Abstract
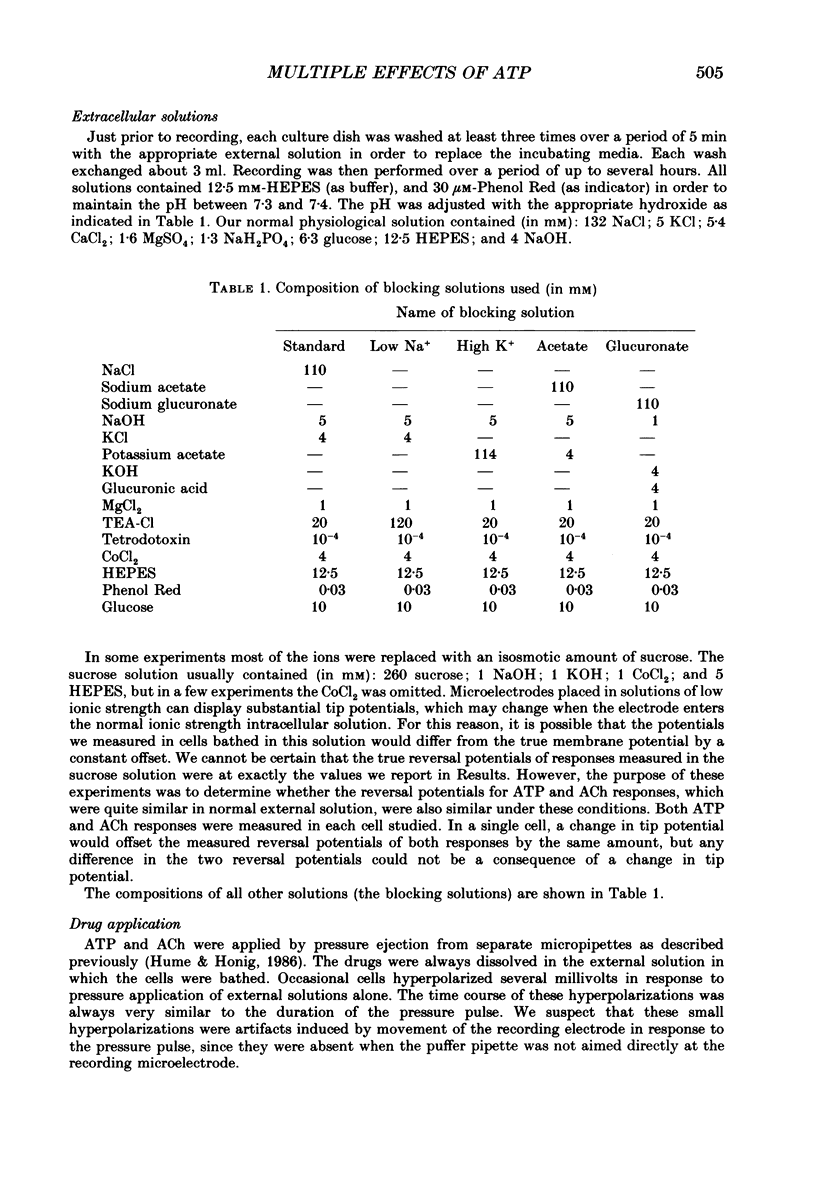
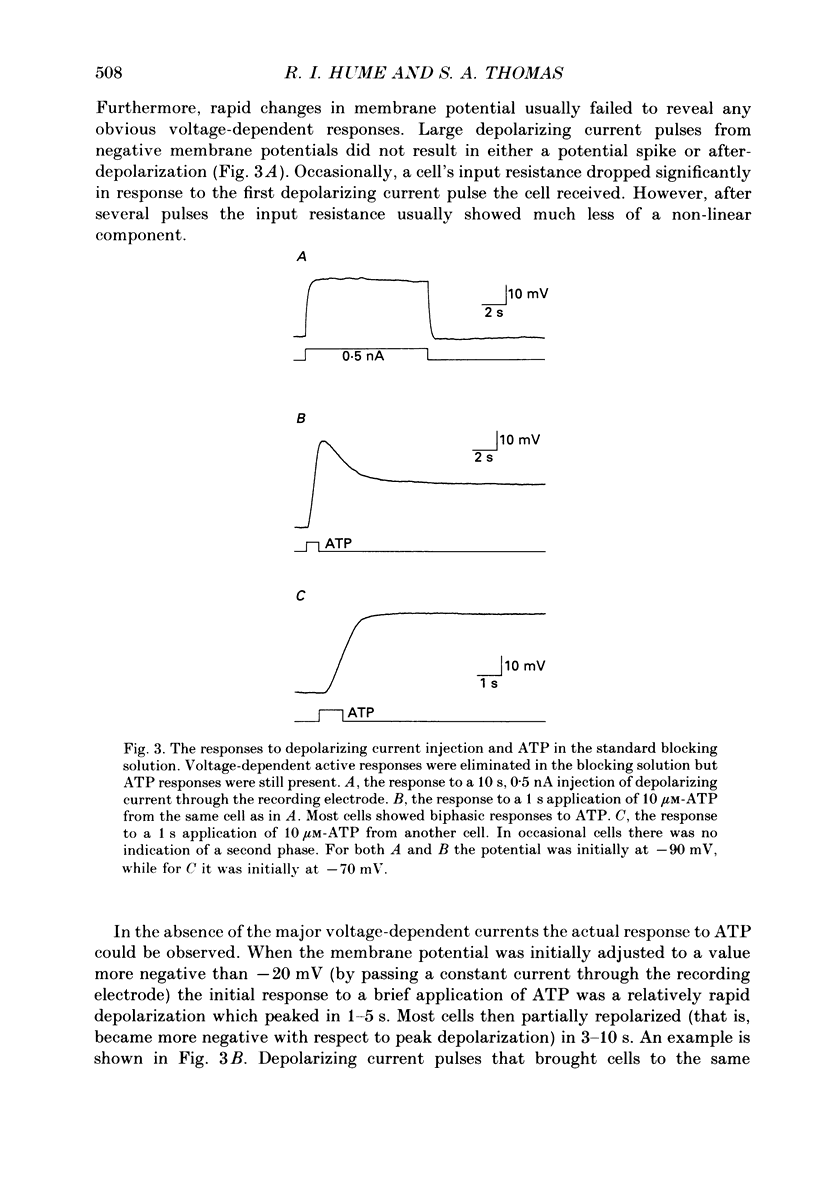
1. Extracellularly applied adenosine 5'-triphosphate (ATP) is known to have an excitatory action on chick skeletal muscle. By making intracellular recordings from cultured chick myotubes bathed with blockers of several types of voltage-dependent channels, the direct action of ATP could be observed. 2. When muscle cells were studied near their resting potential, ATP usually produced a biphasic response. There was a rapid initial depolarization, followed by a slower repolarization. The repolarization could drive cells negative to their initial resting potential, indicating that it was not due simply to desensitization of the process that produced the depolarization. Thus there are at least two distinct responses to ATP. 3. At room temperature the early response to ATP activated within 20 ms, and the second response activated with a latency of approximately 1 s. In our standard blocking solution, the average reversal potential of the early response was -17 mV, while the late response had a reversal potential that was negative to -70 mV. In a few cells the second response appeared to be absent. 4. The amplitude and time course of the late response were substantially decreased by low temperature (12 degrees C) and increased by high temperature (37 degrees C). In contrast, temperature had much smaller effects on the early response. Both the time course and temperature dependence of the late response suggest that an intracellular second messenger system may be involved in its activation. 5. Ion-substitution experiments were performed to determine the type of conductance changes that evoke each response. These indicated that the early response was due to an increased membrane permeability to sodium, potassium and chloride, but not to large cations or anions, and that the late response was due to an increased permeability to potassium. 6. Measurement of the responses of muscle cells to acetylcholine supported the conclusion that both anions and cations are permeable during the early ATP response. Under conditions in which there was a large negative reversal potential for all cations, and a large positive reversal potential for all anions, the early ATP response reversed approximately 50 mV positive to the acetylcholine response. 8. The possibility that the early ATP response is due to a channel selective for size, but not charge, is discussed.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Castel M., Gainer H., Dellmann H. D. Neuronal secretory systems. Int Rev Cytol. 1984;88:303–459. doi: 10.1016/s0074-7696(08)62760-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Nameroff M., Nelson P. G. Electrical properties of chick skeletal muscle fibers developing in cell culture. J Cell Physiol. 1971 Oct;78(2):289–299. doi: 10.1002/jcp.1040780218. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J. Chloride spike: a third type of action potential in tissue-cultured skeletal muscle cells from the chick. Science. 1974 Jul 5;185(4145):76–78. doi: 10.1126/science.185.4145.76. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Fischbach G. D., Smith T. G., Jr A voltage clamp study of the sodium, calcium and chloride spikes of chick skeletal muscle cells grown in tissue culture. Dev Biol. 1976 Apr;49(2):412–424. doi: 10.1016/0012-1606(76)90184-6. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Honig M. G. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986 Mar;6(3):681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblad J., Eriksson H., Heilbronn E. ATP-induced cation influx in myotubes is additive to cholinergic agonist action. Acta Physiol Scand. 1985 Nov;125(3):389–393. doi: 10.1111/j.1748-1716.1985.tb07734.x. [DOI] [PubMed] [Google Scholar]
- Häggblad J., Heilbronn E. Externally applied adenosine-5'-triphosphate causes inositol triphosphate accumulation in cultured chick myotubes. Neurosci Lett. 1987 Feb 24;74(2):199–204. doi: 10.1016/0304-3940(87)90149-2. [DOI] [PubMed] [Google Scholar]
- Kano M., Shimada Y., Ishikawa K. Electrogenesis of embryonic chick skeletal muscle cells differentiated in vitro. J Cell Physiol. 1972 Jun;79(3):363–366. doi: 10.1002/jcp.1040790306. [DOI] [PubMed] [Google Scholar]
- Kano M., Shimada Y. Tetrodotoxin-resistant electric activity in chick skeletal muscle cells differentiated in vitro. J Cell Physiol. 1973 Feb;81(1):85–89. doi: 10.1002/jcp.1040810110. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Wakelam M. J. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983 Jun 16;303(5918):621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]


