Abstract
Tick-borne diseases are among the major widespread emerging zoonotic diseases, and their circulation in the environment is influenced by a broad range of abiotic and biotic factors, including the abundance of vectors and vertebrate hosts. In this study, we estimated the prevalence of tick-borne pathogens and the impact of wildlife head count on their circulation in a lowland natural area in northwestern Italy. We collected ticks and camera trap pictures from 14 sampling points every 2 weeks for 1 year and identified pathogens through molecular analyses: Babesia capreoli, B. microti-like, Borrelia burgdorferi sensu lato (s.l.), Rickettsia of the spotted fever group (SFG), Theileria capreoli, and Anaplasma phagocytophilum. We modeled the presence of B. capreoli, B. microti-like, B. burgdorferi s.l., and SFG Rickettsia on head counts of wild ungulates and mesocarnivores. We tested a global model including all collected ticks, as well as a model focusing solely on Ixodes ricinus nymphs, the species, and the developmental stage most associated with zoonotic infection risk. The highest prevalence was obtained for B. microti-like (13%) and SFG Rickettsia (11%), and, for most pathogens, no differences were detected among tick species and their developmental stages. Mesocarnivores showed an additive effect on B. microti-like and B. burgdorferi s.l., while wild ungulates, non-competent for transmission of our target pathogens, showed a dilutive effect. These findings confirm the circulation of relevant tick-borne pathogens in the study area and show the use of camera trap data in predicting tick-borne pathogens’ risk by targeting host species which may have an indirect impact and are more easily addressed by monitoring and control strategies.
Keywords: humans, Ixodidae, recreational areas, tick-borne zoonoses, wildlife
1. Introduction
In recent years, tick-borne zoonoses have emerged as significant threats to human health, exhibiting increasing prevalence alongside the geographical expansion of their vectors (1–3). Wildlife species can increase the circulation of these pathogens, serving as both reservoirs and hosts for the vectors. However, scant information exists regarding potential differences in pathogen reservoir competence of various species, which is commonly investigated through xenodiagnosis. For instance, Babesia divergens is detected in red deer (Cervus elaphus) with relevant frequency, similar to B. capreoli in roe deer (Capreolus capreolus) (4–6). In contrast, red fox (Vulpes vulpes) is believed to act as a reservoir for some Babesia microti-like species, such as the previously classified B. vulpes (7, 8), and, moderately, for Borrelia burgdorferi sensu lato (s.l.) (9). The wild boar (Sus scrofa), deer species, and mesocarnivores are all believed to contain Anaplasma phagocytophilum (10–12). Wild boars have tested positive for certain pathogens such as B. vulpes or B. capreoli although they are not considered reservoir hosts for these species (13–15). Spotted fever group (SFG) Rickettsia and B. burgdorferi s.l. are registered as reservoir species for small mammals and birds (16). Deer species are not reservoirs for or commonly infected by B. microti-like (17), B. burgdorferi s.l. (18, 19), or SFG Rickettsia (20). Similarly, tick species might be specialist vectors for selected pathogens; for example, Ixodes ricinus is considered competent for multiple pathogens, while Haemaphysalis punctata is the main vector of some SFG Rickettsia (16, 21).
In the study of wildlife populations, camera traps (CTs) have been recognized to provide high-quality data for characterizing wildlife communities (22). This can be of utmost use in tick-host interaction studies (23, 24). Nevertheless, in the European context, very few studies have linked CT-derived wildlife data to predict the presence of tick-borne pathogens. Among these, Takumi et al. (25, 26) have employed camera trap data to study the correlation between vertebrate host availability and density of tick-borne pathogens, including Borrelia spp. and A. phagocytophilum, showing a positive correlation with bank voles and wild ungulates, respectively.
The objective of this study is to assess how effectively camera trap data, specifically head counts of wildlife, can predict the presence of tick-borne pathogens in environmental ticks. This analysis is centered on species readily monitored by such tools, which are also relevant by indirectly influencing tick-borne disease dynamics.
2. Materials and methods
2.1. Sampling area and study design
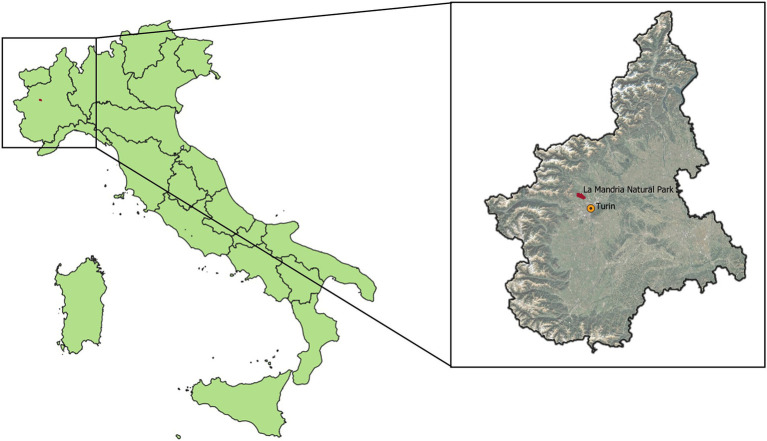
The park (45° 8′ 45″, 7° 36′ 2″, Figure 1) spanning 6,571 hectares at an average elevation of 386 m above sea level, with an elevation gradient of 269 m, is characterized by a temperate lowland climate and is enclosed by fencing (27). The park predominantly features deciduous forests and grasslands, which are managed as hay meadows. The park attracts approximately 2,000 visitors daily and maintains consistent wildlife management throughout the year. While a few horse farms and cultivated plots are situated within the park, access by other domestic animals is restricted (including pets). Data on the density of wild ungulates within the park have been recently established via camera trapping by the European Wildlife Observatory (27).
Figure 1.
Location of La Mandria Natural Park in the Piedmont region (Italy), with reference to the regional capital, Turin.
The camera trap images analyzed in this study originated from a separate field study conducted by Ferroglio et al. (23). This study utilized 14 sampling points, evenly distributed between open (hay meadows) and closed (deciduous forests) habitats. Camera traps at each sampling point were operated continuously for a year, from August 2020 to August 2021; for detailed information on the deployment of these traps, refer to Ferroglio et al. (23). Alongside the camera trapping, ticks were systematically collected every 2 weeks throughout the entire study period using dragging transects. As described by Ferroglio et al. (23), we implemented a 1 m2 cloth, dragged to cover a 10 m2 surface in front of the camera trap and a 26 m circle around it. The cloth was repeatedly checked to collect ticks, which were stored in 70% EtOH for further identification (time of storage: 1 to 2 months).
2.2. Tick-borne pathogen detection and prevalence estimation
Alongside fieldwork, ticks were identified using dichotomous keys (28–30), washed to remove any EtOH residual, which would inhibit the polymerase chain reactions, and stored at −20°C for further analysis (performed after the end of fieldwork). To optimize the effort, the genomic DNA was extracted from ticks grouped into uniform pools based on specific criteria: dragging transect, sampling point, repetition, species, developmental stage, and sex for adult ticks. The extraction was performed on the entire body of the ticks in the pool, using a blackPREP Tick DNA/RNA kit (Biosense, Italy) according to the manufacturer’s instructions. Subsequent PCR tests were conducted on these samples to detect tick-borne pathogens, namely B. divergens/capreoli, B. microti-like, Theileria capreoli, A. phagocytophilum, B. burgdorferi s.l., and SFG Rickettsia. Table 1 illustrates the references and targeted genes of the primers implemented to detect pathogens’ DNA, while specific protocols are detailed in Supplementary Table S1. Our PCR protocol did not differentiate B. capreoli from B. divergens, as it did not target the specific region containing single-nucleotide polymorphisms which is typically used to differentiate the two species (31). However, given the specific features of the study area (the absence of domestic ruminants and the surrounding fence) and the documented extensive circulation of B. capreoli (6), we considered positive samples to represent B. capreoli alone and included them in the statistical analysis. Originally designed to detect B. microti, primers from Persing et al. (32) encompass the whole B. microti-like group, including vulpes-like and Munich-like clades (8), which are of interest both from a zoonotic point of view and for the wildlife species targeted in the present study, specifically the red fox. Primers for T. capreoli were designed in the current study to be species-specific. All primers for Piroplasmid species were intended to avoid the interference of co-infections with other protozoan microorganisms. Similarly, when targeting Anaplasmataceae, we selected specific primers for A. phagocytophilum, the species with the highest public health relevance, to avoid the interference of symbiotic bacteria such as Candidatus Midichloria spp. On the other hand, primers targeting B. burgdorferi s.l. and SFG Rickettsia encompass the whole group of microorganisms (e.g., B. afzelii and B. lusitaniae in the first case, and R. monacensis and R. conorii in the second), which are all relevant for public health as zoonotic pathogens and are not commonly hosted by the wild species targeted in the study (16). All PCR tests included a confirmed positive control for the target pathogen and a no-template negative control. All standard measures were taken to minimize the risk of contamination. Amplicons were analyzed by agarose gel electrophoresis (2%) and visualized by staining with GelRed Nucleic Acid Gel Stain (VWR International Milano, Italy).
Table 1.
Primers implemented in the study, with gene-targeted, primer names and publication reference.
| Pathogen | Primer names (5′–3′) | References |
|---|---|---|
| B. divergens | Gene: 18s (forward and reverse from the paper) | (31) |
| B. microti/microti-like | Gene: 18s (Bab1 and Bab4) | (32) |
| A. phagocytophilum | Gene: groEL [EphplgroEL(569)F and EphplgroEL(1193)R] | (65) |
| B. burgdorferi s.l. | Gene: spacer region between 5S and 23S rRNA genes (23SN1 and 23SC1) | (66) |
| SFG Rickettsia | Gene: surface protein rOmpA (190-70 and 90-701) | (67) |
| T. capreoli | Gene: 18s (TcapreoliF and TcapreoliR) | This study. Reference sequence: AY726011.1 |
For each pool and pathogen, we recorded the binary outcome of the PCR (positive-negative) and estimated the pathogen prevalence within each pool using the package PoolTestR (33) for RStudio (34). This package provides Bayesian estimates of prevalence along with 95% credibility intervals (Cr.I.), based on the number of ticks in each pool and the test outcome (33). Differences in prevalence among tick species and among developmental stages were explored through the chi-squared test or Fisher’s test, depending on the distribution matrix (35).
2.3. Tick-borne pathogen models
For each repetition and sampling point, we computed the total head count of individuals passing by the camera trap. Each instance of an animal exiting and re-entering the camera’s field view was treated as a new individual, as individual recognition was not possible. All age classes were included in the analysis. We extracted data for red deer, roe deer, fallow deer (Dama dama), wild boar, red fox, European badger (Meles meles), pine marten (Martes martes), and beech marten (Martes foina), as CT deployment was not sensitive for distinguishing animal species of smaller size, such as rodents and birds, or for detecting their presence in the whole field of view of the camera trap. We grouped data for mesocarnivores and wild ruminants.
Our database considered, as the response variable, the binary outcome of the PCR test (positive-negative). Explanatory variables involved were as follows:
- Sampling season and point.
- Wildlife head counts (as number of passages) for wild boar, mesocarnivores, and wild ruminants.
- The number of ticks per pool, as the more ticks are tested together, the higher the probability of getting a positive PCR test, i.e. detect an infection.
Vegetation and environmental parameters were not included in the analysis for several reasons. First, the sampling points were uniformly distributed across vegetation types, ensuring homogeneity in coverage. Second, the habitat within the park exhibited overall uniformity due to its limited spatial extent and minimal elevation gradient. Finally, the influence of vegetation on the presence of tick-borne pathogens was likely indirect, as vegetation primarily affects reservoir host distribution or vector abundance rather than directly influencing the pathogen presence.
We implemented a General Linear Mixed Model with a binomial family using the lme4 package in R studio (36). We considered the sampling point as the random variable, and the variance was weighted on the season in which ticks were collected, to account for seasonality in tick and host population.
To test how well the model performed and, consequently, how useful camera trap data could be to predict the presence of tick-borne pathogens, we evaluated three parameters: (i) conditional and marginal R2, to test how much the model was satisfactory in explaining the variance; (ii) accuracy; and (iii) AUC (area under curve, for which threshold interpretation was presented by studies such as Çorbacıoğlu et al. (37)), tested by splitting the database into train (70% of data) and test (30% of data) datasets.
We modeled (i) all tick pools, regardless of species and developmental stages (global model), and (ii) I. ricinus nymph pools, as particularly relevant in terms of zoonotic risk (I. ricinus nymph model) (38, 39). We ultimately created models only for the four most prevalent pathogens (B. capreoli, B. microti-like, B. burgdorferi s.l., and SFG Rickettsia).
3. Results
3.1. Tick-borne pathogen detection and prevalence estimation
We analyzed a total of 2,537 ticks divided into 413 pools, including 282 pools of I. ricinus, of which 112 (424 individuals) were identified in the nymphal stage (23). The two most prevalent pathogens were B. microti-like (Bayesian prevalence 13.12% with Cr.I. 11.05–15.28%) and SFG Rickettsia (10.79%, Cr.I. 9.14–12.64%), followed by B. capreoli (5.47%, Cr.I. 4.2–6.78%) and B. burgdorferi s.l. (2.57%, Cr.I. 1.83–3.45%). Finally, the prevalence was recorded below 1% for T. capreoli and A. phagocytophilum, respectively (0.23%, Cr.I. 0.02–0.71, and 0.34%, Cr.I. 0.14–0.62%).
Ixodes ricinus and H. punctata recorded at least one positive pool for each pathogen (Table 2). B. capreoli, B. microti-like, and SFG Rickettsia were found in samples from every tick species tested, including D. reticulatus, H. concinna, I. hexagonus, and the R. sanguineus complex [R. sanguineus sensu stricto, R. pusillus, and R. turanicus according to the keys implemented in this study (28, 30)]. Additionally, T. capreoli was detected in H. concinna, H. punctata, and I. hexagonus, while B. burgdorferi s.l. was identified in H. concinna and H. punctata. According to the chi-squared test and Fisher’s test, the prevalence rates of only B. burgdorferi s.l. were significantly different (p < 0.05) among developmental stages and those of B. microti-like alone did significantly vary (p < 0.05) among the tick species.
Table 2.
Bayesian estimated the prevalence of pathogens for each tick species and their developmental stages, with 95% Cr.I. within parentheses.
| Tick species | B. capreoli | B. microti-like | B. burgdorferi s.l. | SFG Rickettsia | A. phagocytophilum | T. capreoli |
|---|---|---|---|---|---|---|
| I. ricinus [393] | ||||||
| Larva [154] | 3.5% (2.3–5%) [29] |
6.5% (4.9–8.2%) [54] |
0.4% (0.1–0.8%) [4] |
7.5% (5.8–9.6%) [60] |
0.2% (0–0.6%) [2] |
0.5% (0.2–1%) [5] |
| Nymph [184] | 7.5% (5–10.7%) [26] |
30.9% (24.3–37.8%) [68] |
8.2% (5.5–11.5%) [25] |
31.2% (24–39.1%) [60] |
0.7% (0.1–1.7%) [2] |
0.8% (0.2–2%) [3] |
| Adult [55] | 23.1% (12.1–36.7%) [9] |
53.5% (37.4–69.5%) [20] |
22.9% (11–37.1%) [9] |
38.4% (24.1–52.7%) [15] |
6% (1.1–14%) [2] |
[0] |
| I. hexagonus [4] | ||||||
| Nymph [4] | 24.4% (2.5–60.1%) [1] |
42.1% (8.7–77.2%) [2] |
[0] | 24.9% (3.1–62.8%) [1] |
[0] | [0] |
| H. concinna [27] | ||||||
| Larva [9] | 1.1% (0.2–3%) [2] |
1.1% (0.2–2.9%) [2] |
0.7% (0–2.1%) [1] |
2.1% (0.7–4.4%) [4] |
[0] | [0] |
| Nymph [15] | 12.8% (4.3–25.8%) [4] |
4.2% (0.3–13%) [1] |
4.3% (0.4–13.1%) [1] |
19.3% (8.2–33.8%) [6] |
[0] | 10.2% (2.4–22.2%) [3] |
| Adult [3] | 49.8% (5.6–94.3%) [1] |
49.7% (5–95%) [1] |
50.4% (7.2–93.8%) [1] |
[0] | [0] | [0] |
| H. punctata [43] | ||||||
| Larva [33] | 1.6% (0.7–2.7%) [9] |
0.5% (0.1–1.2%) [3] |
0.2% (0–1%) [4] |
3.9% (2.2–6.2%) [16] |
[0] | 0.2% (0–0.7%) [1] |
| Nymph [10] | 16.5% (3–36.9%) [2] |
16.6% (2.7–38.7%) [2] |
9.6% (0.8–29.1%) [1] |
29.5% (10.4–53.7%) [4] |
9.8% (0.7–27.8%) [1] |
[0] |
| R. sanguineus complex [11] | ||||||
| Larva [8] | 9.7% (1.9–23.6%) [2] |
14.3% (3.6–29.8%) [3] |
[0] | 14.5% (3.8–31.8%) [3] |
[0] | [0] |
| Nymph [3] | 50.3% (6.2–94.1%) [1] |
49.6% (5–93.8%) [1] |
[0] | 50% (5.4–95.2%) [1] |
[0] | [0] |
| D. reticulatus [2] | ||||||
| Larva [1] | 35.1% (2.6–80.6%) [1] |
[0] | [0] | [0] | [0] | [0] |
| Adult [1] | [0] | [0] | [0] | 36.5% (3.5–82%) [1] |
[0] | [0] |
The number of pools per group (left column) and the number of positive pools (other columns) are given in squared brackets.
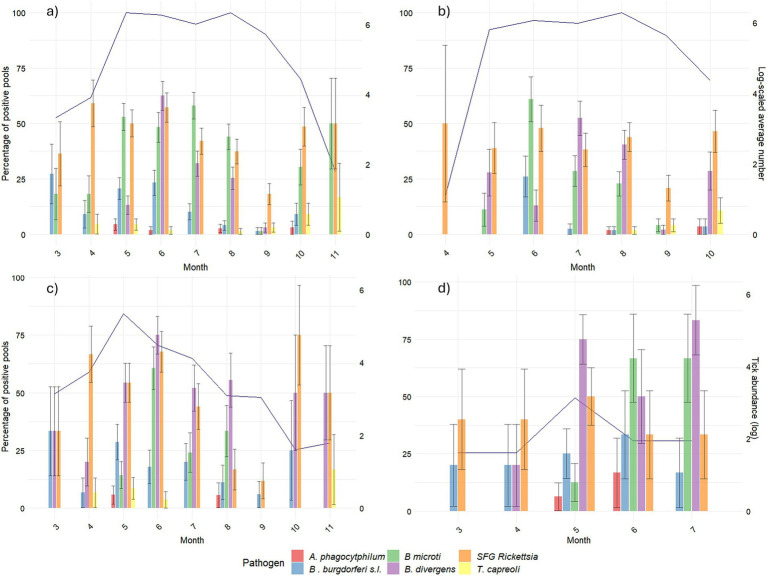
While the percentages of the positive pools of SFG Rickettsia and A. phagocytophilum were persistent throughout the study period, we observed a peak during the summer months for B. burgdorferi s.l. and the two Babesia species (Figure 2). However, some differences may be spotted while decomposing the trend according to developmental stages. For B. burgdorferi, positive larvae peaked in June, while among the other stages, positive pools were more uniformly distributed in warmer months (May–September). For B. microti-like, they peaked in all stages in June and July. Finally, for B. divergens, the peak in nymphs and adults occurred earlier in the year (from May to July) than in larvae (July). Theileria capreoli, which was almost only recorded in larvae, showed higher positivity later in the year.
Figure 2.
Pathogen positivity across the months per developmental stage. For each of the targeted pathogens, the percentage of positive pools over the total number of collected pools in each month is shown on the primary y-axis. Winter months (December, January, and February) were excluded due to a lack of tick activity. The blue line (secondary y-axis) represents ticks’ abundance (log-scaled average of number of ticks collected in each sampling point). (A) Represents the total number of ticks. (B) Represents the number of larvae. (C) Represents the number of nymphs. (D) Represents the number of adults.
3.2. Tick-borne pathogen models
Based on the best performance of the models, the number of ticks in the pool and the wild boar and mesocarnivore head counts were included in all models (Table 3), while deer species head counts were included in the models for B. capreoli, SFG Rickettsia and in the global model of B. burgdorferi s.l.
Table 3.
Variables included in each model.
| Model | Pathogen | Wild boar | Deer species | Mesocarnivores | Tick pool |
|---|---|---|---|---|---|
| Global model | B. capreoli | ✓ | ✓ | ✓ | ✓ |
| B. microti-like | ✓ | ✓ | ✓ | ||
| B. burgdorferi s.l. | ✓ | ✓ | ✓ | ✓ | |
| SFG Rickettsia | ✓ | ✓ | ✓ | ✓ | |
| I. ricinus nymph model | B. capreoli | ✓ | ✓ | ✓ | ✓ |
| B. microti-like | ✓ | ✓ | ✓ | ||
| B. burgdorferi s.l. | ✓ | ✓ | ✓ | ||
| SFG Rickettsia | ✓ | ✓ | ✓ | ✓ |
Considered explanatory variables are wild boar, deer species, mesocarnivores head counts, and the number of ticks in the pool. Both the global model and the model for I. ricinus nymphs are described.
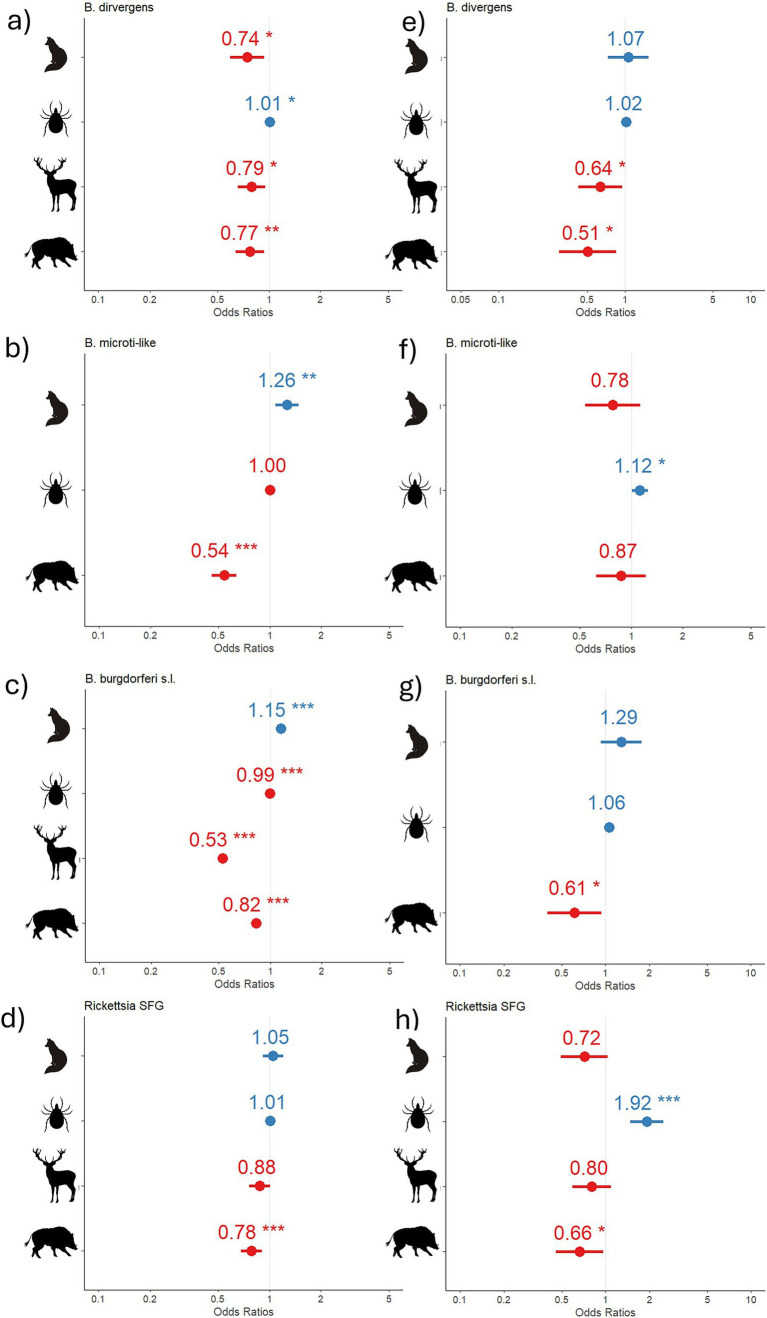
Overall, the coefficients indicated a similar effect (either dilutive or additive) of the same host species across both the global model and the I. ricinus nymph model, despite statistical significance (Figure 3). Wild ruminants and wild boar exhibited a dilutive effect (negative coefficient) on the prevalence of all assessed pathogens. In contrast, the influence of mesocarnivores varied between the global model and the I. ricinus nymph model. Overall, mesocarnivores demonstrated a statistically significant dilutive effect for B. divergens, while showing a statistically significant additive effect for B. burgdorferi s.l. and B. microti-like. Furthermore, the total number of ticks in the pool had a positive association with the presence of pathogens within the pool, except in the global model of B. burgdorferi s.l., where a detractive impact was detected.
Figure 3.
Model result coefficients. Coefficients (blue, positive; red, negative) of the models for each pathogen in the global model (A–D) and in the I. ricinus nymph model (E–H). Asterisks indicate statistical significance. Silhouettes on the x-axis represent mesocarnivores head count (fox), total number of ticks in the pool (tick), wild ruminants head count (red deer), and wild boar head count (wild boar).
The model performance, evaluated using R2, accuracy, and AUC, was the highest for the models focused on I. ricinus nymphs, apart from those predicting B. burgdorferi s.l. Notably, SFG Rickettsia exhibited the lowest accuracy and AUC, but the highest R2 among the models analyzed. The model for B. capreoli demonstrated the highest accuracy, while the B. burgdorferi model achieved the highest AUC, exceeding 0.7, marking it as the only model to surpass this threshold of good predictivity (Table 4).
Table 4.
R2 values, accuracy, and AUC for each model.
| Global model | I. ricinus nymph model | |
|---|---|---|
| B. divergens | ||
| Marginal R2/conditional R2 | 0.070/0.204 | 0.188/0.227 |
| Accuracy | 0.74 | 0.9 |
| AUC | 0.65 | 0.65 |
| B. microti-like | ||
| Marginal R2/conditional R2 | 0.098/0.171 | 0.137/0.485 |
| Accuracy | 0.6 | 0.69 |
| AUC | 0.62 | 0.67 |
| B. burgdorferi s.l. | ||
| Marginal R2/conditional R2 | 0.098/0.388 | 0.118/0.429 |
| Accuracy | 0.91 | 0.86 |
| AUC | 0.66 | 0.74 |
| SFG Rickettsia | ||
| Marginal R2/conditional R2 | 0.031/0.049 | 0.862/0.876 |
| Accuracy | 0.56 | 0.57 |
| AUC | 0.59 | 0.63 |
4. Discussion
Our models showed that the wildlife presence affects the prevalence of tick-borne pathogens, and consequently, camera trap data can be useful for predicting their risk in the environment.
The prevalence of Rickettsia, A. phagocytophilum, Babesia spp., and B. burgdorferi s.l. in this study was consistent with that reported in studies on I. ricinus ticks collected from dogs (40), humans (41), and wildlife (6, 42) in northwestern Italy and in previous studies in the same study area (43). The prevalence detected for T. capreoli was consistent with that reported in wild deer species in Spain (44, 45). Temporal fluctuations in the pathogen presence did follow the seasonal peaks of the developmental stages in which they were more often detected (23), which is also in accordance with the pool size variable in the model showing an additive effect on the presence of the pathogens. Pathogens capable of transovarial transmission, such as B. divergens and SFG Rickettsia (46, 47), exhibit a more evenly distributed positivity rate throughout the months of tick activity. In contrast, B. microti-like, which lacks transovarial transmission (48), shows a peak in positivity rates concentrated in June. Despite its transovarial transmission, B. burgdorferi s.l., in accordance with results obtained by Szekeres et al. (49) in Germany, was predominantly found in nymphal pools and its temporal fluctuations varied accordingly with this developmental stage, with a peak in June. This concurrence was also highlighted by Hartemink et al. in the Netherlands (50).
A limitation of the minimum infection rate (MIR) estimation is that it is derived from the number of ticks within a pooled sample (MIR = 1/n° ticks). Consequently, as the number of ticks tested together increases, the denominator rises, leading to a lower calculated prevalence. Although the Bayesian approach partially mitigates this issue, it does not eliminate the bias introduced by the pool size: for instance, a study on tick-borne zoonoses in the same study area testing individual ticks detected higher prevalence for SFG Rickettsia and B. burgdorferi s.l. (43). This is particularly relevant for larval pools, which may encompass a substantial number of ticks, potentially resulting in an underestimation of the true prevalence. This approach is however more precise than other traditionally implemented methods and still allows comparison among tick species or different locations, especially in situations where single tick testing would be poorly effort-effective (51). In addition to these considerations, it is noteworthy that an increased number of ticks positively affects the probability of a positive outcome of a biomolecular test, remarking how tick hotspots may represent a major risk for tick-borne disease transmission. The sole exception was the effect observed for B. burgdorferi s.l. in the global model, where this effect was highlighted as detractive. The scarcity of positive detection during periods of higher larval abundance may have influenced this outcome.
In some cases, pathogens found in tick species were not recognized as competent vectors: for instance, Babesia spp. were found in all species, although only I. ricinus has been demonstrated to be capable of transmission back to the vertebrate host (16, 21). Even though little is known about the actual competence of less common tick species, it is important to clarify that the presence of pathogen DNA in the vector is not proof of the transmission capability, and it just mirrors a pathogen’s circulation in the study area. This can explain the absence of a statistically significant prevalence difference among tick species for most pathogens. According to the literature, B. burgdorferi was detected in H. punctata (52), B. microti in D. reticulatus (53), and B. bigemina in H. punctata (54), thus confirming our findings that pathogen infection and transmission do not necessarily coincide.
While the wild ungulate species targeted by our camera trap data collection are likely not reservoirs for the four modeled pathogens (13, 15, 17, 18, 20), they serve as maintenance hosts for the tick population (23, 55, 56). In light of informing management actions aimed at reducing the number of ticks, we chose them as relevant variables to model. Despite B. capreoli having been reported in red deer (6), there are several cases where only roe deer was found positive for this pathogen (57–59). Additionally, B. capreoli was not reported in red deer in areas where roe deer was absent (4), suggesting a limited role of this species as a maintenance host. This probably explains our finding of a dilutive effect of deer species altogether, as roe deer density in the park was 1.90 ± 0.97 ind/km2, much lower than that of other species (27), and the ratio between roe and red deer head counts in our study was 171:1,212 and that between roe and fallow deer was 171:854. In Europe, B. microti-like species include B. vulpes and the B. microti Munich strain (8). Red fox, the mesocarnivore species mainly recorded in our study, may harbor B. vulpes, which is a possible reason for the additive effect of its presence on B. microti-like in environmental ticks. Additionally, the presence of red fox may be linked to its micromammal prey, which can be an underlying factor for the positive coefficient on B. burgdorferi s.l. and on B. microti-like. Indeed, both pathogens have micromammals, such as ground-dwelling rodents and shrews, as major reservoirs (8, 16). Including small mammals in such types of studies will be a fundamental step forward in understanding the ecology of these tick-borne pathogens, as performed by Takumi et al. (25).
In this study, and for the reasons outlined, we did not incorporate environmental variables into the analysis. While temperature and humidity significantly influence tick activity and abundance (60), and consequently the prevalence of tick-borne pathogens by increasing tick population density and contact rates between hosts, habitat characteristics primarily affect host presence, density, and temporal occupancy (23, 61, 62). These, in turn, indirectly influence tick abundance and pathogen presence when hosts serve as competent reservoirs (23, 24, 63, 64). Although parameters such as vegetation types or habitat fragmentation indices could be included in the analysis as proxies for the presence of other host species, they would lack the precision of data obtained directly from camera traps. Our results highlighted how camera trap data represent a valid tool to predict the presence of tick-borne pathogens and, consequently, draw insights about the zoonotic risk and further control strategies. The model performance in predicting the presence of pathogens is improved by decomposing the response variable, indicating that pathogen-host association may vary depending on the developmental stage and species of the vector. More accurate predictions and new insights on the pathogen-host interaction would benefit from ad hoc models targeting the single species and developmental stages. This approach was not possible in the current study due to limited numbers of other tick species.
5. Conclusion
Our study verified the presence of tick-borne pathogens in a fenced natural park, a site frequented by many visitors engaging in various outdoor activities. While rodents are known to be primary reservoirs and maintenance hosts for several of these pathogens, our research concentrated on species that indirectly influence pathogen transmission. These species (wild ungulates in particular and red fox to a lesser degree) are more readily observed and managed, particularly through camera trapping and hunting. Our findings demonstrate a clear connection between pathogen prevalence and these species, underscoring the value of camera trap data in providing detailed insights into wildlife populations for studies in disease ecology.
Acknowledgments
The authors acknowledge the park wardens of La Mandria Natural Park for granting access to the park and support in data collection. Also, the authors acknowledge the contribution of Dr. Mattia Fracchia and Dr. Federica De Cicco in fieldwork, tick identification, and testing.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the MUR PNRR Extended Partnership Initiative on Emerging Infectious Diseases (INF-ACT), Project No. PE00000007, and by Enetwild Consortium funded by EFSA, OC/EFSA/BIOHAW/2022/01.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
RV: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SZ: Data curation, Investigation, Methodology, Writing – review & editing. FO: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AT: Investigation, Writing – review & editing. AV: Investigation, Writing – review & editing. EF: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1536260/full#supplementary-material
Protocols and primers implemented in the study.
References
- 1.European Centre for Disease Prevention and Control . Communicable disease threats report, 2–8 August 2020, week 32. Stockholm: European Centre for Disease Prevention and Control; (2020). [Google Scholar]
- 2.Eremeeva ME, Dasch GA. Challenges posed by tick-borne rickettsiae: eco-epidemiology and public health implications. Front Public Health. (2015) 3:55. doi: 10.3389/fpubh.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroffolini G, Segala FV, Lupia T, Faraoni S, Rossi L, Tomassone L, et al. Serology for Borrelia spp. in Northwest Italy: a climate-matched 10-year trend. Life. (2021) 11:1310. doi: 10.3390/life11121310, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zintl A, Finnerty EJ, Murphy TM, de Waal T, Gray JS. Babesias of red deer (Cervus elaphus) in Ireland. Vet Res. (2011) 42:7. doi: 10.1186/1297-9716-42-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hrazdilová K, Rybářová M, Široký P, Votýpka J, Zintl A, Burgess H, et al. Diversity of Babesia spp. in cervid ungulates based on the 18S rDNA and cytochrome c oxidase subunit I phylogenies. Infect Genet Evol. (2020) 77:104060. doi: 10.1016/j.meegid.2019.104060, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Zanet S, Trisciuoglio A, Bottero E, De Mera IGF, Gortazar C, Carpignano MG, et al. Piroplasmosis in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasit Vectors. (2014) 7:70. doi: 10.1186/1756-3305-7-70, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso L, Cortes HCE, Reis A, Rodrigues P, Simões M, Lopes AP, et al. Prevalence of Babesia microti-like infection in red foxes (Vulpes vulpes) from Portugal. Vet Parasitol. (2013) 196:90–5. doi: 10.1016/j.vetpar.2012.12.060, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Goethert HK. What Babesia microti is now. Pathogens. (2021) 10:1168. doi: 10.3390/pathogens10091168, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidrich J, Schönberg A, Steuber S, Nöckler K, Schulze P, Voigt W-P, et al. Investigation of skin samples from red foxes (Vulpes vulpes) in eastern Brandenburg (Germany) for the detection of Borrelia burgdorferi s.l. Zentralbl Bakteriol. (1999) 289:666–72. doi: 10.1016/S0934-8840(99)80026-7, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Cafiso A, Bazzocchi C, Cavagna M, Di Lorenzo E, Serra V, Rossi R, et al. Molecular survey of Babesia spp. and Anaplasma phagocytophilum in roe deer from a Wildlife Rescue Center in Italy. Animals. (2021) 11:3335. doi: 10.3390/ani11113335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebani VV, Verin R, Fratini F, Poli A, Cerri D. Molecular survey of Anaplasma phagocytophilum and Ehrlichia canis in red foxes (Vulpes vulpes) from central Italy. J Wildl Dis. (2011) 47:699–703. doi: 10.7589/0090-3558-47.3.699, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Nahayo A, Bardiau M, Volpe R, Pirson J, Paternostre J, Fett T, et al. Molecular evidence of Anaplasma phagocytophilum in wild boar (Sus scrofa) in Belgium. BMC Vet Res. (2014) 10:80. doi: 10.1186/1746-6148-10-80, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo-Contreras R, Magen L, Birtles R, Varela-Castro L, Hall JL, Conejero C, et al. Ticks on wild boar in the metropolitan area of Barcelona (Spain) are infected with spotted fever group rickettsiae. Transbound Emerg Dis. (2022) 69:E82–95. doi: 10.1111/tbed.14268, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Kurtenbach K, Sewell H-S, Ogden NH, Randolph SE, Nuttall PA. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. (1998) 66:1248–51. doi: 10.1128/IAI.66.3.1248-1251.1998, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sgroi G, D’Alessio N, Auriemma C, Salant H, Gallo A, Riccardi MG, et al. First molecular detection of Babesia vulpes and Babesia capreoli in wild boars from southern Italy. Front Vet Sci. (2023) 10:1201476. doi: 10.3389/fvets.2023.1201476, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salman M, Tarrés-Call J. Ticks and tick-borne diseases: geographical distribution and control strategies in the Euro-Asia region. Wallingford: CABI; (2013). [Google Scholar]
- 17.Piesman J, Spielman A, Etkind P, Ruebush TK, Juranek DD. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J Med Entomol. (1979) 15:537–40. doi: 10.1093/jmedent/15.5-6.537, PMID: [DOI] [PubMed] [Google Scholar]
- 18.De Keukeleire M, Vanwambeke SO, Cochez C, Heyman P, Fretin D, Deneys V, et al. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Francisella tularensis infections in Belgium: results of three population-based samples. Vector Borne Zoonotic Dis. (2017) 17:108–15. doi: 10.1089/vbz.2016.1954, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Jaenson TGT, Tälleklint L. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J Med Entomol. (1992) 29:813–7. doi: 10.1093/jmedent/29.5.813, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Skarphedinsson S, Jensen PM, Kristiansen K. Survey of tickborne infections in Denmark. Emerg Infect Dis. (2005) 11:1055–61. doi: 10.3201/eid1107.041265, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajer A, Dwużnik-Szarek D. The specificity of Babesia-tick vector interactions: recent advances and pitfalls in molecular and field studies. Parasit Vectors. (2021) 14:507. doi: 10.1186/s13071-021-05019-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grignolio S, Apollonio M, Brivio F, Vicente J, Acevedo P, Palencia P, et al. Guidance on estimation of abundance and density data of wild ruminant population: methods, challenges, possibilities. EFSA Support Publ. (2020) 17:1876E. doi: 10.2903/sp.efsa.2020.EN-1876, PMID: 39691501 [DOI] [Google Scholar]
- 23.Ferroglio E, Vada R, Occhibove F, Fracchia M, De Cicco F, Palencia P, et al. An integrated approach to an emerging problem: implementing a whole year of camera trap survey in evaluating the impact of wildlife on tick abundance. Transbound Emerg Dis. (2024) 2024:4064855. doi: 10.1155/2024/4064855 [DOI] [Google Scholar]
- 24.Vada R, Zanet S, Occhibove F, Fantini E, Palencia P, Ferroglio E. Relating wildlife camera trap data to tick abundance: testing the relationship in different habitats. Animals. (2024) 14:2749. doi: 10.3390/ani14182749, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takumi K, Sprong H, Hofmeester TR. Impact of vertebrate communities on Ixodes ricinus-borne disease risk in forest areas. Parasit Vectors. (2019) 12:434. doi: 10.1186/s13071-019-3700-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takumi K, Hofmeester TR, Sprong H. Red and fallow deer determine the density of Ixodes ricinus nymphs containing Anaplasma phagocytophilum. Parasit Vectors. (2021) 14:59. doi: 10.1186/s13071-020-04567-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrasio T, Pelayo Acevedo P, Apollonio M, Arnon A, Barroqueiro C, Belova O, et al. Wild ungulate density data generated by camera trapping in 37 European areas: first output of the European Observatory of Wildlife (EOW). EFSA Support Publ. (2023) 20:7892E. doi: 10.2903/sp.efsa.2023.EN-7892 [DOI] [Google Scholar]
- 28.Manilla G, Iori A. Chiave illustrata delle zecche d’Italia. II: Stadi ninfali delle specie della sottofamiglia Ixodinate (Acari, Ixodoidea, Ixodidae). Parassitologia. (1993) 35:37. [PubMed] [Google Scholar]
- 29.Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa: a guide to species identification. Cham: Springer; (2018). [Google Scholar]
- 30.Manilla G, Iori A. Chiave illustrata delle zecche d’Italia. I: stadi larvali delle specie della sottofamiglia Ixodinae (Acari, Ixodoidea, Ixodidae). Parassitologia. (1992) 34:83–95. PMID: [PubMed] [Google Scholar]
- 31.Hilpertshauser H, Deplazes P, Schnyder M, Gern L, Mathis A. Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Appl Environ Microbiol. (2006) 72:6503–7. doi: 10.1128/AEM.00823-06, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, et al. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. (1992) 30:2097–103. doi: 10.1128/jcm.30.8.2097-2103.1992, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLure A, O’Neill B, Mayfield H, Lau C, McPherson B. PoolTestR: an R package for estimating prevalence and regression modelling for molecular xenomonitoring and other applications with pooled samples. Environ Model Softw. (2021) 145:105158. doi: 10.1016/j.envsoft.2021.105158 [DOI] [Google Scholar]
- 34.R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; (2023). [Google Scholar]
- 35.ML MH. The chi-square test of independence. Biochem Med. (2013) 23:143–9. doi: 10.11613/BM.2013.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, et al. Package ‘lme4’. Convergence. (2015) 12:2. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 37.Çorbacıoğlu ŞK, Aksel G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: a guide to interpreting the area under the curve value. Turk J Emerg Med. (2023) 23:195–8. doi: 10.4103/tjem.tjem_182_23, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battisti E, Zanet S, Boraso F, Minniti D, Giacometti M, Duscher GG, et al. Survey on tick-borne pathogens in ticks removed from humans in northwestern Italy. Vet Parasitol Reg Stud Rep. (2019) 18:100352. doi: 10.1016/j.vprsr.2019.100352, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Wilhelmsson P, Lindblom P, Fryland L, Nyman D, Jaenson TGT, Forsberg P, et al. Ixodes ricinus ticks removed from humans in Northern Europe: seasonal pattern of infestation, attachment sites and duration of feeding. Parasit Vectors. (2013) 6:362. doi: 10.1186/1756-3305-6-362, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanet S, Battisti E, Pepe P, Ciuca L, Colombo L, Trisciuoglio A, et al. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: a country-wide molecular survey. BMC Vet Res. (2020) 16:46. doi: 10.1186/s12917-020-2263-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Audino T, Pautasso A, Bellavia V, Carta V, Ferrari A, Verna F, et al. Ticks infesting humans in North-Western Italy and associated pathogens: a cross-sectional study in a three-year period (2017–2019) in North-Western Italy. Parasit Vectors. (2020) 14:136. doi: 10.1186/s13071-021-04603-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battisti E, Zanet S, Khalili S, Trisciuoglio A, Hertel B, Ferroglio E. Molecular survey on vector-borne pathogens in alpine wild carnivorans. Front Vet Sci. (2020) 7:1. doi: 10.3389/fvets.2020.00001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellato A, Pintore MD, Catelan D, Pautasso A, Torina A, Rizzo F, et al. Risk of tick-borne zoonoses in urban green areas: a case study from Turin, northwestern Italy. Urban For Urban Green. (2021) 64:127297. doi: 10.1016/j.ufug.2021.127297 [DOI] [Google Scholar]
- 44.Díaz-Cao JM, Adaszek Ł, Dzięgiel B, Paniagua J, Caballero-Gómez J, Winiarczyk S, et al. Prevalence of selected tick-borne pathogens in wild ungulates and ticks in southern Spain. Transbound Emerg Dis. (2022) 69:1084–94. doi: 10.1111/tbed.14065, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Hornok S, Sugár L, Horváth G, Kovács T, Micsutka A, Gönczi E, et al. Evidence for host specificity of Theileria capreoli genotypes in cervids. Parasit Vectors. (2017) 10:473. doi: 10.1186/s13071-017-2403-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hauck D, Jordan D, Springer A, Schunack B, Pachnicke S, Fingerle V, et al. Transovarial transmission of Borrelia spp., Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus under field conditions extrapolated from DNA detection in questing larvae. Parasit Vectors. (2020) 13:176. doi: 10.1186/s13071-020-04049-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnet S, Jouglin M, Malandrin L, Becker C, Agoulon A, L’hostis M, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. (2007) 134:197–207. doi: 10.1017/S0031182006001545 [DOI] [PubMed] [Google Scholar]
- 48.Gray J, von Stedingk LV, Gürtelschmid M, Granström M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J Clin Microbiol. (2002) 40:1259–63. doi: 10.1128/JCM.40.4.1259-1263.2002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szekeres S, Lügner J, Fingerle V, Margos G, Földvári G. Prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in questing ticks from a recreational coniferous forest of East Saxony, Germany. Ticks Tick Borne Dis. (2017) 8:922–7. doi: 10.1016/j.ttbdis.2017.08.002, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Hartemink N, van Vliet AJH, Gort G, Gassner F, Jacobs F, Fonville M, et al. Seasonal patterns and spatial variation of Borrelia burgdorferi (sensu lato) infections in Ixodes ricinus in the Netherlands. Parasit Vectors. (2021) 14:121. doi: 10.1186/s13071-021-04607-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fracasso G, Grillini M, Grassi L, Gradoni F, da Rold G, Bertola M. Effective methods of estimation of pathogen prevalence in pooled ticks. Pathogens. (2023) 12:557. doi: 10.3390/pathogens12040557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tälleklint L. Lyme borreliosis spirochetes in Ixodes ricinus and Haemaphysalis punctata ticks (Acari: Ixodidae) on three islands in the Baltic Sea. Exp Appl Acarol. (1996) 20:467–76. doi: 10.1007/BF00053310 [DOI] [Google Scholar]
- 53.Wójcik-Fatla A, Bartosik K, Buczek A, Dutkiewicz J. Babesia microti in adult Dermacentor reticulatus ticks from eastern Poland. Vector Borne Zoonotic Dis. (2012) 12:841–3. doi: 10.1089/vbz.2011.0904, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Yin H, Lu W, Luo J, Zhang Q, Lu W, Dou H. Experiments on the transmission of Babesia major and Babesia bigemina by Haemaphysalis punctata. Vet Parasitol. (1996) 67:89–98. doi: 10.1016/S0304-4017(96)01022-9, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Hofmeester TR, Rowcliffe JM, Jansen PA. Quantifying the availability of vertebrate hosts to ticks: a camera-trapping approach. Front Vet Sci. (2017) 4:115. doi: 10.3389/fvets.2017.00115, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofmeester TR, Sprong H, Jansen PA, Prins HHT, van Wieren SE. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasit Vectors. (2017) 10:433. doi: 10.1186/s13071-017-2370-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kauffmann M, Rehbein S, Hamel D, Lutz W, Heddergott M, Pfister K, et al. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol Cell Probes. (2017) 31:46–54. doi: 10.1016/j.mcp.2016.08.008, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Silaghi C, Fröhlich J, Reindl H, Hamel D, Rehbein S. Anaplasma phagocytophilum and Babesia species of sympatric roe deer (Capreolus capreolus), fallow deer (Dama dama), sika deer (Cervus nippon) and red deer (Cervus elaphus) in Germany. Pathogens. (2020) 9:968. doi: 10.3390/pathogens9110968, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tampieri MP, Galuppi R, Bonoli C, Cancrini G, Moretti A, Pietrobelli M. Wild ungulates as Babesia hosts in northern and central Italy. Vector Borne Zoonotic Dis. (2008) 8:667–74. doi: 10.1089/vbz.2008.0001, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Zajac Z, Kulisz J, Bartosik K, Wozniak A, Dzierzak M, Khan A, et al. Environmental determinants of the occurrence and activity of Ixodes ricinus ticks and the prevalence of tick-borne diseases in eastern Poland. Sci Rep. (2021) 11:15472. doi: 10.1038/s41598-021-95079-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cargnelutti B, Reby D, Desneux L, Angibault J-M, Joachim J, Hewison AJ. Space use by roe deer in a fragmented landscape: some preliminary results. Rev Ecol. (2002) 57:29–37. doi: 10.3406/revec.2002.2379 [DOI] [Google Scholar]
- 62.Lovari S, Serrao G, Mori E. Woodland features determining home range size of roe deer. Behav Process. (2017) 140:115–20. doi: 10.1016/j.beproc.2017.04.012, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Diuk-Wasser MA, VanAcker MC, Fernandez MP. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J Med Entomol. (2021) 58:1546–64. doi: 10.1093/jme/tjaa209, PMID: [DOI] [PubMed] [Google Scholar]
- 64.Ehrmann S, Ruyts SC, Scherer-Lorenzen M, Bauhus J, Brunet J, Cousins SAO, et al. Habitat properties are key drivers of Borrelia burgdorferi (sl) prevalence in Ixodes ricinus populations of deciduous forest fragments. Parasit Vectors. (2018) 11:23. doi: 10.1186/s13071-017-2590-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alberti A, Addis MF, Sparagano O, Zobba R, Chessa B, Cubeddu T, et al. Anaplasma phagocytophilum, Sardinia, Italy. Emerg Infect Dis. (2005) 11:1322–4. doi: 10.3201/eid1108.050085, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rijpkema SG, Molkenboer MJ, Schouls LM, Jongejan F, Schellekens JF. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. (1995) 33:3091–5. doi: 10.1128/jcm.33.12.3091-3095.1995, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roux V, Fournier P-E, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. (1996) 34:2058–65. doi: 10.1128/jcm.34.9.2058-2065.1996, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocols and primers implemented in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.