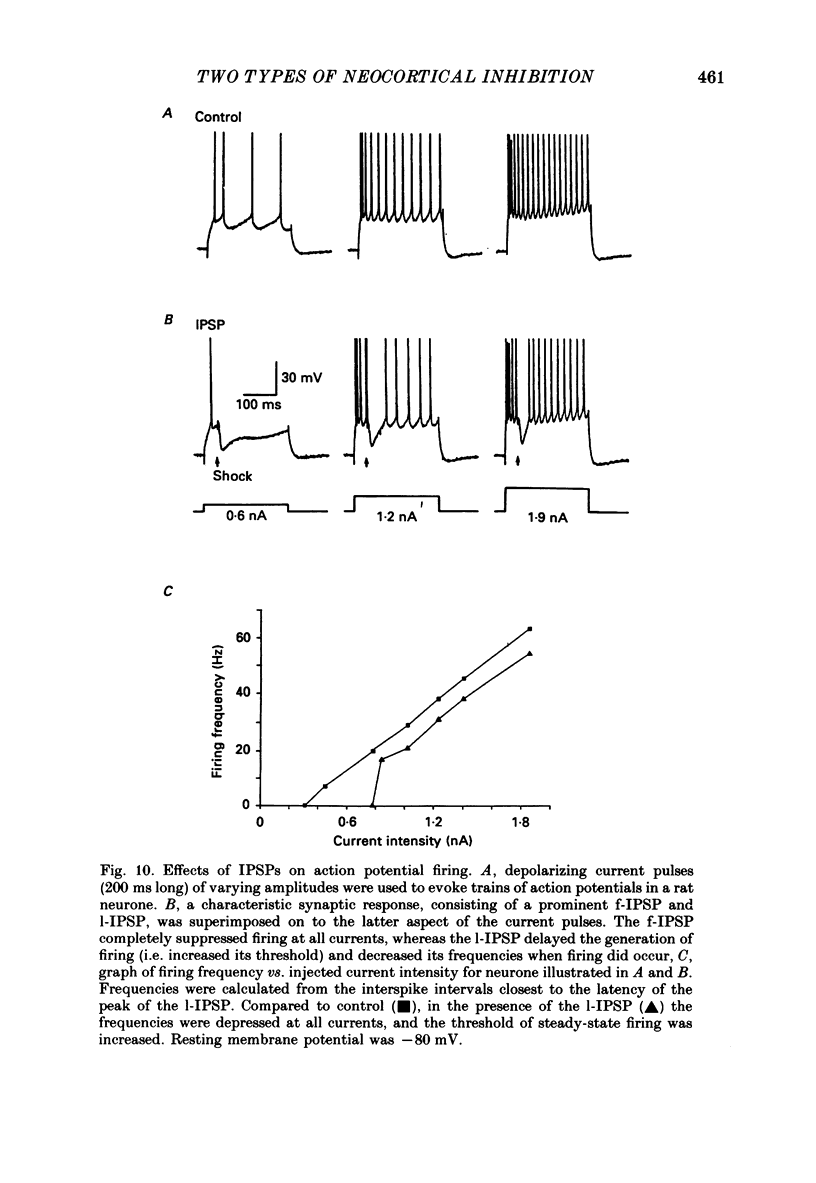







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1984 Nov;52(5):892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Baclofen selectively inhibits transmission at synapses made by axons of CA3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982 Nov;223(2):291–297. [PubMed] [Google Scholar]
- Avoli M. Inhibitory potentials in neurons of the deep layers of the in vitro neocortical slice. Brain Res. 1986 Apr 2;370(1):165–170. doi: 10.1016/0006-8993(86)91118-2. [DOI] [PubMed] [Google Scholar]
- Bolz J., Gilbert C. D. Generation of end-inhibition in the visual cortex via interlaminar connections. 1986 Mar 27-Apr 2Nature. 320(6060):362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Price G. W., Hudson A. L., Hill D. R., Wilkin G. P., Turnbull M. J. GABA receptor multiplicity. Visualization of different receptor types in the mammalian CNS. Neuropharmacology. 1984 Feb;23(2B):219–231. doi: 10.1016/0028-3908(84)90063-7. [DOI] [PubMed] [Google Scholar]
- Brady R. J., Swann J. W. Postsynaptic actions of baclofen associated with its antagonism of bicuculline-induced epileptogenesis in hippocampus. Cell Mol Neurobiol. 1984 Dec;4(4):403–408. doi: 10.1007/BF00733601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Connors B. W. Initiation of synchronized neuronal bursting in neocortex. Nature. 1984 Aug 23;310(5979):685–687. doi: 10.1038/310685a0. [DOI] [PubMed] [Google Scholar]
- Dichter M. A. Physiological identification of GABA as the inhibitory transmitter for mammalian cortical neurons in cell culture. Brain Res. 1980 May 19;190(1):111–121. doi: 10.1016/0006-8993(80)91163-4. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dykes R. W., Landry P., Metherate R., Hicks T. P. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol. 1984 Dec;52(6):1066–1093. doi: 10.1152/jn.1984.52.6.1066. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Houser C. R., Jones E. G., Vaughn J. E. Synaptic organization of immunocytochemically identified GABA neurons in the monkey sensory-motor cortex. J Neurocytol. 1983 Aug;12(4):639–660. doi: 10.1007/BF01181528. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Characteristics of long-duration inhibitory postsynaptic potentials in rat neocortical neurons in vitro. Cell Mol Neurobiol. 1987 Mar;7(1):1–18. doi: 10.1007/BF00734986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring P., Stähli C., Schoch P., Takács B., Staehelin T., Möhler H. Monoclonal antibodies reveal structural homogeneity of gamma-aminobutyric acid/benzodiazepine receptors in different brain areas. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4837–4841. doi: 10.1073/pnas.82.14.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Characterization of pre- and postsynaptic actions of (-)-baclofen in the guinea-pig hippocampus in vitro. Br J Pharmacol. 1985 Apr;84(4):843–851. doi: 10.1111/j.1476-5381.1985.tb17378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Mitchell J. F., Srinivasan V. The release of gamma-aminobutyric acid during inhibition in the cat visual cortex. J Physiol. 1971 Jan;212(2):519–534. doi: 10.1113/jphysiol.1971.sp009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Kriegstein A. R., Connors B. W. Cellular physiology of the turtle visual cortex: synaptic properties and intrinsic circuitry. J Neurosci. 1986 Jan;6(1):178–191. doi: 10.1523/JNEUROSCI.06-01-00178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. Pharmacology of cortical inhibition. J Physiol. 1966 May;184(1):78–105. doi: 10.1113/jphysiol.1966.sp007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Mori K., Nowycky M. C., Shepherd G. M. Analysis of synaptic potentials in mitral cells in the isolated turtle olfactory bulb. J Physiol. 1981 May;314:295–309. doi: 10.1113/jphysiol.1981.sp013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needler M. C., Shaw C., Cynader M. Characteristics and distribution of muscimol binding sites in cat visual cortex. Brain Res. 1984 Aug 13;308(2):347–353. doi: 10.1016/0006-8993(84)91076-x. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Baudry M., Fagni L., Lynch G. The blocking action of baclofen on excitatory transmission in the rat hippocampal slice. J Neurosci. 1982 Jun;2(6):698–703. doi: 10.1523/JNEUROSCI.02-06-00698.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984 Nov 1;229(3):374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Ribak C. E. Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol. 1978 Aug;7(4):461–478. doi: 10.1007/BF01173991. [DOI] [PubMed] [Google Scholar]
- Satou M., Mori K., Tazawa Y., Takagi S. F. Two types of postsynaptic inhibition in pyriform cortex of the rabbit: fast and slow inhibitory postsynaptic potentials. J Neurophysiol. 1982 Nov;48(5):1142–1156. doi: 10.1152/jn.1982.48.5.1142. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. Responses to gamma-aminobutyric acid applied to cell bodies and dendrites of rat visual cortical neurons. Brain Res. 1985 Dec 9;358(1-2):385–389. doi: 10.1016/0006-8993(85)90990-4. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Vogel S. M. Independent control of channel closure and block of open channels by methylxanthines at acetylcholine receptors in frog. J Physiol. 1987 Sep;390:33–44. doi: 10.1113/jphysiol.1987.sp016684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Repetitive firing in layer V neurons from cat neocortex in vitro. J Neurophysiol. 1984 Aug;52(2):264–277. doi: 10.1152/jn.1984.52.2.264. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Further studies on the action of baclofen on neurons of the dorsolateral septal nucleus of the rat, in vitro. Brain Res. 1985 Dec 9;358(1-2):360–363. doi: 10.1016/0006-8993(85)90984-9. [DOI] [PubMed] [Google Scholar]
- Tseng G. F., Haberly L. B. Characterization of synaptically mediated fast and slow inhibitory processes in piriform cortex in an in vitro slice preparation. J Neurophysiol. 1988 May;59(5):1352–1376. doi: 10.1152/jn.1988.59.5.1352. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Hablitz J. J. Interaction of penicillin and pentobarbital with inhibitory synaptic mechanisms in neocortex. Cell Mol Neurobiol. 1984 Dec;4(4):301–317. doi: 10.1007/BF00733594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]


