Abstract
Objective
The aim of this study was to determine the impact of margin width and boost radiotherapy on the local recurrence risk of pure ductal carcinoma in situ (DCIS).
Methods and analysis
This is a prospectively registered systematic review and meta-analysis reporting relative risk (RR), OR and HR margin width outcomes. Eligible studies included prospective and retrospective case series with defining margin widths and 48 months of minimum follow-up. All patients (100%) received adjuvant whole breast radiotherapy (WBRT).
Results
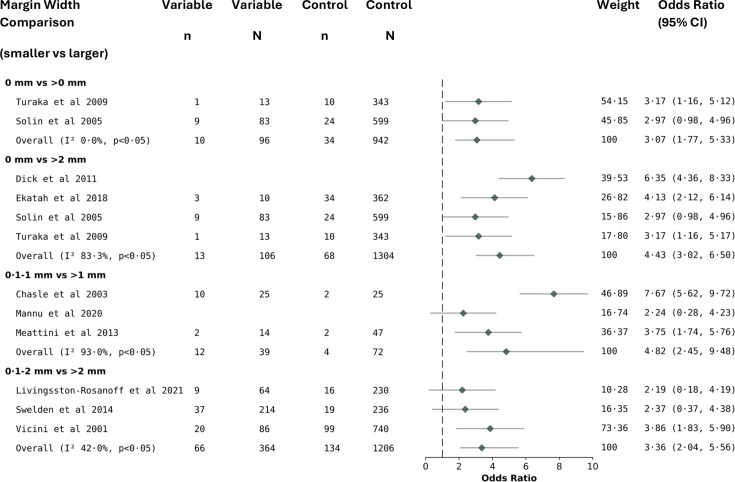
A total of 40 265 patients with pure DCIS in 31 studies were included. ORs and RR were calculated from 15 studies in 12 519 patients, and HRs were calculated from 12 studies in 12 946 patients. Local recurrence was significantly greater with narrower ‘close’ margins; 0.1–1 mm versus >1 mm in RR (2.88, 95% CI 1.86 to 3.90; p<0.05), OR (4.82, 95% CI 2.45 to 9.48; p<0.05) and HR analysis (1.34, 95% CI 1.01 to 1.67; p<0.05). Compared with margins >2 mm, significantly greater local recurrence was observed in margins 0.1–2 mm in RR (1.72, 95% CI 1.09 to 2.35; p<0.05) and OR (4.43, 95% CI 3.02 to 6.50; p<0.05). Comparing 0.1–1 mm versus >1 mm and 0.1–2 mm versus >2 mm, differences in local recurrence were not statistically significant, once adjusted for boost radiotherapy.
Conclusions
In pure DCIS with WBRT, the local recurrence risk reduces as margin width increases up to 2 mm. The strength of the recommendation for a minimum clear margin of 2 mm is limited by a lack of data comparing 1.1–2 mm with >2 mm. The association between recurrence and close margins is not significant following boost radiotherapy, suggesting a possible alternative to re-excision in patients with close margins <2 mm.
Systematic review registration
CRD42022308524.
Keywords: Radiation oncology, Breast cancer (female)
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is no international consensus on the definition of a minimum clear resection margin following breast-conserving surgery (BCS) for pure ductal carcinoma in situ (DCIS). Across 79 breast departments in the United Kingdom, 53.2% of units accepted a 1 mm and 38% accepted a 2 mm negative margin width. Marinovich et al (2016) suggested that margins <2 mm were inadequate; however, the specific local recurrence risk based on margin widths of 0.1–1 mm and >1 mm remains unclear. Whether boost radiotherapy impacts local recurrence risk within close margin widths 0.1–1 mm and 0.1–2 mm for DCIS is also unknown.
WHAT THIS STUDY ADDS
Reported findings within the comprehensive meta-analysis are commensurate with NICE, ASCO/ASTRO, ESMO and the newly revised Association of Breast Surgery (ABS) guidance (2024) which recommend a minimum radial margin of 2 mm. Critically, the statistically significant association between increased risk of local recurrence and ‘close’ margin widths (<1 mm and <2 mm) is apparent in patients not receiving boost radiotherapy. Once boost radiotherapy was considered in these groups, associations between margin width and local recurrence were no longer statistically significant. This finding supports the latest ASTRO guidelines in the use of boost radiotherapy in patients with ‘close’ (<2 mm) or positive margins.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides evidence to support boost radiation as an alternative to re-excision in patients with close margins (<2 mm) after BCS for DCIS with comparable local control. Our findings highlight a gap in the NICE and ABS recommendations in order to consider the use of boost radiotherapy in patients with DCIS.
Introduction
Ductal carcinoma in situ (DCIS) is a highly prevalent form of noninvasive breast cancer affecting over 56 000 women per year in the United States, over 7000 in the United Kingdom (UK) and over 2300 in the Netherlands.1,4 The Sloane Project in the UK reported that of 9938 women with screen-detected DCIS, 70% underwent breast-conserving surgery (BCS).5 The aim of BCS is to excise the DCIS with an adequate margin of clearance between disease and the healthy tissue edge. Clinically, it is accepted that an insufficient margin leads to a greater risk of local and distant recurrence and impacts overall survival.6 7 Despite systematic reviews and meta-analyses of the literature, historically there was no international consensus on the minimum distance required between DCIS and the healthy resection edge that defines a ‘clear margin’. The lack of standardisation in the definitions of clear margin manifests as a variation in the practice of re-operative intervention.7,9 Moreover, validated national registries such as the US National Cancer Institute’s Surveillance, Epidemiology, and End Results database are limited by the absence of data studying the impact of surgical margins on patient outcomes.10 11
Data suggest that the reduction in the risk of local recurrence is no longer seen beyond a 2 mm clear margin, but the risk of recurrence between 1 and 2 mm is widely debated.712,14 A prior OR meta-analysis concluded that compared with >2 mm, close margins 0.1–1 mm are inadequate with a significant odds of local recurrence (0.50, 95% CI 0.32 to 0.79; p<0.05) and that there is minimal additional risk reduction in margins wider than 2 mm.7 This led to the Society of Surgical Oncology (SSO)–American Society for Radiation Oncology (ASTRO) consensus guidance to recommend a 2 mm minimum clear margin for breast conservation for DCIS, with adjuvant whole breast radiotherapy (WBRT).12 13 The European Society for Medical Oncology (ESMO) also recommends a minimum clear margin of 2 mm for DCIS following BCS and WBRT, following a shared decision-making approach.15 In January 2024, the National Institute for Health and Care Excellence (NICE) in the UK updated its guidance, advising a minimum clear margin of 2 mm for DCIS, along with a discussion surrounding the benefits and risks of adjuvant WBRT.12 More recently, in October 2024, the Association of Breast Surgery (ABS) changed its recommendations on clear minimum margins from 1 mm to 2 mm.14 16 The recent shift in guidance to achieve wider margins in DCIS raises the concern that this may result in a greater proportion of women undergoing re-operative intervention, poses questions regarding the strength of the evidence on which these guidelines were established and also whether there are alternative strategies to manage the elevated risk associated with close resection margins (eg, boost radiation).
To address these concerns and move towards consensus on protocols for the treatment of DCIS, a revised analysis of the literature on margin width and local recurrence is required. Current guidance is informed by historical meta-analyses.7 9 These are now at least a decade old at the time of the literature search (October 2014).7 Ten important trials with data on local recurrence have been published since the prior meta-analysis.517,25 Moreover, in prior analyses, not all patients received WBRT.7 9 Indeed, 29% of patients in prior analyses did not receive radiation potentially confounding the findings of prior analyses.7 WBRT, following shared multidisciplinary decision-making, is accepted as a standard of care following BCS for the treatment of DCIS.26 Accepting the limitation that not all patients receiving BCS for DCIS undergo radiation,6 22 the advantage of ensuring an analysis exclusively in patients receiving WBRT is that it permits assessment of optimal margin widths independent of confounding differences in delivery of radiation therapy. Critically, prior meta-analyses evaluating margin width and local recurrence failed to adjust for boost radiation.7 This is important given recent data that suggest boost radiation may offset the additional risk associated with close margins in DCIS.27,30 Additionally, in prior analyses several margin widths were combined (eg, tumour on ink combined with margins <1 mm),7 and due to expired guidance, margin widths of at least 1 mm were not evaluated (eg, 0 mm vs 0.1–1 mm and vs >2 mm, and 0 mm vs >1 mm), making it impossible to accurately quantify risk between 0.1 and 1 mm.7 9 Prior meta-analyses focused solely on OR for recurrence7 but failed to include other important metrics such as relative risk (RR) and HRs which have not been incorporated into data synthesis until now.
Our aim was to systematically review and meta-analyse the literature on margin width and local recurrence in women undergoing breast conservation, followed by WBRT for DCIS. We sought to advance the previous work by Marinovich et al,7 through precise quantitative margin thresholds for comparison (ie, 0.1–1 mm and 0.1 mm–2 mm), while simultaneously adjusting for boost radiation. We consider the current analysis to be the most comprehensive interrogation of associations between resection margin width and local recurrence outcome data conducted to date, not simply through contemporaneous literature search and data extraction, but also because we comprehensively evaluated all metrics of local recurrence outcome (ie, OR, RR and HR).31,33
Methods
Search strategy and selection criteria
We performed a meta-analysis of original research studies that assessed the impact of margin width on local recurrence following breast conservation in patients with biopsy-proven DCIS. The systematic review was registered using the International Prospective Register of Systematic Reviews (ID CRD42022308524). MEDLINE and Embase were searched for articles up to 01 November 2023 (online supplemental appendix 1). Eligible studies were full journal articles written in English with a clearly defined margin status and specified width (mm). Studies included BCS only, followed by WBRT with at least 48 months of follow-up. Studies in which margin width was not clearly defined were excluded. Patients requiring completion mastectomy were excluded from the meta-analysis on local recurrence risk. Key search terms included ‘ductal’, ‘margin’ and ‘recurrence’. We screened additional references by reviewing bibliographies of included articles and previous meta-analyses.
Data extraction and checking
Study selection and data extraction regarding margin status and association with local breast recurrence in studies published until 01 November 2023 were done in parallel by two co-authors (AE and DS). Full-text review was done in parallel by AE, DS, MB, MJ, RP, YG, RN, NACB, HE, SH, JH and DRL. A secondary full-text review was completed to resolve an outstanding conflict by AE, DS and NL. Quality assessment was performed using the Risk Of Bias In Non-randomized Studies – of Interventions, Version 2 (ROBINS-2) quality assessment tool by co-authors (AE, DS and NL).34 Where conflicts could not be resolved, the final decision was made by discussion with the senior author (DRL).
Outcomes
The primary outcome of the study was the risk/odds of ipsilateral in-breast recurrence based on a quantitative margin width range (mm–mm). The secondary outcome of the study was to determine if adjuvant boost radiotherapy significantly reduces the risk/odds of ipsilateral in-breast recurrence.
Margins were reported within original studies and defined as either positive (tumour on ink or 0 mm), negative (there is no disease within a predefined agreed margin width) or close (no disease on ink but the defined negative margin clearance has not been achieved). As the margin width (mm) defining these terms varies internationally, we extracted data based on exact quantitative thresholds where they were reported. Resection margin widths were quantitatively subcategorised based on the distance between the inked margin and DCIS as follows: (a) DCIS on ink (0 mm), (b) >0.1 mm to 1 mm, (c) >0.1 mm to 2 mm, (d) >1 mm and (e) >2 mm. Within each primary study, local recurrence for each margin width was individually extracted and compared with calculated RR and OR (ie, 0 mm vs >2 mm, 0 mm vs 0.1–2 mm and 0·1–2 mm vs >2 mm). Patient and tumour characteristics were extracted (table 1). Adjuvant therapy included WBRT, boost radiotherapy and endocrine (hormone) therapy.
Table 1. Summary of patient demographics, tumour characteristics and local recurrence rates in relation to excised margin width.
| Variable | Total number (N) | |||
| Studies | Women | |||
| Total no. of patients (N) | 31 | 40 265 | ||
| Absolute number | ||||
| Study design | Studies (n) | Womenn (%) | ||
| Retrospective | 24 | 38 095 (94.6) | ||
| Prospective | 7 | 2170 (5.4) | ||
| Age in years, median (IQR) | 55.5 (53.1–57.8) | 22 | 37 023 (91.9) | |
| Follow-up in months, median (IQR) | 86.4 (69.2–100.2) | 28 | 37 807 (93.9) | |
| Tumour size in cm, median (IQR) | 1.48 (0.82–2.0) | 9 | 3272 (8.1) | |
| Therapy | ||||
| Whole breast radiotherapy | ||||
| Yes | 31 | 40 265 (100) | ||
| No | 0 | 0 (0) | ||
| Boost radiotherapy | ||||
| Yes | 18 | 4620 (59.1) | ||
| No | 20 | 3251 (39.3) | ||
| Unknown | 11 | 31 984 (100) | ||
| Hormone therapy | ||||
| Yes | 13 | 8395 (27.3) | ||
| No | 15 | 21 340 (71.8) | ||
| Unknown | 16 | 10 530 (35.4) | ||
| Receptor status | ||||
| ER positive | 9 | 1983 (56.2) | ||
| ER unknown | 22 | 36 446 (100) | ||
| PR positive | 5 | 1013 (46.7) | ||
| PR unknown | 26 | 38 095 (100) | ||
| Surgery | ||||
| BCS | 31 | 40 265 (100) | ||
| Number of re-excision | ||||
| Re-excision was BCS | 8 | 1214 (26.4) | ||
| Re-excision was mastectomy | 3 | 39 (4.4) | ||
| DCIS grade | ||||
| Low nuclear grade | 15 | 987 (9.3) | ||
| Intermediate nuclear grade | 15 | 3021 (28.4) | ||
| High nuclear grade | 16 | 6698 (54.8) | ||
| Unknown | 8 | 458 (5.0) | ||
| Margin width(as defined by each study) | ||||
| Negative | All negative | 24 | 7412 (48.9) | |
| Negative unknown distance | 2 | 68 (15.0) | ||
| Negative >1 mm | 2 | 1237 (78.9) | ||
| Negative >2 mm | 11 | 2824 (65.8) | ||
| Close | ||||
| Close 0.1–1 mm | 11 | 657 (7.9) | ||
| Close 0.1–2 mm | 12 | 2094 (18.3) | ||
| >0.1 and <10 mm | 11 | 3853 (42.8) | ||
| Positive (tumour on ink/0 mm) | 12 | 456 (4.4) | ||
| Unknown | 8 | 565 (11.6) | ||
| Total number of local recurrence | 27 | 1468 (4.4) | ||
| Type of local recurrence | ||||
| DCIS | 19 | 638 (2.0) | ||
| Invasive | 19 | 738 (2.3) | ||
| Not stated | 4 | 7013 (100) | ||
| Unknown | 5 | 69 (6.8) | ||
| Both | 3 | 23 (1.3) | ||
| Local recurrence per margin width comparison(smaller vs larger) | Absolute risk n/N (%) | Absolute risk reduction % | ||
| Smaller | Larger | |||
| 0 mm vs >0 mm | 25/208 (12.0) | 103/1707 (6.0) | 6 | |
| 0 mm vs 0.1–2 mm | 17/254 (6.7) | 45/988 (4.6) | 2.1 | |
| 0 mm vs >2 mm | 20/264 (7.6) | 121/5114 (2.4) | 5.2 | |
| 0.1–1 mm vs >1 mm | 15/68 (22.1) | 7/98 (7.1) | 14.9 | |
| 0.1–2 mm vs >2 mm | 127/1586 (11.1) | 279/6537 (7.6) | 3.7 | |
Margins are defined as follows: positive (tumour on ink or 0 mm), negative (there is no disease within a predefined agreed margin width) and close (no disease on ink but the defined negative margin clearance has not been achieved).
n is the subgroup patient number.
N is the total patient number.
BCSbreast-conserving surgeryDCISductal carcinoma in situERoestrogen receptorPRprogesterone receptor
Local recurrence was defined as ipsilateral in-breast recurrent DCIS or invasive disease, as reported in the primary article. Where studies described ipsilateral breast recurrence, we documented this as a locally recurrent disease. Raw outcome data on local recurrence were used to compute adjusted OR and RR. Where available, HRs were extracted and included for meta-analysis. Median and mean values are used based on the provided individual data.
Statistical analysis
Meta-analysis was performed with DerSimonian and Laird random effects modelling to derive OR, HR and RR with CIs.35 We assessed statistical heterogeneity within each outcome comparison by calculating the I2 statistic. We considered a value <30% as low heterogeneity, between 30 and 60% as moderate and over 60% as high. Missing data for study-level CIs were accommodated for analysis by single imputation. Here, the assumptions included homogeneity of variances, distribution normality, consistency of effect sizes and correct specification of covariates. We performed subgroup and sensitivity analyses to assess the effect of the highly variable reporting of margin distances and local recurrences by obtaining summary estimates of HR, OR and RR across all reported margins. We were unable to adjust the analysis to control the use of endocrine therapy. For analyses, we applied Stata (V.15.1, StataCorp LCC, TX).36
Role of funding source
This study is an independent research funded by the National Institute for Health Research (NIHR), Imperial Biomedical Research Centre (BRC) and the Cancer Research UK (CRUK) Convergence Science Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, Imperial BRC or CRUK. This work was not informed by patient and public involvement.
Results
As illustrated in the PRISMA flow diagram in figure 1, 2644 records were identified through database searching and 12 further records were identified from other sources up to 01 November 2023, including bibliographic cross-referencing. Duplicate studies were removed (843 studies), 1801 abstracts were screened and a further 1765 records were excluded. (online supplemental appendix 1). A total of 31 studies, published up to 01 November 2023 and involving 40 265 patients undergoing BCS and radiotherapy for DCIS, were eligible for inclusion in the meta-analysis, describing local treatment failure associated with varying margin widths.58 17,25 28 37 Following exclusion of 5 further studies (1 study did not report margin definition, 3 studies did not report 100% WBRT and 1 study reported wrong study outcomes)56,60 (online supplemental appendix 2), there were 24 retrospective and 7 prospective studies. Where sufficient data were available, OR and RR were calculated from 15 studies involving 12 519 patients.817 20,22 24 25 28 41 42 51 53 HR was calculated from 12 studies involving 12 946 patients.5 8 18 19 21 23 25 28 37 40 51 54 Study demographics are summarised in table 1. The median (IQR) age of women was 55.5 years (53.1–57.8), the median (IQR) follow-up was 86.4 months (69.2–100.2) and the median (IQR) tumour size was 1.48 cm (0.8–2.0) (table 1). Where reported, receptor status was oestrogen positive in 3527 patients (56.2%) and progesterone positive in 2170 patients (46.7%) (table 1). Within the meta-analysis of 21 studies, 9 scored as ‘low risk’, 9 as ‘moderate risk’, 2 as ‘serious risk’ and 1 as ‘critical risk’ of bias, using the ROBINS-2 quality assessment (see online supplemental appendix 3).
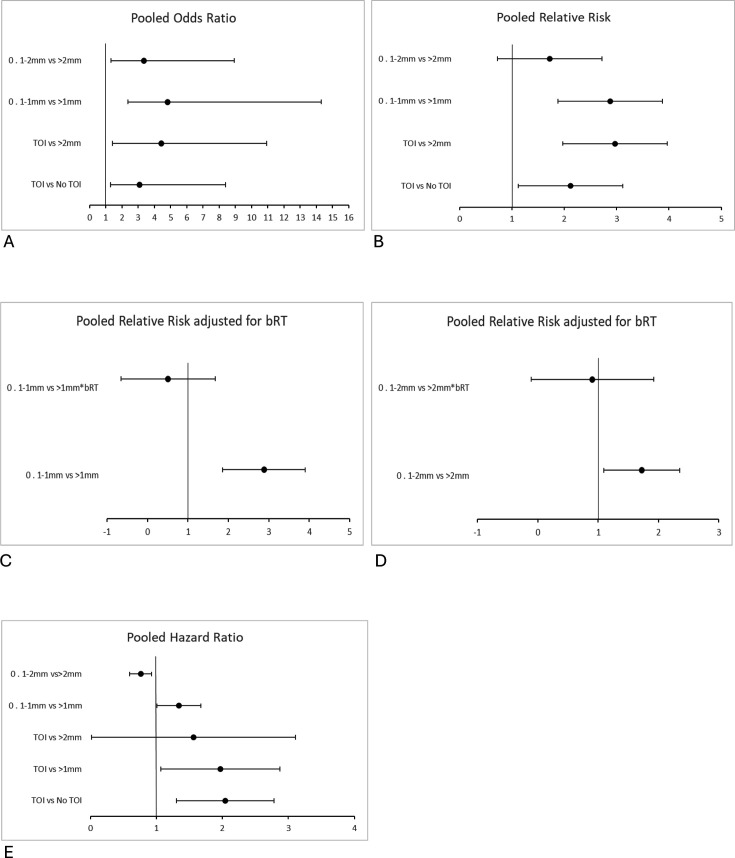
Figure 1. Reporting the relative risk for local recurrence in patients undergoing breast-conserving surgery with whole breast radiotherapy. bRT, boost radiotherapy based on comparisons between different margin widths; I2, heterogeneity score; n, number of patients with local recurrence; N, total number of patients within the margin width group.
Overall, 100% (40 265) of patients received WBRT, 59.1% (4620/7817) received boost radiotherapy and 27.3% (8395/30 807) received hormonal therapy (table 1). The grade of DCIS was reported as low 9.3% (987/10 636), intermediate 28.4% (3021/10 636), high 54.8% (6698/12 224) and unknown 5.0% (458/9161). Notably, 22.8% (1253/5486) of patients required re-operative intervention. In total, 4.4% (1468/33 252) of patients experienced local recurrence.
Overall, although the effect size is less after the first 1 mm, the risk for local recurrence up to a negative margin of >2 mm remains statistically significant (figures14). Comparing 0 mm versus >2 mm, data showed a local recurrence RR (2.97, 95% CI 2.16 to 3.78; p<0.05), OR (4.43, 95% CI 3.02 to 6.50; p<0.05) and HR (1.56, 95% CI 0.02 to 3·11; p<0.05) (figures14). Importantly, the risk for local recurrence in the 0.1–1 mm versus >1 mm demonstrated an RR (2.88, 95% CI 1.86 to 3.90; p<0.05), OR (4.82, 95% CI 2.45 to 9.48; p<0.05) and HR (1.34, 95% CI 1.01 to 1.67; p<0.05) (figures14). Regarding 0.1–2 mm versus >2 mm, data showed an RR (1.72, 95% CI 1.09 to 2.35; p<0.05), OR (3.36, 95% CI 2.04 to 5.56; p<0.05) and HR (0.76, 95% CI 0.59 to 0.93; p<0.05) (figures24). On comparison of 0 mm versus >1 mm, data showed an HR (1.97, 95% CI 1.07 to 2.87; p<0.05) (figure 3). Comparing 0 mm versus 0.1–2 mm, data showed an RR (1.61, 95% CI 0.61 to 2.6; p<0.05) (figure 1). All between-group comparisons (smaller vs larger margin widths) were statistically significant (figures14).
Figure 4. Reporting forest plot meta-analysis of local recurrence risk outcomes in patients undergoing breast-conserving surgery with whole breast radiotherapy, based on comparisons between different margin widths. A. OR. B. RR. C. RR comparing 0.1–1 mm versus >1 mm*bRT D. RR comparing 0·1–2 mm versus >2 mm*bRT. E. HR. bRT, adjusted for boost radiotherapy; RR, relative risk; TOI, tumour on ink.
Figure 2. Reporting the OR for local recurrence in patients undergoing breast-conserving surgery with whole breast radiotherapy, based on comparisons between different margin widths. I2, heterogeneity score; n, number of patients with local recurrence; N, total number of patients within the margin width group.
Figure 3. Reporting the HR for local recurrence in patients undergoing breast-conserving surgery with whole breast radiotherapy, based on comparisons between different margin widths. I2, heterogeneity score; N, total number of patients within the margin width group.
The local recurrence RR was adjusted for boost radiotherapy, as illustrated in figures1 4. The largest reduction in local recurrence was observed on comparison of 0.1–1 mm versus >1 mm RR (2.88, 95% CI 1.86 to 3.90; p<0.05) and 0.1–1 mm versus >1 mm adjusted for boost radiotherapy RR (0.51, 95% CI −0.65 to 1.68; p=0.31) (figures1 4C). A reduced risk for local recurrence was also observed in 0.1–2 mm versus >2 mm RR (1.72, 95% CI 1.09 to 2.35; p<0.05) and 0.1–2 mm versus >2 mm adjusted for boost radiotherapy RR (0.90, 95% CI −0.11 to 1.92; p=0.08) (figures1 4D).
The absolute risk reduction for local recurrence favoured wider margin widths across all between-group comparisons (table 1). The largest absolute risk reduction (14.9%) was reported in 0.1–1 mm (absolute risk for local recurrence=22.1%, 15/68) versus >1 mm (7.1%, 7/98) (table 1). An absolute risk reduction for local recurrence of 3.7% is reported in 0.1–2 mm (11.1%, 127/1586) versus >2 mm (7.6%, 279/6537) (table 1). An absolute risk reduction for local recurrence of 6.0% is reported in 0 mm (12.0%, 25/208) versus >0 mm (6.0%, 103/1707) (table 1).
Discussion
The current study of 40 265 patients with DCIS is the first to concurrently present a comprehensive meta-analysis of local recurrence following BCS and adjuvant WBRT while adjusting for boost radiation. Our findings demonstrate that ‘close’ resection margins (<2 mm) exhibit a significantly increased risk of local recurrence, with the greatest risk within a margin width of 0.1 mm–1 mm versus >1 mm (RR 3.36, OR 4.82 and HR 1.97). Wider margins (0.1–2 vs >2 mm) were associated with an attenuated yet statistically significant risk of local recurrence (RR 1.72, OR 3.36 and HR 0.76). Arguably, it may be hypothesised that the larger risk observed in the first 0.1–1 mm compared with that observed in the first 0.1–2 mm supports the notion that most of the risk is offset by achieving a 1 mm negative margin. Importantly, the elevated risk/odds of recurrence in close margins <2 mm was no longer significant once the effect of boost radiation was considered, implying that boost radiotherapy may present an alternative to re-excision in patients with DCIS with close margins following breast conservation.
Local breast recurrence is the first and the most common outcome failure following breast conservation for DCIS.62 Despite a perceptually low 3% 20-year breast cancer mortality estimate of women undergoing treatment for DCIS, the 25-year cumulative risk of invasive breast cancer in women diagnosed with DCIS is estimated to be more than 1 in 4 (27%).22 63 In fact, one study of nearly 110 000 patients with DCIS showed an 18-fold increased risk of dying from breast cancer, following ipsilateral invasive breast local recurrence.63 Importantly, ipsilateral recurrence following breast conservation and WBRT is usually managed with mastectomy with or without breast reconstruction and second-line systemic treatment with additional healthcare costs.27 This emphasises the importance of our study in understanding and reducing the risk of local recurrence of disease based on resection margin width.
It is a widely accepted disease as an inked resected margin confers an unacceptably high risk of recurrence.13 14 16 63 The NSABP B-17 trial reported that positive margins (tumour on ink or 0 mm) increased RR for local recurrence twofold compared with negative margins (defined as no tumour on ink).42 These findings align with the results of the current meta-analysis for tumour on ink versus wider margins across different metrics of ipsilateral recurrence. Specifically, we observed that patients with tumour on ink compared with wider margins experienced a 3–4.5-fold increase in risk of ipsilateral local recurrence (figures14).
As described previously, our results imply that the risk/odds of recurrence remain significant up to a minimum clear margin of 2 mm. This is in line with another data synthesis,64 which evaluated 4660 patients with DCIS undergoing BCS and WBRT and observed ~50% reduction in the odds of local recurrence in margins >2 mm (vs <2 mm).64 We infer that in breast conservation for DCIS, resection margins >2 mm are associated with an approximately 70% odds reduction in local recurrence compared with a narrower margin width of 0.1–2 mm. However, the greatest risk reduction was observed at a margin width >1 mm compared with a margin width of 0.1–1 mm, with a 79.3% reduction in the odds of local recurrence.
Patients with ‘close’ resection margins exhibited a significantly increased risk of local recurrence, with the greatest risk within a margin width of 0.1–1 mm versus >1 mm (RR 3.36, OR 4.82 and HR 1.97). Wider margins (>2 mm vs 0.1–2 mm) were associated with an attenuated yet statistically significant reduction in the risk of local recurrence (RR 1.72 and OR 3.36). Arguably, it may be hypothesised that the greater risk observed in the first 0.1–1 mm compared with 0.1–2 mm supports the notion that the majority of risk is offset by achieving at least a 1 mm margin. The caveat is that the data for 0.1–1 versus >1 mm and 0.1–2 versus >2 mm extracted for analysis were from different study populations. A statistically significant increase in the risk of local recurrence was observed for close margins up to 2 mm, although our study was limited by a lack of data directly comparing margin width outcomes between 1.1–2 mm versus >1 mm and >2 mm. This is a key evidence gap, which requires further research. Nevertheless, we believe these estimates of risk/odds to be more accurate than prior meta-analyses7 9 given our analysis has a larger sample size for subgroups within 2 mm margins and prior analyses combined margins >0 mm and 1 mm into a single category which may have skewed results and biased outcomes.7
One of the novelties of this analysis is that we only included patients receiving WBRT. This is important since histological65 and anatomical studies66 have shown that DCIS can skip segments up to 10 mm and that to achieve complete resection with surgery alone would necessitate margins >10 mm.65 Since then, to pursue BCS with minimal tissue loss and to maximise breast-related quality of life, the approach has shifted from solely targeting margin width for optimal local recurrence reduction to a multimodal therapeutic approach. Rather than achieving an entirely negative radial margin, where 100% of DCIS excision has been achieved, our view is that the goal is to resect DCIS by the minimum safe radial margin width in combination with adjuvant WBRT and/or hormonal therapy. The principle of DCIS control, as one of several integral parts in a multimodal therapeutic approach, is exemplified by Wong et al,67 who evaluated the use of BCS for DCIS at a minimum clear margin width of 1 cm without adjuvant WBRT.67 The authors closed their study prematurely, due to an unacceptable local recurrence rate of 2.4% per year, corresponding to 12% at 5 years.67 Commensurate with this, the outcomes from the National Surgical Adjuvant Breast Project (NSABP), an 8-year update of protocol B-17 trial, first reported in 1993, observed a 60% lower risk of local recurrence when BCS was followed by WBRT, globally adopted thereafter.42 Similarly, the NSABP trial B-24 trial, one of the largest prospective studies of breast conservation in DCIS, reported administering adjuvant WBRT and tamoxifen, compared with surgery alone, resulted in an overall 50% reduction in invasive breast local recurrence at 15 years of follow-up (8.5% vs 19.4%, respectively).62
Our findings are congruous with the recent change by the ABS in October 2024, replacing the earlier recommendation of a radial margin of 1 mm with 2 mm, as the critical margin width, in DCIS management with BCS.14 16 This updated ABS Best Practice Guideline for DCIS management is commensurate with recommendations by the UK NICE (guideline NG101) and international guidance from ASCO/ASTRO which advocates for a minimum negative clear margin of 2 mm.12 13 Interestingly, despite these guidelines, variation in local practice was evident in a recent multicentre prospective study of 79 breast departments in the UK, which reported that 53.2% of units accepted a 1 mm and only 38% accepted a 2 mm negative margin width.68 This may suggest either a lack of awareness of new national guidance or might reflect views regarding the modest strength of evidence in support of 2 mm margins (given a lack of 1–2 mm comparator) and/or concerns regarding higher rates of re-operative intervention through acceptance of wider margins. In this regard, our findings do support alternative strategies for re-operative intervention in cases of margins <2 mm. Importantly, we found that once adjustment for boost radiotherapy was conducted, differences in local recurrence rates were no longer significant. Indeed, the greatest reduction in the risk of relapse (82%) favouring boost radiotherapy was for margins 0.1–1 mm. For the comparison of 0.1–2 mm versus >2 mm, the RR reduction in local recurrence of boost radiation was nearly 48%. Therefore, the unique finding of our analysis is that patients with intermediate-/high-grade DCIS (represents 83.2% of our study cohort), with a close margin of up to 1 mm and 2 mm, may benefit from boost radiotherapy, as an alternative to re-excision, and an adjunct to adjuvant WBRT.
It is important to note that patients included in our study were predominantly diagnosed with intermediate- or high-grade DCIS (83.2%) which may explain the relatively high proportion of patients receiving boost radiotherapy (59.1%). The relatively high proportion of boost radiation is considered a strength of this study in terms of ensuring sufficient sample size to more precisely detect the effect of boost. It is noteworthy that individual studies in which boost radiation had minimal impact on the risk of local recurrence included comparatively small proportions of patients receiving a boost.69 70 For example, Rakovitch et al69 observed no association between boost radiation and recurrence but only 29% of the patients received a boost. Yerushalmi et al70 found no impact of a boost on recurrence on Cox proportion regression analysis, but only 26.7% received a boost. Similarly, Dreyfuss et al71 found no impact of boost but acknowledged that their findings could be impacted by type II error. The authors noted that studies demonstrating boost reduces recurrence include greater proportions of patients receiving a boost.71
Importantly, our results are commensurate with multiple well-designed studies that highlight boost radiation reduces recurrence risk.27,3067 For example, Moran et al29 observed boost led to a statistically significant reduction in ipsilateral recurrence in a multicentre study of >4000 patients in which the sample size was informed by an a priori computation to determine both the total cohort size and the percentage of patients treated with a boost to detect a 3% difference in recurrence rates. Similarly, in the study by Meattini et al,28 boost radiation led to a reduction in the risk of relapse HR (0.50, 95% CI 0.22 to 1.12) and was a significantly independent predictor of local recurrence (HR 0.17, 95% CI 0.04 to 0.70, p=0.014). Omlin et al30 showed that boost radiation had a significant advantage on local relapse-free survival compared with radiotherapy without boost (HR 0.45, 95% CI 0.23 to 0.90, p=0.024). Finally, in an international, multicentre, randomised trial (TROG 07.01) of boost versus no boost in 1608 DCIS cases with minimum 1 mm clear margins, Chua et al27 found that boost radiation led to a significant decrease in local recurrence (5-year freedom from local recurrence; boost=97.1%, 95% CI 95.6 to 98.1 vs no boost=92.7%, 95% CI 90.6 to 94.4).
While it is widely accepted that WBRT following BCS significantly reduces local recurrence,12,1472 the use of boost radiation is more controversial. Critically, our findings enhance ASTRO 2018 recommendations and ESMO Clinical Practice Guidelines 2024 on the role of boost radiotherapy in patients undergoing BCS/WBRT for intermediate- or high-grade DCIS.15 73 ASTRO recommended consideration of tumour bed boost for patients with DCIS who were ≤50 years of age with high-grade DCIS and/or with close (<2 mm) or positive margins.73 The results of our analysis add further weight to these recommendations, especially for patients with close margins (<2 mm). Specifically, our data suggest that boost radiation may reduce the RR of local recurrence by 48–82%. This is well-timed, given recent changes that recommend wider minimum clear margins in BCS for DCIS from the UK ABS,14 and concerns that the need for wider margins will lead to higher rates of re-operative intervention, and/or resections of larger volume with adverse impact on quality of life and cosmesis. Against this background, we hope the results of our analysis provide evidence of safe and reasonable alternative strategies in scenarios where re-excision is deemed suboptimal or too high risk.
Many studies found an association between close margins (<2 mm) and delivery of boost radiation.28 50 67 71 For example, Wong et al67 found patients receiving boost radiation were significantly more likely to have positive margins (p=0.0001). In the study by Monteau et al,50 patients with involved margins received a significantly higher radiation dosage (p=0.0006). Similarly, Meattini et al28 found a greater proportion of patients with margins <1 mm received boost radiation. Beyond close margins, other factors to consider when deciding on a boost include young age at diagnosis30 and nuclear grade.27 For example, Omlin et al30 demonstrated the benefits of boost for local control in women under the age of 45 years. In the randomised controlled trial by Chua et al,27 which showed a significant benefit of boost radiation, 71% of patients in the boost arm had high-grade, comedo-type necrosis. Taken together, we suggest that young patients, those with a high-grade disease and/or close margins <2 mm, be considered for boost radiation.
It is important to acknowledge that while the RR reduction from boost radiation in close margins may be substantial, the absolute benefit may be limited. This is especially important considering the adverse effects of boost radiation including induration and fibrosis.70 71 In the BIG 307/TROG 07.01 randomised trial, at a follow-up of 6.6 years, 5-year freedom from local recurrence was 97.1% for boost versus 92% in the no boost arm, equating to a 4.4% absolute difference.61 Critically, the current meta-analysis suggests that an absolute risk reduction of a margin >2 mm (vs 0.1–2 mm) is 3.7%, hence akin to that afforded by boost.
Given equity in the benefits of re-operative intervention and boost radiation in reducing recurrence, decisions regarding which strategy to employ will likely be influenced by practical and clinical considerations. For example, re-operative intervention is unlikely to be advocated in elderly patients with multimorbidity or competing risks. Additionally, re-operative intervention is known to impact breast-related quality of life.74 In a study of 2543 patients, re-operative BCS was associated with significantly lower breast satisfaction (p=0·007) and sexual well-being (p=0·049).74 Conversely, boost radiation is associated with an increase in breast induration in randomised trials (BIG 307/TROG 07.01).27 75 These studies show an increase of severe (grade 3 or 4) tumour bed induration from 1.8% to 5.2% and 5% to 11%, respectively.27 75 These differences are numerically small, but statistically significant. In the BIG 3 trial, grade 2 and above and breast pain increased with a boost from 10 to 14%.27 Moreover, planning boost therapy may not be trivial, especially in oncoplastic cases involving parenchymal rearrangement. We also acknowledge that boost radiotherapy may adversely affect cosmesis.76 The EORTC trial in 5178 early invasive breast cancers undergoing BCS and WBRT comparing boost without reported that boost radiation was associated with breast fibrosis.77 These factors should be weighed against the risks of re-excision or completion mastectomy, which may be complicated by breast reconstruction and physical/psychosexual patient morbidity.27 78 79 None of the boost radiation studies in our meta-analysis reported on the psychosexual impact of re-excision nor on the rate of breast fibrosis. Rather than a one-size-fits-all approach in patients with close margins <2 mm, we advocate for a case-by-case discussion in which the risks and benefits of re-operation versus boost radiation are weighed up, based on shared decision-making.
Limitations
While many of the patients internationally undergoing BCS for DCIS receive postoperative radiotherapy,2280,82 the proportion treated varies by centre.2280,82 The UK Sloane Project, relating to 2003 to 2006, reported that radiotherapy was given to roughly 57% of cases with the planned use of adjuvant radiotherapy significantly higher for tumours >40 mm, or a high nuclear grade or/and with central comedo-type necrosis present.82 Radiotherapy usage has also changed over time, increasing between 1990 and 2018.22 80 81 There are ongoing efforts to identify groups of patients who remain at low risk of recurrence without radiotherapy based on clinical and genomic profiles.83 Given the importance of adequate margin width in those receiving radiotherapy, it is likely that this would also be of importance in those treated with breast conservation alone.
Direct subgroup analysis of extracted data between 0.1–1 versus >1 mm and 0.1–2 versus >2 mm was limited, given data were from different study populations. Due to a lack of data for margin width 1.1–2 mm, an analysis of 1.1–2 mm versus >2 mm was not possible at the present time. Given that the difference in rates of local recurrence for margin threshold 1.1 mm–2 mm versus >2 mm remains a critical clinical question, we strongly advocate for precise quantitative reporting of margin width in future studies that evaluate resection margins and oncological outcomes.
While we ensured that all patients in this study received WBRT, we acknowledge a shift in management based on nuclear grade, whereby low-grade DCIS with <10% local recurrence risk at 10 years of follow-up may be treated along a de-escalated pathway with safe WBRT avoidance.26 In studies in which boost radiotherapy was delivered, we frequently found that the dose of boost radiotherapy was often not reported. The absolute risk of local recurrence with and without boost radiotherapy per margin width was limited by the unavailability of relevant data. Of the six studies8 24 28 53 55 61 reporting the administration of boost radiotherapy, only two explicitly reported the dose administered (10 Gy) and selection criteria based on positive or ‘close’ margins.24 61 One study reported a boost dose of 16–20 Gy,28 and one study reported an overall dose of radiotherapy (WBRT and boost radiotherapy) ranging from 45.0 to 71.8 Gy.8 Two studies did not report the boost radiotherapy dose.53 55 Four of six studies did not report selection criteria for boost radiotherapy administration.8 28 53 55 One study stated that boost was controversial and left to the discretion of the treating clinician.24
Statistical analysis is limited by sample heterogeneity and sample size differences. Local practice variations, such as radiotherapy regimen and dosage, are important confounding variables, often not explicitly quantified. Meta-analysis data for OR, HR and RR have computed CIs, which poses a statistical risk. These include the introduction of analytical bias, overestimation or underestimation of effect sizes, mass inconsistency effects across studies and sensitivity to outliers. In certain cases, OR analysis was not possible, resulting from logarithmic computation, yielding negative values. This is evident in the discordance between calculated OR in 15 studies and OR meta-analysis in 10 studies (online supplemental file 3, figure 2). The HR meta-analysis results for margin widths 0.1–2 mm versus >2 mm are limited by the inclusion of only two studies. Finally, we were unable to adjust for hormonal therapy since most studies did not report its use (table 1). We accept that variation in endocrine use may have confounded associations between margin width and recurrence.
Conclusions
In breast conservation for DCIS, resection >2 mm is associated with an approximately 70% odds reduction in local recurrence compared with a narrower margin width of 0.1–2 mm. The greatest risk reduction is observed at a margin width >1 mm compared with a margin width of 0.1–1 mm, with a 79.3% reduction in the odds of local recurrence. A statistically significant increase in the risk of local recurrence is observed for close margins up to 2 mm, although our study was limited by a lack of data directly comparing margin width outcomes between 1.1–2 mm versus >1 mm and >2 mm. This is a key evidence gap, which requires further research.
The novel finding is that the increased risk/odds of ipsilateral breast recurrence is offset through boost radiation in patients with intermediate- and high-grade DCIS. We suggest bringing into alignment international recommendations on the role of boost radiation for close margins in intermediate- and high-grade DCIS. Ultimately, decision-making for re-excision versus boost radiations represents a trade-off between the benefits and harms of these interventions, especially considering that their impact on reduction in absolute risk of ipsilateral recurrences appears broadly equivalent.
supplementary material
Footnotes
Funding: This paper is independent research funded by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC) and the Cancer Research UK (CRUK) Convergence Science Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, Imperial BRC or CRUK. This work was not informed by patient and public involvement
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.American Cancer Society Cancer facts and figures 2024. [2-Dec-2024]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf Available. Accessed.
- 2.Cancer Research UK Ductal carcinoma in situ (DCIS) 2023. [10-Dec-2024]. https://www.cancerresearchuk.org/about-cancer/breast-cancer/types/ductal-carcinoma-in-situ-dcis Available. Accessed.
- 3.van Seijen M, Lips EH, Thompson AM, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer. 2019;121:285–92. doi: 10.1038/s41416-019-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Netherlands comprehensive cancer organisation (iknl) 2023. [10-Dec-2024]. https://nkr-cijfers.iknl.nl/viewer/incidentie-per-jaar?language=en_GB&viewerId=b814ee10-e6a5-495b-aab1-8e31799ad248 Available. Accessed.
- 5.Thompson AM, Clements K, Cheung S, et al. Management and 5-year outcomes in 9938 women with screen-detected ductal carcinoma in situ: the UK Sloane Project. Eur J Cancer. 2018;101:210–9. doi: 10.1016/j.ejca.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Giannakeas V, Sopik V, Narod SA. Association of a Diagnosis of Ductal Carcinoma In Situ With Death From Breast Cancer. JAMA Netw Open . 2020;3:e2017124. doi: 10.1001/jamanetworkopen.2020.17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marinovich ML, Azizi L, Macaskill P, et al. The Association of Surgical Margins and Local Recurrence in Women with Ductal Carcinoma In Situ Treated with Breast-Conserving Therapy: A Meta-Analysis. Ann Surg Oncol. 2016;23:3811–21. doi: 10.1245/s10434-016-5446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicini FA, Recht A. Age at diagnosis and outcome for women with ductal carcinoma-in-situ of the breast: a critical review of the literature. J Clin Oncol. 2002;20:2736–44. doi: 10.1200/JCO.2002.07.137. [DOI] [PubMed] [Google Scholar]
- 9.Wang S-Y, Chu H, Shamliyan T, et al. Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst. 2012;104:507–16. doi: 10.1093/jnci/djs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surveillance, epidemiology, and end results program. 2024. [1-Sep-2024]. https://seer.cancer.gov/statfacts/html/breast.html Available. Accessed.
- 11.Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst. 2015;107:djv263. doi: 10.1093/jnci/djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (NICE) Early and locally advanced breast cancer: diagnosis and management. 2018. [1-Sep-2024]. https://www.nice.org.uk/guidance/ng101/evidence/evidence-review-a-surgery-to-the-breast-pdf-4904666606 Available. Accessed. [PubMed]
- 13.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma In Situ. J Clin Oncol. 2016;34:4040–6. doi: 10.1200/JCO.2016.68.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association of breast surgery recommendations for the management of radial surgical margins in patients undergoing breast conserving surgery for ductal carcinoma in situ (dcis) 2024. https://associationofbreastsurgery.org.uk/media/3z5bk0nj/dcis-margins-guidance-2024-v2.pdf Available.
- 15.Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35:159–82. doi: 10.1016/j.annonc.2023.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Association of breast surgery consensus statement: margin width in breast conservation surgery 2015. [10-Dec-2024]. https://associationofbreastsurgery.org.uk/media/64245/final-margins-consensus-statement.pdf Available. Accessed.
- 17.Ekatah GE, Turnbull AK, Arthur LM, et al. Margin width and local recurrence after breast conserving surgery for ductal carcinoma in situ. Eur J Surg Oncol. 2017;43:2029–35. doi: 10.1016/j.ejso.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Hennigs A, Fuchs V, Sinn H-P, et al. Do Patients After Reexcision Due to Involved or Close Margins Have the Same Risk of Local Recurrence as Those After One-Step Breast-Conserving Surgery? Ann Surg Oncol. 2016;23:1831–7. doi: 10.1245/s10434-015-5067-1. [DOI] [PubMed] [Google Scholar]
- 19.Jobsen JJ, Scheijmans LJEE, Smit WGJM, et al. Breast-conserving therapy for primary Ductal Carcinoma in Situ in The Netherlands: A multi-center study and population-based analysis. Breast. 2018;42:3–9. doi: 10.1016/j.breast.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Kuru B, Yuruker S, Sullu Y, et al. Does a Close Surgical Margin for Ductal Carcinoma In Situ Associated with Invasive Breast Carcinoma Affect Breast Cancer Recurrence? J Invest Surg. 2020;33:627–33. doi: 10.1080/08941939.2018.1539791. [DOI] [PubMed] [Google Scholar]
- 21.Livingston-Rosanoff D, Trentham-Dietz A, Hampton JM, et al. Does margin width impact breast cancer recurrence rates in women with breast conserving surgery for ductal carcinoma in situ? Breast Cancer Res Treat. 2021;189:463–70. doi: 10.1007/s10549-021-06278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannu GS, Wang Z, Broggio J, et al. Invasive breast cancer and breast cancer mortality after ductal carcinoma in situ in women attending for breast screening in England, 1988-2014: population based observational cohort study. BMJ. 2020;369:m1570. doi: 10.1136/bmj.m1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz RSJM, van den Belt-Dusebout AW, Clements K, et al. Association of DCIS size and margin status with risk of developing breast cancer post-treatment: multinational, pooled cohort study. BMJ. 2023;383:e076022. doi: 10.1136/bmj-2023-076022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaikh T, Li T, Murphy CT, et al. Importance of Surgical Margin Status in Ductal Carcinoma In Situ. Clin Breast Cancer. 2016;16:312–8. doi: 10.1016/j.clbc.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Van Zee KJ, Subhedar P, Olcese C, et al. Relationship Between Margin Width and Recurrence of Ductal Carcinoma In Situ: Analysis of 2996 Women Treated With Breast-conserving Surgery for 30 Years. Ann Surg. 2015;262:623–31. doi: 10.1097/SLA.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaloge S, Khan SA, Wesseling J, et al. Ductal carcinoma in situ of the breast: finding the balance between overtreatment and undertreatment. Lancet. 2024;403:2734–46. doi: 10.1016/S0140-6736(24)00425-2. [DOI] [PubMed] [Google Scholar]
- 27.Chua BH, Link EK, Kunkler IH, et al. Radiation doses and fractionation schedules in non-low-risk ductal carcinoma in situ in the breast (BIG 3-07/TROG 07.01): a randomised, factorial, multicentre, open-label, phase 3 study. Lancet. 2022;400:431–40. doi: 10.1016/S0140-6736(22)01246-6. [DOI] [PubMed] [Google Scholar]
- 28.Meattini I, Livi L, Franceschini D, et al. Role of radiotherapy boost in women with ductal carcinoma in situ: A single-center experience in a series of 389 patients. European Journal of Surgical Oncology (EJSO) 2013;39:613–8. doi: 10.1016/j.ejso.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Moran MS, Zhao Y, Ma S, et al. Association of Radiotherapy Boost for Ductal Carcinoma In Situ With Local Control After Whole-Breast Radiotherapy. JAMA Oncol. 2017;3:1060–8. doi: 10.1001/jamaoncol.2016.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omlin A, Amichetti M, Azria D, et al. Boost radiotherapy in young women with ductal carcinoma in situ: a multicentre, retrospective study of the Rare Cancer Network. Lancet Oncol. 2006;7:652–6. doi: 10.1016/S1470-2045(06)70765-3. [DOI] [PubMed] [Google Scholar]
- 31.George A, Stead TS, Ganti L. What’s the Risk: Differentiating Risk Ratios, Odds Ratios, and Hazard Ratios? Cureus. 2020;12:e10047. doi: 10.7759/cureus.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenzie DP, Thomas C. Relative risks and odds ratios: Simple rules on when and how to use them. Eur J Clin Invest. 2020:e13249. doi: 10.1111/eci.13249. [DOI] [PubMed] [Google Scholar]
- 33.Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J. 2008;101:730–4. doi: 10.1097/SMJ.0b013e31817a7ee4. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.StataCrop . College Station, TX: StataCorp LLC; Stata statistical software: release 18. [Google Scholar]
- 37.Ben-David MA, Sturtz DE, Griffith KA, et al. Long-term results of conservative surgery and radiotherapy for ductal carcinoma in situ using lung density correction: the University of Michigan experience. Breast J. 2007;13:392–400. doi: 10.1111/j.1524-4741.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 38.Cambra MJ, Farrús B, Moreno F, et al. Management of breast ductal carcinoma in situ in Catalonia, Spain: Results from the Grup Oncologic Calalà-Occità-Catalonia survey with 9-year follow up. Breast. 2017;35:196–202. doi: 10.1016/j.breast.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Co M, Kwong A. Ductal carcinoma in situ of the breast - Long term results from a twenty-year cohort. Cancer Treat Res Commun. 2018;14:17–20. doi: 10.1016/j.ctarc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Cutuli B, Cohen-Solal-Le Nir C, De Lafontan B, et al. Ductal carcinoma in situ of the breast results of conservative and radical treatments in 716 patients. Eur J Cancer. 2001;37:2365–72. doi: 10.1016/s0959-8049(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 41.Dick AW, Sorbero MS, Ahrendt GM, et al. Comparative effectiveness of ductal carcinoma in situ management and the roles of margins and surgeons. J Natl Cancer Inst. 2011;103:92–104. doi: 10.1093/jnci/djq499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher ER, Costantino J, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). The National Surgical Adjuvant Breast and Bowel Project Collaborating Investigators. Cancer. 1995;75:1310–9. doi: 10.1002/1097-0142(19950315)75:6<1310::aid-cncr2820750613>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Fowble B, Hanlon AL, Fein DA, et al. Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS) Int J Radiat Oncol Biol Phys. 1997;38:949–57. doi: 10.1016/s0360-3016(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 44.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–7. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 45.Halasz LM, Sreedhara M, Chen Y-H, et al. Improved outcomes of breast-conserving therapy for patients with ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2012;82:e581–6. doi: 10.1016/j.ijrobp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Hathout L, Hijal T, Théberge V, et al. Hypofractionated radiation therapy for breast ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2013;87:1058–63. doi: 10.1016/j.ijrobp.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Kim K, Kim JH, Kim YB, et al. Selective Radiation Therapy for Ductal Carcinoma In Situ Following Breast-Conserving Surgery According to Age and Margin Width: Korean Radiation Oncology Group 11-04 and 16-02 Studies. J Breast Cancer. 2017;20:327–32. doi: 10.4048/jbc.2017.20.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macdonald HR, Silverstein MJ, Lee LA, et al. Margin width as the sole determinant of local recurrence after breast conservation in patients with ductal carcinoma in situ of the breast. Am J Surg. 2006;192:420–2. doi: 10.1016/j.amjsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Mathew J, Karia R, Morgan DAL, et al. Factors influencing local control in patients undergoing breast conservation surgery for ductal carcinoma in situ. Breast. 2017;31:181–5. doi: 10.1016/j.breast.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Monteau A, Sigal-Zafrani B, Kirova YM, et al. Ductal carcinoma in situ of the breast with close or focally involved margins following breast-conserving surgery: treatment with reexcision or radiotherapy with increased dosage. Int J Radiat Oncol Biol Phys. 2009;75:1021–8. doi: 10.1016/j.ijrobp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Rudloff U, Brogi E, Reiner AS, et al. The influence of margin width and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Ann Surg. 2010;251:583–91. doi: 10.1097/SLA.0b013e3181b5931e. [DOI] [PubMed] [Google Scholar]
- 52.Shikama N, Sekiguchi K, Nakamura N, et al. Final results from a multicenter prospective study (JROSG 05-5) on postoperative radiotherapy for patients with ductal carcinoma in situ with an involved surgical margin or close margin widths of 1 mm or less. J Radiat Res. 2015;56:830–4. doi: 10.1093/jrr/rrv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solin LJ, Fourquet A, Vicini FA, et al. Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast. Cancer. 2005;103:1137–46. doi: 10.1002/cncr.20886. [DOI] [PubMed] [Google Scholar]
- 54.Sweldens C, Peeters S, van Limbergen E, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20:1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 55.Turaka A, Freedman GM, Li T, et al. Young age is not associated with increased local recurrence for DCIS treated by breast-conserving surgery and radiation. J Surg Oncol. 2009;100:25–31. doi: 10.1002/jso.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler-Henderson K, Lee AH, Lenzo NP, et al. Epidemiology of ductal carcinoma in situ in Western Australia: implications for surgical margins and management. Breast Cancer. 2015;22:641–7. doi: 10.1007/s12282-014-0531-5. [DOI] [PubMed] [Google Scholar]
- 57.Fregatti P, Gipponi M, Depaoli F, et al. No Ink on Ductal Carcinoma In Situ: A Single Centre Experience. Anticancer Res. 2019;39:459–66. doi: 10.21873/anticanres.13134. [DOI] [PubMed] [Google Scholar]
- 58.Neuschatz AC, DiPetrillo T, Safaii H, et al. Margin width as a determinant of local control with and without radiation therapy for ductal carcinoma in situ (DCIS) of the breast. Int J Cancer. 2001;96 Suppl:97–104. doi: 10.1002/ijc.10357. [DOI] [PubMed] [Google Scholar]
- 59.Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455–61. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 60.Solin LJ, Fourquet A, Vicini FA, et al. Mammographically detected ductal carcinoma in situ of the breast treated with breast-conserving surgery and definitive breast irradiation: long-term outcome and prognostic significance of patient age and margin status. Int J Radiat Oncol Biol Phys. 2001;50:991–1002. doi: 10.1016/s0360-3016(01)01517-6. [DOI] [PubMed] [Google Scholar]
- 61.Chasle J, Delozier T, Denoux Y, et al. Immunohistochemical study of cell cycle regulatory proteins in intraductal breast carcinomas--a preliminary study. Eur J Cancer. 2003;39:1363–9. doi: 10.1016/s0959-8049(02)00774-8. [DOI] [PubMed] [Google Scholar]
- 62.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–88. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narod SA, Iqbal J, Giannakeas V, et al. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015;1:888–96. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 64.Dunne C, Burke JP, Morrow M, et al. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–20. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 65.Holland R, Hendriks JH, Vebeek AL, et al. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990;335:519–22. doi: 10.1016/0140-6736(90)90747-s. [DOI] [PubMed] [Google Scholar]
- 66.Faverly DR, Burgers L, Bult P, et al. Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol. 1994;11:193–8. [PubMed] [Google Scholar]
- 67.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–6. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 68.Tang S-K, Kaptanis S, Haddow JB, et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur J Cancer. 2017;84:315–24. doi: 10.1016/j.ejca.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 69.Rakovitch E, Parpia S, Koch A, et al. DUCHESS: an evaluation of the ductal carcinoma in situ score for decisions on radiotherapy in patients with low/intermediate-risk DCIS. Breast Cancer Res Treat. 2021;188:133–9. doi: 10.1007/s10549-021-06187-7. [DOI] [PubMed] [Google Scholar]
- 70.Yerushalmi R, Sulkes A, Mishaeli M, et al. Radiation treatment for ductal carcinoma in situ (DCIS): is a boost to the tumor bed necessary? Neoplasma. 2006;53:507–10. [PubMed] [Google Scholar]
- 71.Dreyfuss AD, Max D, Flynn J, et al. Locoregional Control Benefit of a Tumor Bed Boost for Ductal Carcinoma In Situ. Adv Radiat Oncol. 2023;8:101254. doi: 10.1016/j.adro.2023.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viani GA, Stefano EJ, Afonso SL, et al. Breast-conserving surgery with or without radiotherapy in women with ductal carcinoma in situ: a meta-analysis of randomized trials. Radiat Oncol. 2007;2:28. doi: 10.1186/1748-717X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaidar-Person O, Offersen BV, Tramm T, et al. The King is in the altogether: Radiation therapy after oncoplastic breast surgery. Breast. 2023;72:103584. doi: 10.1016/j.breast.2023.103584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matar-Ujvary R, Haglich K, Flanagan MR, et al. The Impact of Breast-Conserving Surgery Re-excision on Patient-Reported Outcomes Using the BREAST-Q. Ann Surg Oncol. 2023;30:5341–9. doi: 10.1245/s10434-023-13592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 76.Kindts I, Laenen A, Depuydt T, et al. Tumour bed boost radiotherapy for women after breast-conserving surgery. Cochrane Database Syst Rev. 2017;11:CD011987. doi: 10.1002/14651858.CD011987.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collette S, Collette L, Budiharto T, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 “boost versus no boost”. Eur J Cancer. 2008;44:2587–99. doi: 10.1016/j.ejca.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 78.Hanson SE, Lei X, Roubaud MS, et al. Long-term Quality of Life in Patients With Breast Cancer After Breast Conservation vs Mastectomy and Reconstruction. JAMA Surg. 2022;157:e220631. doi: 10.1001/jamasurg.2022.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martins Maia C, Siderides C, Jaffer S, et al. Mastectomy or Margin Re-excision? A Nomogram for Close/Positive Margins After Lumpectomy for DCIS. Ann Surg Oncol. 2022;29:3740–8. doi: 10.1245/s10434-021-11293-3. [DOI] [PubMed] [Google Scholar]
- 80.Giannakeas V, Sopik V, Narod SA. Association of Radiotherapy With Survival in Women Treated for Ductal Carcinoma In Situ With Lumpectomy or Mastectomy. JAMA Netw Open. 2018;1:e181100. doi: 10.1001/jamanetworkopen.2018.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mannu GS, Wang Z, Dodwell D, et al. Invasive breast cancer and breast cancer death after non-screen detected ductal carcinoma in situ from 1990 to 2018 in England: population based cohort study. BMJ. 2024;384:e075498. doi: 10.1136/bmj-2023-075498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dodwell D, Clements K, Lawrence G, et al. Radiotherapy following breast-conserving surgery for screen-detected ductal carcinoma in situ: indications and utilisation in the UK. Interim findings from the Sloane Project. Br J Cancer. 2007;97:725–9. doi: 10.1038/sj.bjc.6603945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rakovitch E, Gray R, Baehner FL, et al. Refined estimates of local recurrence risks by DCIS score adjusting for clinicopathological features: a combined analysis of ECOG-ACRIN E5194 and Ontario DCIS cohort studies. Breast Cancer Res Treat. 2018;169:359–69. doi: 10.1007/s10549-018-4693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.