Abstract
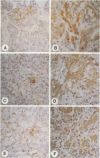
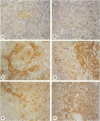
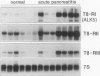
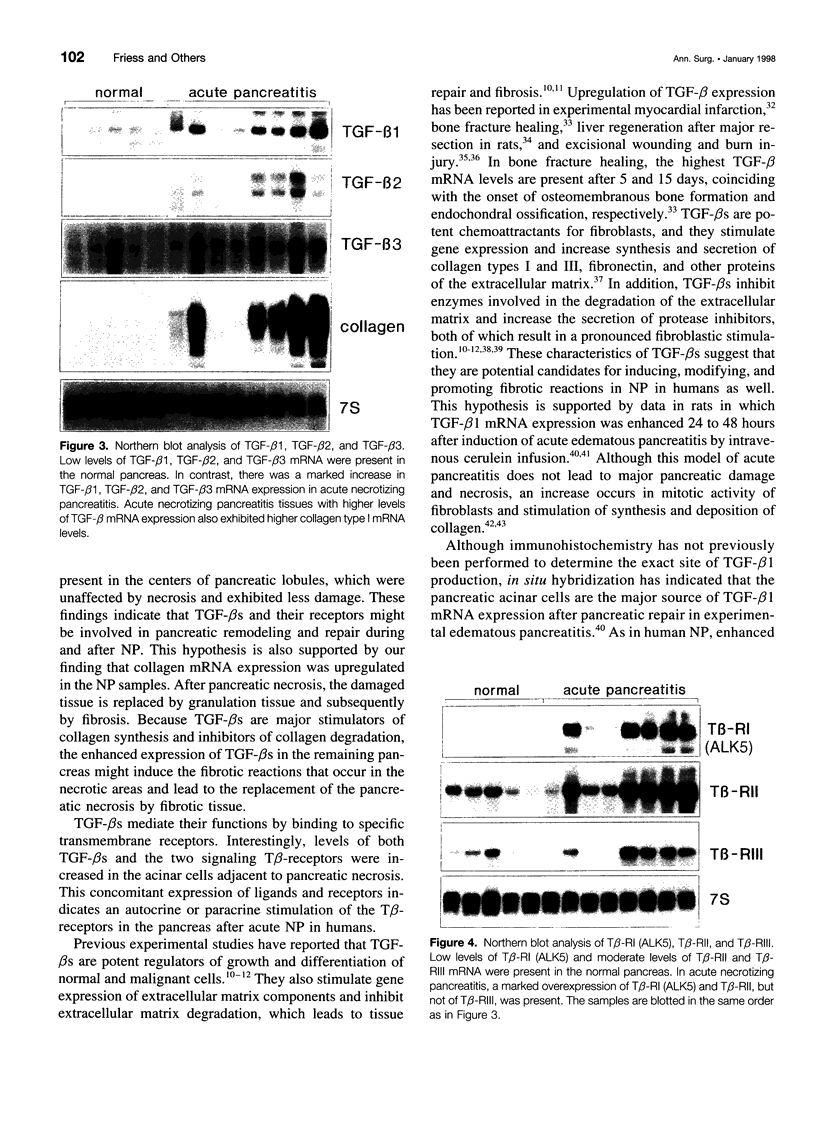
OBJECTIVES: To determine which mechanisms are involved in pancreatic remodeling, repair, and fibrosis after acute necrotizing pancreatitis (NP) in humans. SUMMARY BACKGROUND DATA: Transforming growth factor betas (TGF-betas) are multifunctional polypeptides that have been implicated in the regulation and formation of extracellular matrix and fibrosis. They exert their functions by binding to specific receptors. In this study, we analyze the expression of TGF-beta1, TGF-beta2, and TGF-beta3 and their receptors type I (Tbeta-RI [ALK5]), type II (Tbeta-RII), and type III (Tbeta-RIII) in NP. PATIENTS: Pancreatic tissue samples were obtained from 6 female and 8 male patients with a median age of 65 years (range, 37 to 77 years) undergoing surgery for NP. The median Ranson score of the patients was 6 (range, 2 to 9). The operation was performed a median 5.5 days (range, 4 to 17 days) after the onset of acute pancreatitis. Pancreatic tissue obtained from 12 previously healthy organ donors (6 male, 6 female; median age of 43 years) served as controls. METHODS: The expression of TGF-beta1, TGF-beta2, TGF-beta3, Tbeta-RI (ALK5), Tbeta-RII, Tbeta-RIII, and collagen type I mRNA was analyzed by Northern blot analysis. In addition, immunohistochemical analysis using polyclonal antibodies was performed to detect TGF-beta1, TGF-beta2, TGF-beta3, Tbeta-RI (ALK5), and Tbeta-RII. RESULTS: Northern blot analysis showed an increase in TGF-betas and their receptors in NP tissue samples compared with samples from normal controls. The increase was 3.5-fold for TGF-beta1 (p < 0.05), 2.7-fold for TGF-beta2 (p < 0.05), 3.5-fold for TGF-beta3 (p < 0.05), 10-fold for Tbeta-RI (ALK5) (p < 0.05), 5.7-fold for Tbeta-RII (p < 0.05), and 1.4-fold for Tbeta-RIII (not significant). Collagen type I mRNA was also markedly increased in NP samples and correlated with the level of TGF-betas. Immunohistochemical analysis demonstrated intense TGF-beta1, TGF-beta2, TGF-beta3, Tbeta-RI (ALK5), and Tbeta-RII immunoreactivity in the remaining acinar and ductal cells in most NP samples; in the normal control pancreas, there was weak to moderate immunoreactivity for these factors only in some acinar cells and a few ductal cells. CONCLUSION: The marked increase in expression of TGF-betas and their signaling receptors Tbeta-RI (ALK5) and Tbeta-RII suggests a role for TGF-betas in the repair process after the onset of NP in humans and raises the possibility that TGF-betas might be involved in tissue remodeling and the fibrotic reaction that occurs in the pancreas after necrosis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Hupp T., Kern H. F. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch A Pathol Anat Histol. 1979 May 14;382(1):31–47. doi: 10.1007/BF01102739. [DOI] [PubMed] [Google Scholar]
- Aho H. J., Ahola R. A., Tolvanen A. M., Nevalainen T. J. Experimental pancreatitis in the rat. Changes in pulmonary phospholipids during sodium taurocholate-induced acute pancreatitis. Res Exp Med (Berl) 1983;182(1):79–84. doi: 10.1007/BF01852290. [DOI] [PubMed] [Google Scholar]
- Aho H. J., Koskensalo S. M., Nevalainen T. J. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15(4):411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- Attisano L., Cárcamo J., Ventura F., Weis F. M., Massagué J., Wrana J. L. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993 Nov 19;75(4):671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Friess H., Yokoyama M., Lopez M. E., Kobrin M. S., Büchler M. W., Korc M. Attenuated ALK5 receptor expression in human pancreatic cancer: correlation with resistance to growth inhibition. Int J Cancer. 1996 Jul 17;67(2):283–288. doi: 10.1002/(SICI)1097-0215(19960717)67:2<283::AID-IJC21>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Becker V. Pathological anatomy and pathogenesis of acute pancreatitis. World J Surg. 1981 May;5(3):303–313. doi: 10.1007/BF01657981. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Paradis V. Transforming growth factor-beta (TGF-beta): a key-role in liver fibrogenesis. J Hepatol. 1995;22(2 Suppl):37–42. [PubMed] [Google Scholar]
- Border W. A., Noble N. A. Fibrosis linked to TGF-beta in yet another disease. J Clin Invest. 1995 Aug;96(2):655–656. doi: 10.1172/JCI118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Noble N. A. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994 Nov 10;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Border W. A. Transforming growth factor-beta and the pathogenesis of glomerular diseases. Curr Opin Nephrol Hypertens. 1994 Jan;3(1):54–58. doi: 10.1097/00041552-199401000-00007. [DOI] [PubMed] [Google Scholar]
- Bozkurt T., Maroske D., Adler G. Exocrine pancreatic function after recovery from necrotizing pancreatitis. Hepatogastroenterology. 1995 Feb;42(1):55–58. [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield A. P., Cooper M. J., Williamson R. C. Acute pancreatitis: a lethal disease of increasing incidence. Gut. 1985 Jul;26(7):724–729. doi: 10.1136/gut.26.7.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrin B., Butcher D., McAnulty B. J., Dubois R. M., Black C. M., Laurent G. J., Harrison N. K. Immunohistochemical localization of transforming growth factor-beta 1 in the lungs of patients with systemic sclerosis, cryptogenic fibrosing alveolitis and other lung disorders. Histopathology. 1994 Feb;24(2):145–150. doi: 10.1111/j.1365-2559.1994.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas. 1989;4(2):169–178. doi: 10.1097/00006676-198904000-00005. [DOI] [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas. 1986;1(5):421–429. doi: 10.1097/00006676-198609000-00006. [DOI] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Beger H. G., Do D. A., Kobrin M. S., Korc M. Increased expression of acidic and basic fibroblast growth factors in chronic pancreatitis. Am J Pathol. 1994 Jan;144(1):117–128. [PMC free article] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Berger H. G., Kobrin M. S., Baldwin R. L., Korc M. Enhanced expression of the type II transforming growth factor beta receptor in human pancreatic cancer cells without alteration of type III receptor expression. Cancer Res. 1993 Jun 15;53(12):2704–2707. [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Ebert M., Beger H. G., Gold L. I., Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993 Dec;105(6):1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- Gress T., Müller-Pillasch F., Elsässer H. P., Bachem M., Ferrara C., Weidenbach H., Lerch M., Adler G. Enhancement of transforming growth factor beta 1 expression in the rat pancreas during regeneration from caerulein-induced pancreatitis. Eur J Clin Invest. 1994 Oct;24(10):679–685. doi: 10.1111/j.1365-2362.1994.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Isenmann R., Büchler M. W. Infection and acute pancreatitis. Br J Surg. 1994 Dec;81(12):1707–1708. doi: 10.1002/bjs.1800811203. [DOI] [PubMed] [Google Scholar]
- Joyce M. E., Terek R. M., Jingushi S., Bolander M. E. Role of transforming growth factor-beta in fracture repair. Ann N Y Acad Sci. 1990;593:107–123. doi: 10.1111/j.1749-6632.1990.tb16104.x. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Levine J. H., Moses H. L., Gold L. I., Nanney L. B. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993 Aug;143(2):368–380. [PMC free article] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Wrana J. L., Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993 Jul 2;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Laiho M., Ralph D. A., Weis F. M., Zentella A. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Xu J., Wang F., McKeehan W. L., Krummen L., Kan M. A widely expressed transmembrane serine/threonine kinase that does not bind activin, inhibin, transforming growth factor beta, or bone morphogenic factor. J Biol Chem. 1993 Jun 15;268(17):12719–12723. [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesle E., Friess H., Zhao L., Wagner M., Uhl W., Baczako K., Gold L. I., Korc M., Büchler M. W. Increased expression of transforming growth factor beta s after acute oedematous pancreatitis in rats suggests a role in pancreatic repair. Gut. 1997 Jan;40(1):73–79. doi: 10.1136/gut.40.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana A., Saxena B., Noble N. A., Gold L. I., Marshall B. C. Increased expression of transforming growth factor beta isoforms (beta 1, beta 2, beta 3) in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1995 Jul;13(1):34–44. doi: 10.1165/ajrcmb.13.1.7541221. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Compton C. C., Rattner D. W., Lewandrowski K., Warshaw A. L. Late histopathologic changes and healing in an improved rodent model of acute necrotizing pancreatitis. Digestion. 1995;56(3):246–252. doi: 10.1159/000201251. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Wong H. L., Wahl S. M. Selective induction of Fc gamma RIII on human monocytes by transforming growth factor-beta. J Immunol. 1990 May 1;144(9):3444–3448. [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Zhang K., Garner W., Cohen L., Rodriguez J., Phan S. Increased types I and III collagen and transforming growth factor-beta 1 mRNA and protein in hypertrophic burn scar. J Invest Dermatol. 1995 May;104(5):750–754. doi: 10.1111/1523-1747.ep12606979. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Ichijo H., Franzén P., Laiho M., Miyazono K., Heldin C. H. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994 Apr 1;264(5155):101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]