Abstract
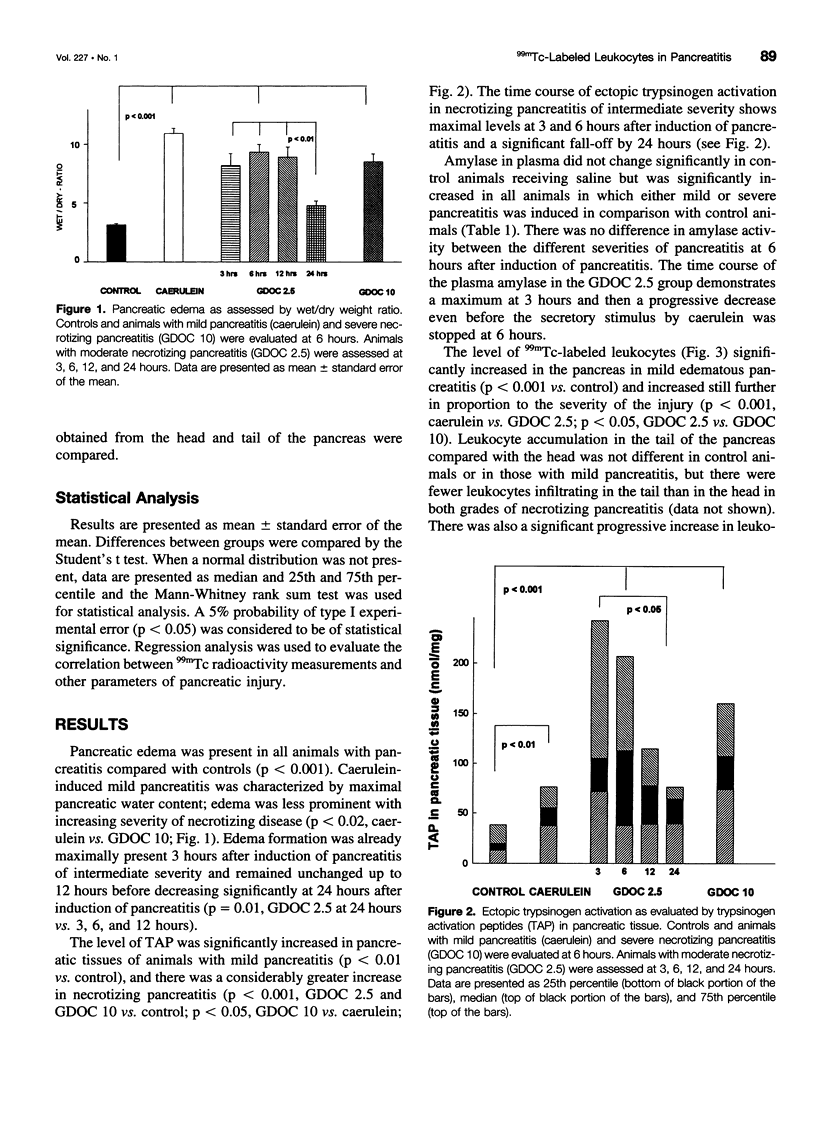
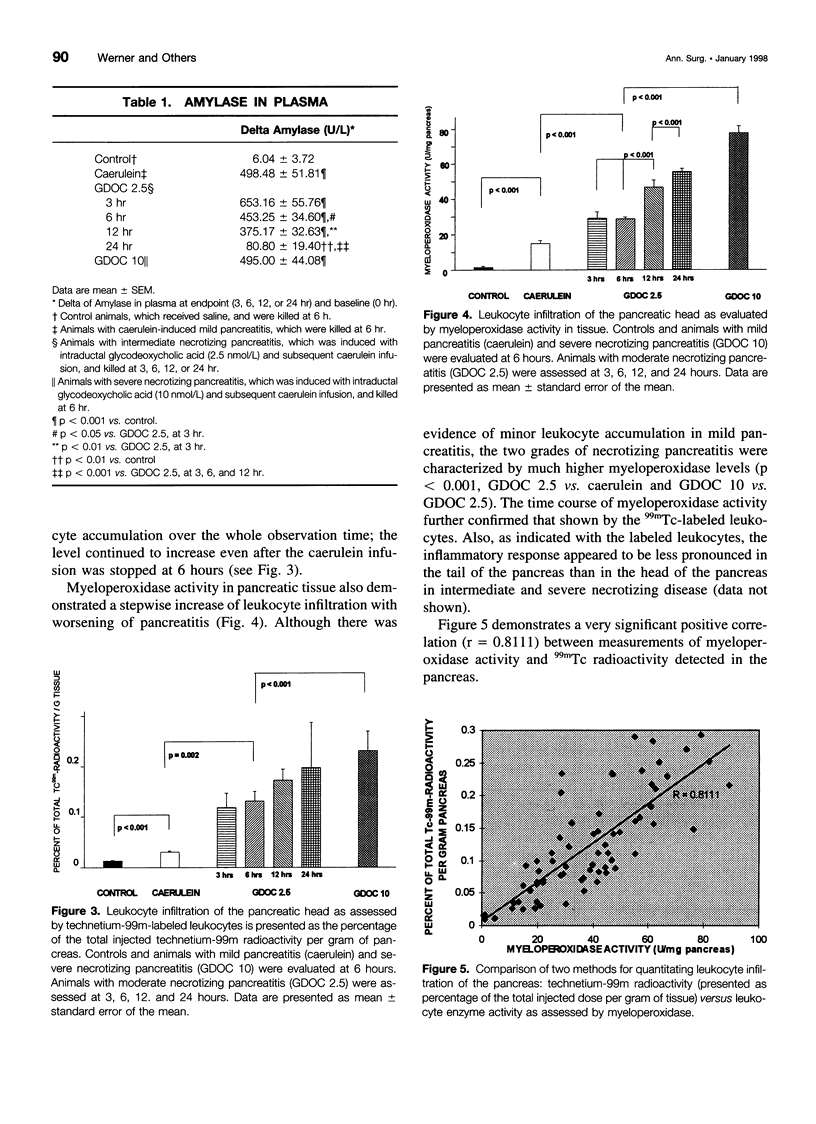
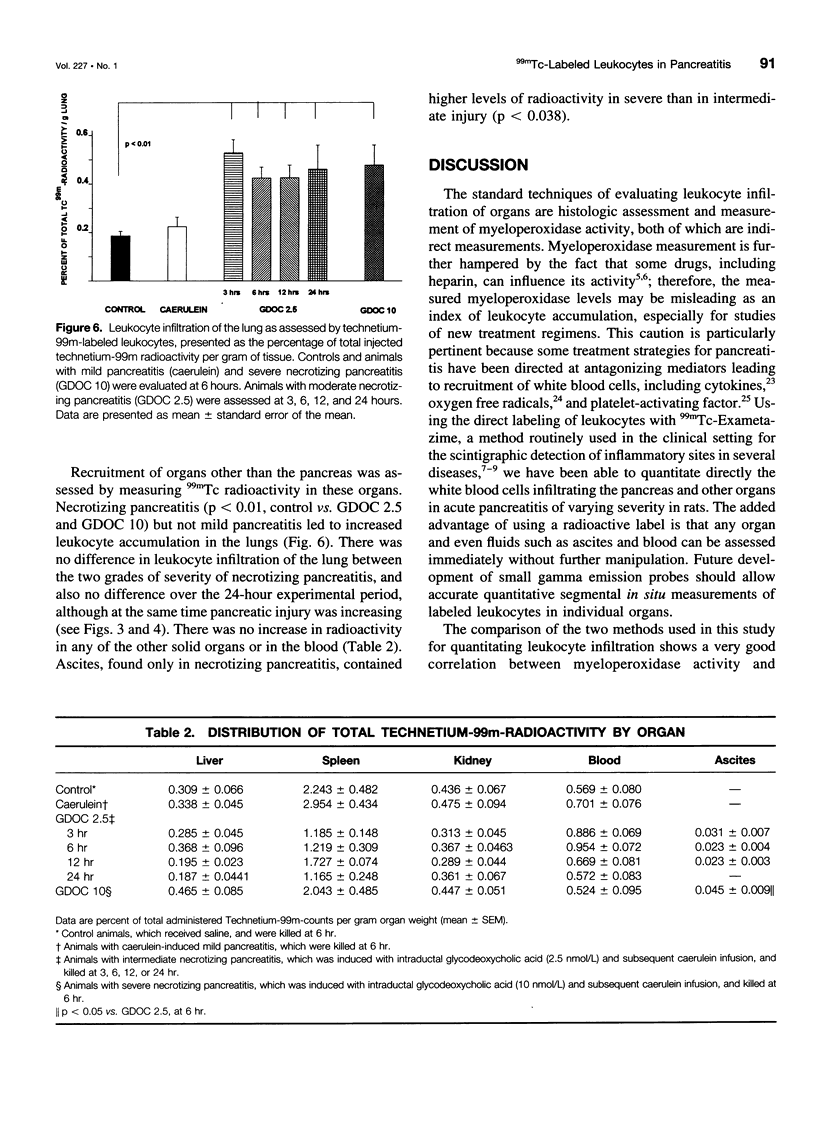
OBJECTIVE: We developed a new method to quantitate leukocyte accumulation in tissues and used it to examine the time course and severity of acute experimental pancreatitis. BACKGROUND: Leukocyte activation and infiltration are believed to be critical steps in the progression from mild to severe pancreatitis and responsible for many of its systemic complications. METHODS: Pancreatitis of graded severity was induced in Sprague-Dawley rats with a combination of caerulein and controlled intraductal infusion. Technetium-99m (99mTc)-labeled leukocytes were quantified in pancreas, lung, liver, spleen, and kidney and compared with myeloperoxidase activity. The severity of pancreatitis was ascertained by wet/dry weight ratio, plasma amylase, and trypsinogen activation peptide in the pancreas. The time course of leukocyte accumulation was determined over 24 hours. RESULTS: Pancreatic leukocyte infiltration correlated well with tissue myeloperoxidase concentrations. In mild pancreatitis, leukocytes accumulated only in the pancreas. Moderate and severe pancreatitis were characterized by much greater leukocyte infiltration in the pancreas than in mild disease (p < 0.01), and increased 99mTc radioactivity was detectable in the lung as early as 3 hours. 99mTc radioactivity correlated directly with the three levels of pancreatitis. CONCLUSIONS: Mild pancreatitis is characterized by low-level leukocyte activation and accumulation in the pancreas without recruitment of other organs; marked leukocyte accumulation was found in the pancreas and in the lung in more severe grades of pancreatitis. These findings provide a basis for the pathophysiologic production of cytokines and oxygen free radicals, which potentiate organ injury in severe pancreatitis. This study validates a new tool to study local and systemic effects of leukocytes in pancreatitis as well as new therapeutic hypotheses.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ais G., López-Farre A., Gomez-Garre D. N., Novo C., Romeo J. M., Braquet P., López-Novoa J. M. Role of platelet-activating factor in hemodynamic derangements in an acute rodent pancreatic model. Gastroenterology. 1992 Jan;102(1):181–187. doi: 10.1016/0016-5085(92)91799-a. [DOI] [PubMed] [Google Scholar]
- Almer S., Bodemar G., Lindström E., Peters A. M., Ström M. Air enema radiology compared with leukocyte scintigraphy for imaging inflammation in active ulcerative colitis. Eur J Gastroenterol Hepatol. 1995 Jan;7(1):59–64. [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. II. Cytochemistry and electron microscopy of bone marrow cells. J Cell Biol. 1968 Nov;39(2):299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow S. A., Graham W., Jyawook S., Dragotakes S. C., Solomon H. F., Babich J. W., Rubin R. H., Fischman A. J. Localization of indium-111-immunoglobulin G, technetium-99m-immunoglobulin G and indium-111-labeled white blood cells at sites of acute bacterial infection in rabbits. J Nucl Med. 1993 Nov;34(11):1975–1979. [PubMed] [Google Scholar]
- Basran G. S., Ramasubramanian R., Verma R. Intrathoracic complications of acute pancreatitis. Br J Dis Chest. 1987 Oct;81(4):326–331. doi: 10.1016/0007-0971(87)90180-x. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Danpure H. J., Osman S., Carroll M. J. The development of a clinical protocol for the radiolabelling of mixed leucocytes with 99Tcm-hexamethylpropyleneamine oxime. Nucl Med Commun. 1988 Jun;9(6):465–475. doi: 10.1097/00006231-198806000-00010. [DOI] [PubMed] [Google Scholar]
- Fernández-del Castillo C., Schmidt J., Warshaw A. L., Rattner D. W. Interstitial protease activation is the central event in progression to necrotizing pancreatitis. Surgery. 1994 Sep;116(3):497–504. [PubMed] [Google Scholar]
- Foitzik T., Hotz H. G., Schmidt J., Klar E., Warshaw A. L., Buhr H. J. Effect of microcirculatory perfusion on distribution of trypsinogen activation peptides in acute experimental pancreatitis. Dig Dis Sci. 1995 Oct;40(10):2184–2188. doi: 10.1007/BF02209003. [DOI] [PubMed] [Google Scholar]
- Gaboury J. P., Anderson D. C., Kubes P. Molecular mechanisms involved in superoxide-induced leukocyte-endothelial cell interactions in vivo. Am J Physiol. 1994 Feb;266(2 Pt 2):H637–H642. doi: 10.1152/ajpheart.1994.266.2.H637. [DOI] [PubMed] [Google Scholar]
- Gross V., Leser H. G., Heinisch A., Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993 Dec;40(6):522–530. [PubMed] [Google Scholar]
- Gudgeon A. M., Heath D. I., Hurley P., Jehanli A., Patel G., Wilson C., Shenkin A., Austen B. M., Imrie C. W., Hermon-Taylor J. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990 Jan 6;335(8680):4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Johnson K. J., Kunkel R. G., Morganroth M. L., Ward P. A. Pancreatitis-induced acute lung injury. An ARDS model. Ann Surg. 1988 Jul;208(1):71–77. doi: 10.1097/00000658-198807000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House S. D., Lipowsky H. H. Leukocyte-endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc Res. 1987 Nov;34(3):363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- Hurley P. R., Cook A., Jehanli A., Austen B. M., Hermon-Taylor J. Development of radioimmunoassays for free tetra-L-aspartyl-L-lysine trypsinogen activation peptides (TAP). J Immunol Methods. 1988 Jul 22;111(2):195–203. doi: 10.1016/0022-1759(88)90127-5. [DOI] [PubMed] [Google Scholar]
- Klar E., Messmer K., Warshaw A. L., Herfarth C. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990 Nov;77(11):1205–1210. doi: 10.1002/bjs.1800771104. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Waltersdorph A. M., Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- Knoefel W. T., Kollias N., Warshaw A. L., Waldner H., Nishioka N. S., Rattner D. W. Pancreatic microcirculatory changes in experimental pancreatitis of graded severity in the rat. Surgery. 1994 Nov;116(5):904–913. [PubMed] [Google Scholar]
- Lantto E. H., Lantto T. J., Vorne M. Fast diagnosis of abdominal infections and inflammations with technetium-99m-HMPAO labeled leukocytes. J Nucl Med. 1991 Nov;32(11):2029–2034. [PubMed] [Google Scholar]
- Liras G., Carballo F. An impaired phagocytic function is associated with leucocyte activation in the early stages of severe acute pancreatitis. Gut. 1996 Jul;39(1):39–42. doi: 10.1136/gut.39.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus B. C., Wyble C. W., Hynes K. L., Gewertz B. L. Cytokine-induced increases in endothelial permeability occur after adhesion molecule expression. Surgery. 1996 Aug;120(2):411–417. doi: 10.1016/s0039-6060(96)80317-5. [DOI] [PubMed] [Google Scholar]
- Milani Júnior R., Pereira P. M., Dolhnikoff M., Saldiva P. H., Martins M. A. Respiratory mechanics and lung morphometry in severe pancreatitis-associated acute lung injury in rats. Crit Care Med. 1995 Nov;23(11):1882–1889. doi: 10.1097/00003246-199511000-00015. [DOI] [PubMed] [Google Scholar]
- Mithöfer K., Fernández-del Castillo C., Frick T. W., Lewandrowski K. B., Rattner D. W., Warshaw A. L. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995 Jul;109(1):239–246. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Montravers P., Chollet-Martin S., Marmuse J. P., Gougerot-Pocidalo M. A., Desmonts J. M. Lymphatic release of cytokines during acute lung injury complicating severe pancreatitis. Am J Respir Crit Care Med. 1995 Nov;152(5 Pt 1):1527–1533. doi: 10.1164/ajrccm.152.5.7582288. [DOI] [PubMed] [Google Scholar]
- Murakami H., Nakao A., Kishimoto W., Nakano M., Takagi H. Detection of O2- generation and neutrophil accumulation in rat lungs after acute necrotizing pancreatitis. Surgery. 1995 Sep;118(3):547–554. doi: 10.1016/s0039-6060(05)80372-1. [DOI] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Borchard F., Ude K., Lüthen R., Strohmeyer G., Ferrell L. D., Grendell J. H. Effects of antioxidants and free radical scavengers in three different models of acute pancreatitis. Pancreas. 1992;7(4):486–496. doi: 10.1097/00006676-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Norman J. G., Fink G., Franz M., Guffey J., Carter G., Davison B., Sexton C., Glaccum M. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg. 1996 Feb;223(2):163–169. doi: 10.1097/00000658-199602000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988 Mar;3(2-3):105–112. doi: 10.1007/BF02798921. [DOI] [PubMed] [Google Scholar]
- Robertson C. S., Basran G. S., Hardy J. G. Lung vascular permeability in patients with acute pancreatitis. Pancreas. 1988;3(2):162–165. doi: 10.1097/00006676-198804000-00009. [DOI] [PubMed] [Google Scholar]
- Saluja A., Saito I., Saluja M., Houlihan M. J., Powers R. E., Meldolesi J., Steer M. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol. 1985 Dec;249(6 Pt 1):G702–G710. doi: 10.1152/ajpgi.1985.249.6.G702. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Usami S., Skalak R., Chien S. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc Res. 1980 Jan;19(1):45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Fernández-del Castillo C., Rattner D. W., Lewandrowski K., Compton C. C., Warshaw A. L. Trypsinogen-activation peptides in experimental rat pancreatitis: prognostic implications and histopathologic correlates. Gastroenterology. 1992 Sep;103(3):1009–1016. doi: 10.1016/0016-5085(92)90036-x. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Lewandrowsi K., Warshaw A. L., Compton C. C., Rattner D. W. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int J Pancreatol. 1992 Aug;12(1):41–51. doi: 10.1007/BF02927069. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Rattner D. W., Lewandrowski K., Compton C. C., Mandavilli U., Knoefel W. T., Warshaw A. L. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992 Jan;215(1):44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölmerich J., Schümichen C., Lausen M., Gross V., Leser H. G., Lay L., Farthmann E. H., Gerok W. Scintigraphic assessment of leukocyte infiltration in acute pancreatitis using technetium-99m-hexamethyl propylene amine oxine as leukocyte label. Dig Dis Sci. 1991 Jan;36(1):65–70. doi: 10.1007/BF01300089. [DOI] [PubMed] [Google Scholar]
- Simms H. H., D'Amico R. Polymorphonuclear leukocyte dysregulation during the systemic inflammatory response syndrome. Blood. 1994 Mar 1;83(5):1398–1407. [PubMed] [Google Scholar]
- Uetrecht J. P. Myeloperoxidase as a generator of drug free radicals. Biochem Soc Symp. 1995;61:163–170. doi: 10.1042/bss0610163. [DOI] [PubMed] [Google Scholar]
- Videm V. Heparin in clinical doses 'primes' granulocytes to subsequent activation as measured by myeloperoxidase release. Scand J Immunol. 1996 Apr;43(4):385–390. doi: 10.1046/j.1365-3083.1996.d01-57.x. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Werner J., Rivera J., Fernandez-del Castillo C., Lewandrowski K., Adrie C., Rattner D. W., Warshaw A. L. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery. 1997 Jan;121(1):23–30. doi: 10.1016/s0039-6060(97)90178-1. [DOI] [PubMed] [Google Scholar]
- Willemer S., Feddersen C. O., Karges W., Adler G. Lung injury in acute experimental pancreatitis in rats. I. Morphological studies. Int J Pancreatol. 1991 May;8(4):305–321. doi: 10.1007/BF02952723. [DOI] [PubMed] [Google Scholar]
- Willemer S., Klöppel G., Kern H. F., Adler G. Immunocytochemical and morphometric analysis of acinar zymogen granules in human acute pancreatitis. Virchows Arch A Pathol Anat Histopathol. 1989;415(2):115–123. doi: 10.1007/BF00784348. [DOI] [PubMed] [Google Scholar]
- de Beaux A. C., Ross J. A., Maingay J. P., Fearon K. C., Carter D. C. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br J Surg. 1996 Aug;83(8):1071–1075. doi: 10.1002/bjs.1800830811. [DOI] [PubMed] [Google Scholar]


