Abstract
Background
Unexplained cardiac arrest is often attributed to a patient’s underlying disease. However, it is well known that an autopsy can reveal pathologies that were not noticed before death.
Case Summary
Multiple micro-scars (MMS) found in the myocardium of 3 patients who died of unexplained cardiac arrest were presented at our clinicopathology conference. Upon review of the clinical record, patients with MMS before death had arrhythmia (ie, atrial fibrillation and nonsustained ventricular tachycardia, including new onset). Interestingly, MMS were found in the left ventricle, the junction of the pulmonary vein and left atrium, and the right ventricle and right atrium. All 3 patients had histories of COVID-19 booster vaccination, and 1 of the 3 patients had a history of COVID-19.
Discussion
For patients with unexplained cardiac arrest complicated with arrhythmia, cardiac MMS is given as the differential background disease.
Key Words: acute heart failure, cardiomyopathy, thrombosis
Visual Summary
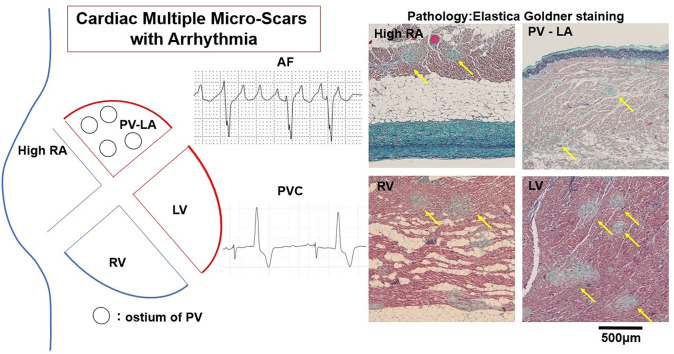
During clinicopathology conferences at our hospital from August 2023 to April 2024, multiple micro-scars (MMS) were observed in the myocardium by hematoxylin and eosin stain followed by elastica-Goldner stain (Figure 1) in 3 pathologic specimens from patients with cardiac arrest. At our clinicopathology conferences spanning about 30 years, MMS in the myocardium, other than in cases of myocardial infarction, had never been observed. Moreover, there had been no reports of MMS in the myocardium by pathology as far as we were able to find. Reports of myocardial scars other than those resulting from myocardial infarction are cardiac scars detected by magnetic resonance imaging (MRI) in patients associated with COVID-19.1, 2, 3 However, the areas diagnosed as scarring seen on MRI are of a certain size, and MRI could not clearly detect even microscopic scarring (ie, diagnosis using imaging technology is inferior to pathologic diagnosis for micro-scars). Therefore, we describe a detailed investigation of cardiac MMS by pathologic diagnosis in patients with cardiac arrest.
Take-Home Messages
-
•
An autopsy study showed that cardiac MMS were detected in patients who died of unexplained cardiac arrest and who had arrhythmias before death.
-
•
For patients with unexplained cardiac arrest complicated with arrhythmia, cardiac MMS is given as the differential background disease.
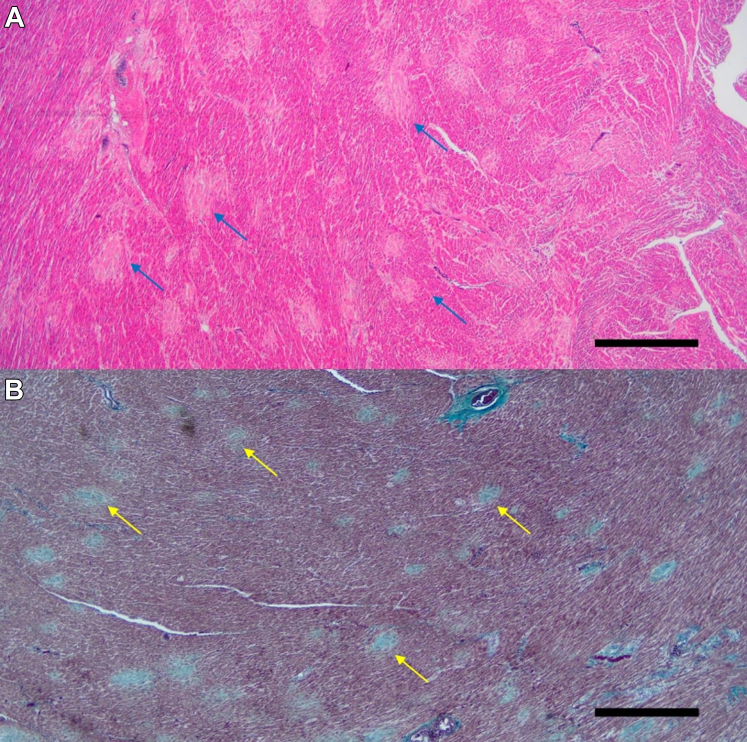
Figure 1.
Multiple Micro-Scars in the Left Ventricle
Representative pathologic image of the left ventricular myocardium with hematoxylin and eosin (A) and elastica-Goldner (B) staining in a patient with unexplained cardiac arrest. Round, slightly blurred scars (blue arrows in A) and stained light blue (yellow arrows in B) are seen at approximately similar intervals. Scale bar, 1 mm.
Patient 1
The patient was a 75-year-old woman who had been attending our hospital for bronchiectasis. She had been transported by ambulance to the emergency department because of cardiopulmonary arrest. Cardiopulmonary resuscitation was immediately initiated, and return of spontaneous circulation was restored after 3 cycles of pulse checks. Twelve-lead electrocardiography showed no signs of ischemic heart disease but revealed premature ventricular contraction and nonsustained ventricular tachycardia. Her cardiac enzymes were within the normal limits on blood testing. Her left ventricular ejection fraction was maintained. After admission, the patient underwent bronchoscopy with blood pressure support with catecholamines. On bronchoscopy, a blood clot was aspirated from the left lower lobe, but no active bleeding was noted. Despite treatment with oxygen and intravenous catecholamines, the patient subsequently developed multiorgan failure, and she went into cardiac arrest and died. The patient was in cardiopulmonary arrest when she arrived at the hospital, the cause of which was not clear, and although she subsequently developed multiple organ failure, the appearance of new ventricular arrhythmias, the cause of the cardiac arrest, and other aspects of her condition were unclear. Therefore, we explained the situation to the family and obtained their consent to perform an autopsy.
Patient 2
The patient was a 91-year-old woman who had been hospitalized for heart failure 2 years earlier and was rehospitalized because of worsening heart failure. She had been prescribed cardioprotective drugs since her last discharge. No apparent cause for the heart failure could be identified during the first admission. Upon second admission, the patient presented with new-onset atrial fibrillation. The left ventricular ejection fraction was maintained, and heart rate was controlled at about 60 beats/min. Despite treatment with oxygen, intravenous diuretic agents, and catecholamines, the patient did not respond well, and her urine output decreased. After consulting with her family, a policy of palliative care was chosen. Thereafter, on the 12th day after admission, her blood pressure dropped, and she went into cardiac arrest and died. We explained that the patient was elderly, but the background disease of heart failure was unknown, and that by donating the body, we could contribute to the advancement of medical science if we could find even a partial cause of these diseases, and the family gave their consent for an autopsy.
Patient 3
The patient was a 73-year-old man who had been receiving 3 courses of chemotherapy using cyclophosphamide, doxorubicin, vincristine, and prednisone for T-cell lymphoma with subcutaneous nodules at our hospital. His nodules disappeared, and the patient was discharged from the hospital. After 1 year and 3 months, subcutaneous nodules reappeared, and he was admitted to the hospital for a fourth course of chemotherapy. Gemcitabine, dexamethasone, and cisplatin therapy was started, but 1 week later, the patient developed a fever in the 38 °C range and was diagnosed with COVID-19. His general condition worsened because of COVID-19 with a lymphoma flare-up, and arrhythmias were recorded on electrocardiographic monitoring, so the family agreed to palliative care. His general condition deteriorated further, and he went into cardiac arrest. The patient’s family requested that an autopsy be performed if it would be more useful for the development of medicine and medical education than investigating the cause of death, as the patient had died of cancer.
We obtained written informed consent from all 3 patients or their families to perform the autopsies. This work was conducted in accordance with the Declaration of Helsinki.
Characteristics of the 3 patients are shown in Table 1. All patients had booster COVID-19 vaccination histories, having received up to 6 COVID-19 vaccinations. The diagnosis of COVID-19 infection was made by testing the patient’s saliva or nasopharyngeal swab upon admission using both the Takara SARS-CoV-2 direct polymerase chain reaction detection kit (Takara Bio) and SARS-CoV-Ag (Roch Diagnostics Japan).
Table 1.
Patient Characteristics
| Patien 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Sex | Female | Female | Male |
| Age, y | 75 | 91 | 73 |
| Heart weight, g | 255 | 500 | 330 |
| COVID-19 vaccinations | 5 | 5 | 6 |
| History of COVID-19 | − | − | + |
| CTR, % | 59 | 59 | 51 |
| LVEF, % | 65 | 58 | 65 |
| PAF/PAC | + | + | + |
| PVC/VT | − | + | + |
| BNP, pg/dL | 116 | 127 | NA |
| Cre, mg/dl | 0.77 | 2.47 | 0.63 |
| Platelet, ×103/μL | 237 | 157 | 200 |
| Cause of death | Not determined | Not determined | Lymphoma |
| Malignancy | None | HCC | Lymphoma |
BNP = brain natriuretic peptide; Cre = creatinine; CTR = cardiothoracic ratio; HCC = hepatocellular carcinoma; LVEF = left ventricular ejection fraction; NA = not available; PAC = premature atrial contraction; PAF = paroxysmal atrial fibrillation; PVC = premature ventricular contraction, VT = ventricular tachycardia.
In pathologic analyses for the 3 patients, the organs searched for pathology were the heart, lung, liver, kidney, and muscle, using standard methods. Regarding the heart, both the right and left ventricles, the high right atrium, and the junction of the pulmonary vein and left atrium and epicardial coronary artery were examined. On autopsy, macroscopic findings revealed no major abnormalities in the myocardium. There were no significant stenoses or occlusive lesions in the epicardial coronary arteries in the respective hearts. However, microscopic findings unexpectedly revealed MMS in the myocardium by hematoxylin and eosin stain and by elastica-Goldner stain (Figure 1). Imaging by elastica-Goldner staining showed mottled, evenly distributed, slightly edematous, pale blue scarring of the same aspect throughout the myocardium of both ventricles, and these scars were observed to be similarly spaced. Direct fast scarlet staining was performed to differentiate cardiac amyloidosis due to the presence of microscarring in the myocardium and no amyloid deposition was observed in the micro-scars in all 3 cases (Figure 2). For the MMS, to identify and understand the characteristics of the disease state, the circular scars in the left ventricular myocardium delineated by elastica-Goldner staining were manually traced one by one, and their maximum and minimum diameters were determined (Figure 3). From these values, the area of each scar was calculated. The distance from the center of the scar to the adjacent scars was measured, and the average was calculated. To represent the distribution of scars, we measured and compared the number of scars on the anterior, lateral, and posterior walls in left ventricle and septal and right ventricle wall of the heart, as well as on the endocardial and epicardial sides of the myocardium. To observe hemolysis in the microvessel, the capillary system was examined in each organ.
Figure 2.
Elastica-Goldner Staining and Direct Fast Scarlet Staining in Micro-Scars
(A) Micro-scars of the left ventricular myocardium by elastica-Goldner staining (arrows). (B) No amyloid deposition was observed in the microscar area by direct fast scarlet staining. Scale bar, 500 μm.
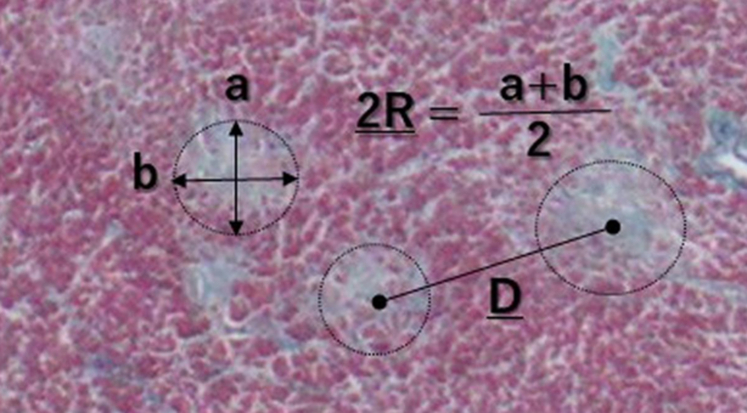
Figure 3.
Diagram of the Scars
Diagram of the scar shown in Figure 1B. Circular scars were traced, their minimum (A) and maximum (B) diameters were measured, and average diameter of the scar was calculated as 2R. A representation of the center-to-center distance (D) between adjacent scars is also shown.
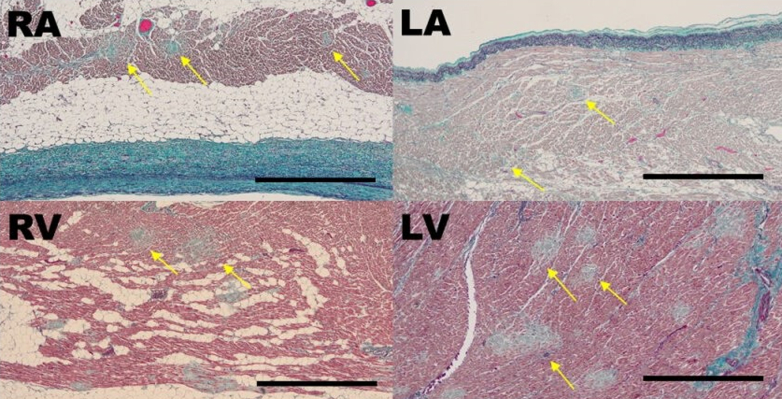
MMS were observed in both the right and left ventricles, the high right atrium, and the junction of the pulmonary vein and left atrium. Representative pathologic images in patient 2 are shown in Figure 4. A high-power field image of the heart shows fragmented erythrocytes in capillary vessels (Figure 5). αCD42b was not stained with all myocardia. For other organs, including the lung and kidney, no MMS were observed in all patients. Regarding micro-scars in the left ventricle, 64 scars were traced, and their average diameter was 211 μm, the average of their area was 143,810 μm2, and the average of their distance was 383 μm. Nevertheless, when the distribution of the scar within the myocardium was measured in the anterior, lateral, and posterior walls of the left ventricle, septum, and right ventricle, there was a tendency for MMS to be more common on the endocardial side of the circulatory terminals (Table 2).
Figure 4.
Micro-Scars in the Entire Myocardium
Representative images of myocardium with elastica-Goldner staining in patient 2. Micro-scars (yellow arrows) are seen in the high right atrium (RA), the right ventricle (RV), the junction of pulmonary vein and left atrium (LA), and the left ventricle (LV). Scale bar, 1 mm.
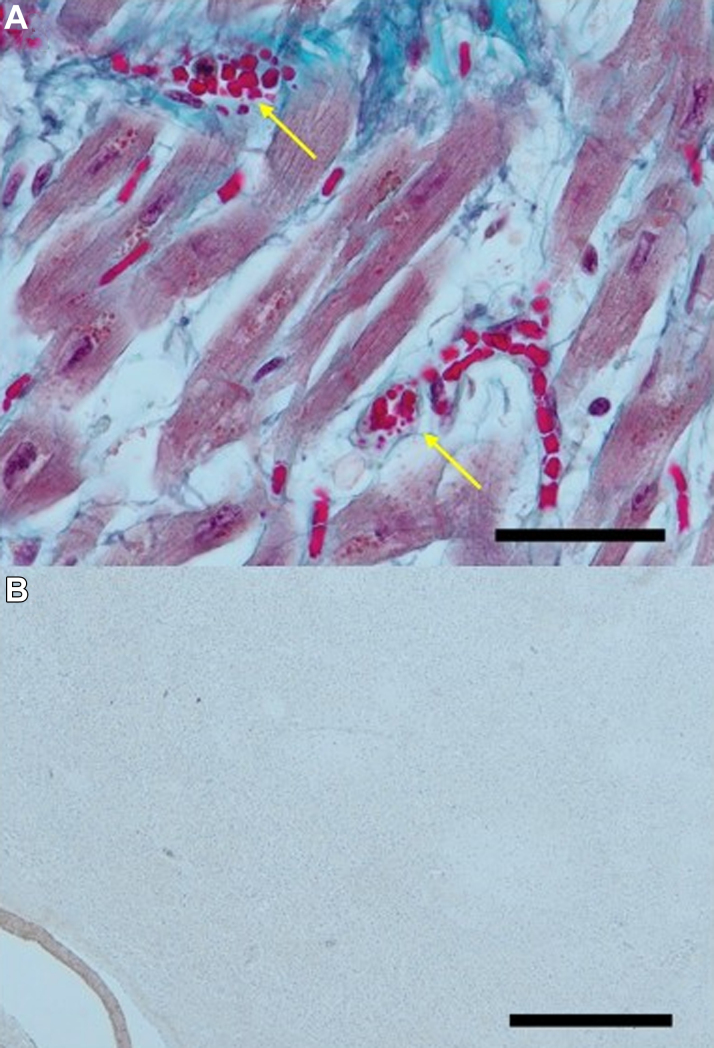
Figure 5.
Magnified Image of Capillary in Microscar in Myocardium
(A) Magnified image of Figure 1B. Thrombi, including stagnant crushed erythrocytes (yellow arrows), are seen in the capillaries. Scale bar, 100 μm. (B) αCD42b staining of myocardium; αCD42b was not stained. Scale bar, 100 μm.
Table 2.
Regional Densities of Scar in Myocardial Tissue
| Observed Region | Scar Density, n/mm2a | Scar Count Rangeb | |
|---|---|---|---|
| Anterior | Outerc | 1.12 | 7-15 |
| Innerc | 1.24 | 3-25 | |
| Lateral | Outer | 0.84 | 3-10 |
| Inner | 1.24 | 0-21 | |
| Posterior | Outer | 0.76 | 2-13 |
| Inner | 1.90 | 11-30 | |
| Septal | 1.96 | 10-24 | |
| Right ventricle | 0.50 | 1-5 | |
Calculated as count of the scar of interest (n) in myocardial area observed (mm2).
Minimum and maximum counts of the scar in a microscopic field (7 mm2/field) among no fewer than 4 fields.
Outer: epicardial half of myocardium; inner: endocardial half of myocardium.
Discussion
This is likely the first report of patients with cardiac MMS who died of cardiac arrest. Of note, the left ventricular ejection fraction in all 3 patients was not reduced despite their having MMS in the entire myocardium. Two patients did not have histories of COVID-19, and 1 had COVID-19. Regarding COVID-19 vaccination history, all 3 patients had histories of booster vaccinations up to the last admission. An association between arrhythmia and COVID-19 vaccination has been reported recently.4,5 A global survey showed that any type of COVID-19 vaccine appears to instigate cardiac arrhythmias, and COVID-19 vaccines may lead to cardiac conduction abnormalities. These mechanisms are speculated to arise from molecular mimicry or spike protein production, an escalated inflammatory response, and the eventual scar and fibrosis. Interestingly, in the present pathologic case study, microscarring was also observed at the junction of the left atrium with the pulmonary artery and high right atrium, which is also a common site of catheter ablation for atrial fibrillation. In the future, we hope to see research that will make it possible to diagnose the pathophysiology of cardiac MMS through cardiac imaging and/or blood tests prior to death.
Why were MMS seen only in the myocardium? The distance and size of the adjacent scars within the myocardium suggest that the scars formed following inflammation at the level of the microvascular bed of the capillaries. The distance from the terminal arteriole to the beginning of the venule has been reported to be about 300 to 500 μm in past studies,6,7 like the distance between the scars in the present cases. The facts that these scars are caused by inflammation due to thromboembolism only at the level of capillary bed and that each scar has the same aspect suggest that the inflammation occurred all at once and at the same time. αCD42b was not stained in the hearts, indicating no platelet activation state (ie, that is not an acute phase). Although the course of cardiac MMS has not been reported, it represents a poor prognosis unless appropriate treatment is given. As shown in the magnified image of the heart, erythrocyte fragmentation was seen in the microvasculature, indicating thrombotic microangiopathy. This unexpected finding of microthrombosis only in the myocardium, not in the kidneys, suggests that these cases are not compatible with a diagnosis of thrombotic thrombocytopenic purpura or atypical hemolytic uremia syndrome,8 yet it is a thrombotic microangiopathy, pathologically. The cause of the cardiac MMS has not been clarified. The fact that these rare cardiac MMS continue to be found at autopsy within a short period of time, approximately 6 months, compels us to consider the involvement of current trends in the background. Thrombotic microangiopathy in myocardium was likely to be shown in these cardiac MMS cases as a preceding condition, but we have not been able to demonstrate a relationship to COVID-19. Thrombosis after COVID-19 vaccination has been reported,9 and our patients received booster vaccinations against COVID-19. Although it is possible that MMS are induced by the vaccine, the direct relationship between the vaccination and these MMS with capillary-level thrombi could not be proved in the present study. Further study should investigate its involvement.
Visual Summary.
Multiple Micro Scarring Throughout Myocardium
AF = atrial fibrillation; LA = left atrium; LV = left ventricle; PV = pulmonary vein; PVC = premature ventricular contraction; RA = right atrium; RV = right ventricle.
Conclusions
Cardiac MMS should be considered one of the differential findings of cardiac arrest. Further study is needed to identify and understand the characteristics of this disease state.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors appreciate the review of this report by Heidi N. Bonneau, RN, MS, CCA. The authors thank the attending physicians for obtaining consent for these autopsies from the patients and their families.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Artico J., Shiwani H., Moon J.C., et al. Myocardial involvement after hospitalization for COVID-19 complicated by troponin elevation: a prospective, multicenter, observational study. Circulation. 2023;147:364–374. doi: 10.1161/CIRCULATIONAHA.122.060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myhre P.L., Heck S.L., Skranes J.B., et al. Cardiac pathology 6 months after hospitalization for COVID-19 and association with the acute disease severity. Am Heart J. 2021;242:61–70. doi: 10.1016/j.ahj.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuber M., Baggish A.L. Acute myocardial injury in the COVID-HEART study: emphasizing scars while reassuring scares. Circulation. 2023;147:375–377. doi: 10.1161/CIRCULATIONAHA.122.062508. [DOI] [PubMed] [Google Scholar]
- 4.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pari B., Babbili A., Kattubadi A., et al. COVID-19 vaccination and cardiac arrhythmias: a review. Curr Cardiol Rep. 2023;25:925–940. doi: 10.1007/s11886-023-01921-7. [DOI] [PubMed] [Google Scholar]
- 6.Fung Y.C. Biomechanics: Circulation. 2nd ed. Springer; New York: 1997. Mean capillary length. Coronary capillaries; pp. 470–471. [Google Scholar]
- 7.Kassab G.S. Topology and dimensions of pig coronary capillary network. Am J Physiol. 1994;267:H319–H325. doi: 10.1152/ajpheart.1994.267.1.H319. [DOI] [PubMed] [Google Scholar]
- 8.Mannucci P.M., Cugno M. The complex differential diagnosis between thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: laboratory weapons and their impact on treatment choice and monitoring. Thromb Res. 2015;136:851–854. doi: 10.1016/j.thromres.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Greinacher A., Thiele T., Warkentin T.E., et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]