Abstract
1. Identified neurones in the abdominal ganglion of Aplysia californica were voltage clamped in order to investigate how guanosine 5'-O-(3-thiotriphosphate) (GTP-gamma-S), a GTP analogue that irreversibly activates guanine nucleotide-binding (G) proteins, modifies activation by the neuropeptide FMRFamide (Phe-Met-Arg-Phe-NH2) of a slow K+ current resembling the serotonin- and adenosine 3',5'-cyclic monophosphate (cyclic AMP)-sensitive 'S' current, and a similar response to acetylcholine. 2. Ionophoretic or pressure injection of GTP-gamma-S into the cell triggered the slow and irreversible development of a large K+ current, rendered the K+ current responses to FMRFamide and acetylcholine irreversible, and finally, once the GTP-gamma-S-induced current had fully developed, occluded the neurotransmitter responses altogether. 3. The K+ currents activated by GTP-gamma-S and acetylcholine had properties identical to those previously found for the FMRFamide-induced 'S'-like K+ current: they were Ca2+ and voltage independent, relatively insensitive to block by extracellular tetraethylammonium (TEA) and 4-aminopyridine (when high concentrations of acetylcholine were used to overcome an additional block by these agents of the receptor), and suppressed in Ba2+-containing solution, by injection of TEA+ or Cs+ into the cell, and by serotonin and elevation of the intracellular concentration of cyclic AMP. 4. The K+ current responses to FMRFamide and acetylcholine were not additive when the agonist concentrations used were high enough to activate most of the available current. 5. Desensitization of either response did not affect the other, and the effect of acetylcholine, but not that of FMRFamide, could be blocked by the known acetylcholine-receptor blockers phenyltrimethylammonium and TEA. 6. These results suggest that FMRFamide and acetylcholine, acting through different receptors, activate the same 'S'-like K+ current by a mechanism involving a G protein. 7. In addition to activating the slow K+ current, FMRFamide and acetylcholine each activate a faster current in these cells, carried by Na+ in the case of FMRFamide, and by Cl- in the case of acetylcholine. Neither fast response was affected by GTP-gamma-S.
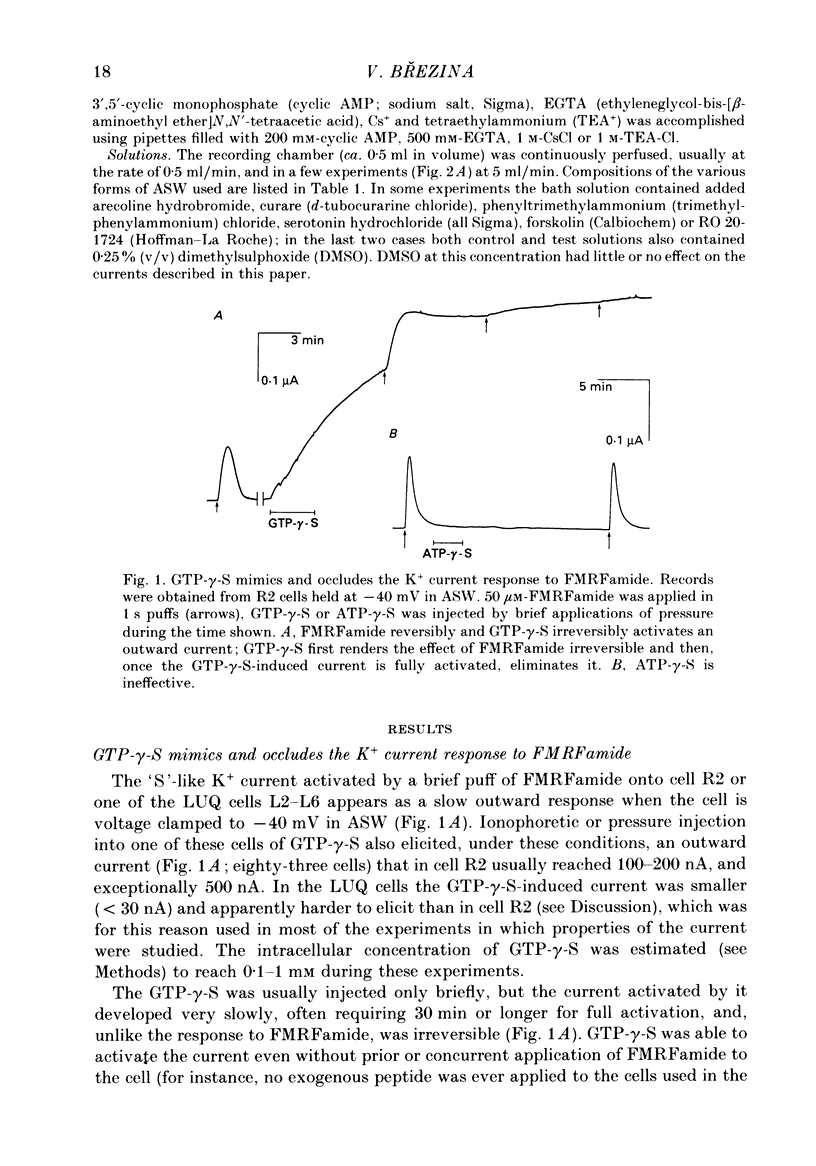
Full text
PDF

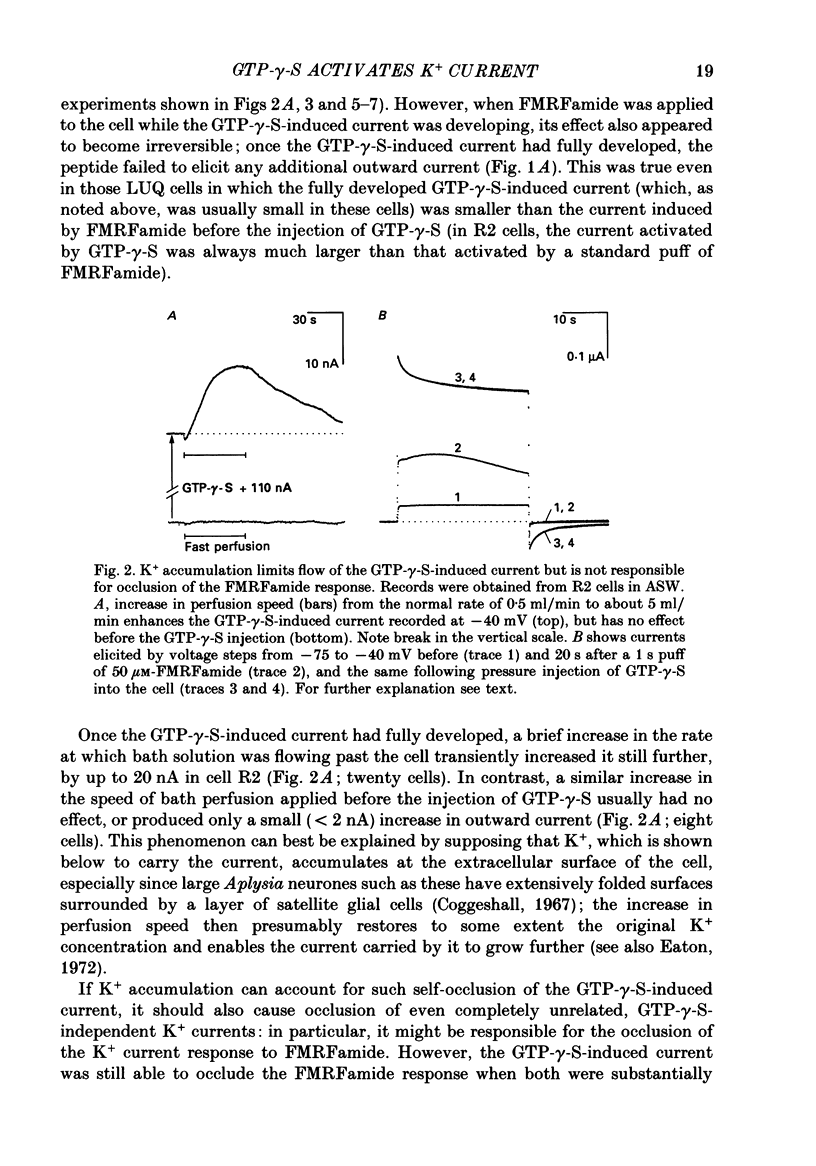


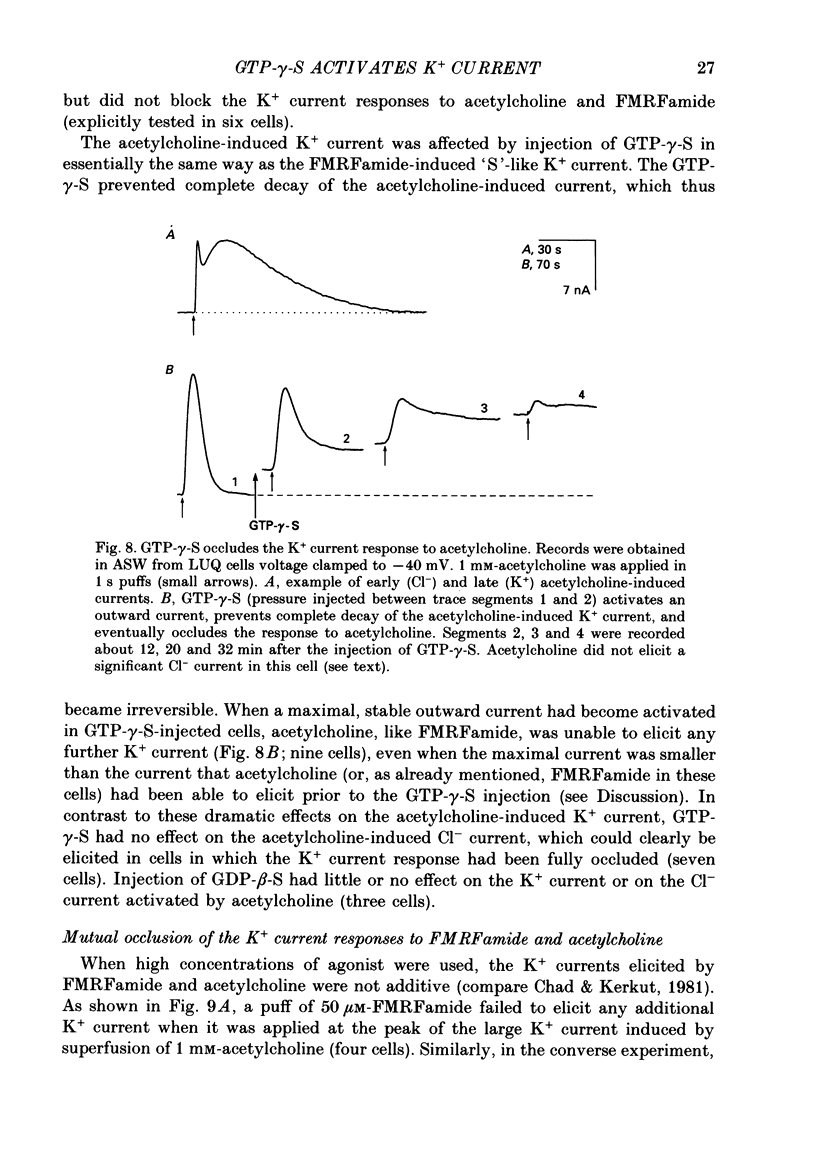
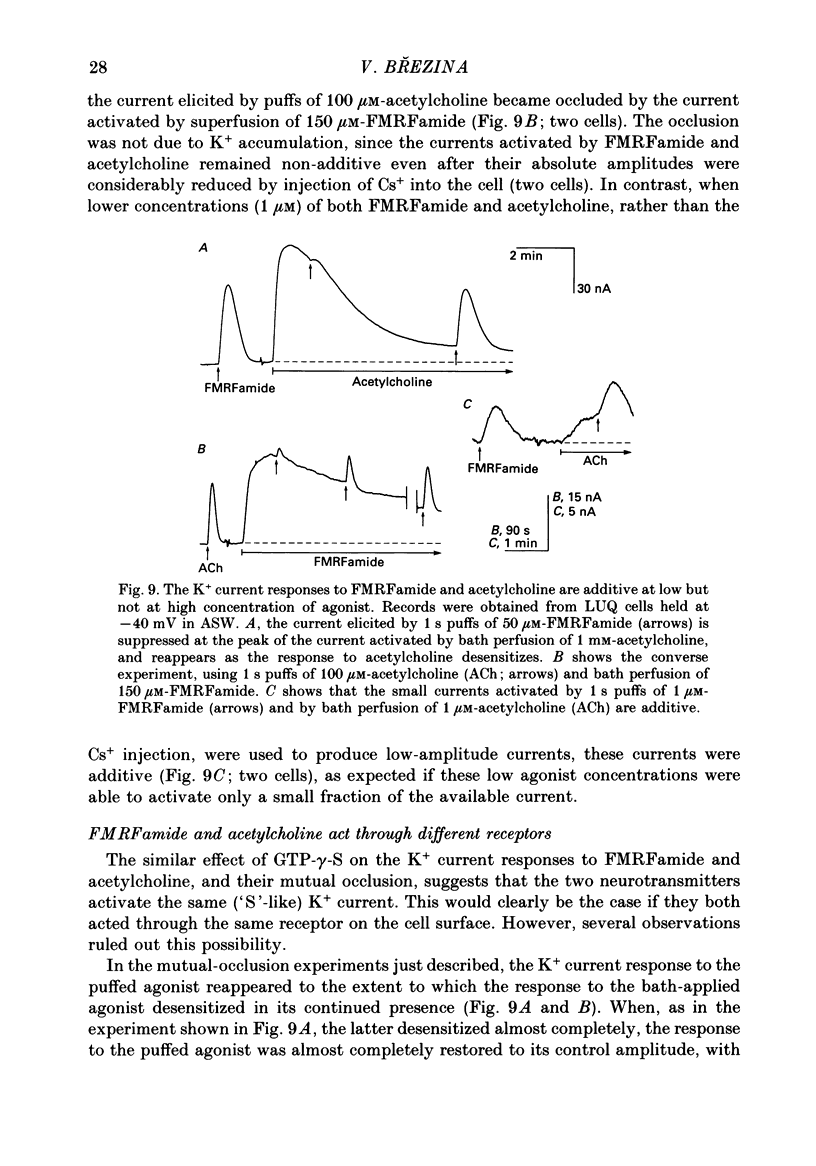
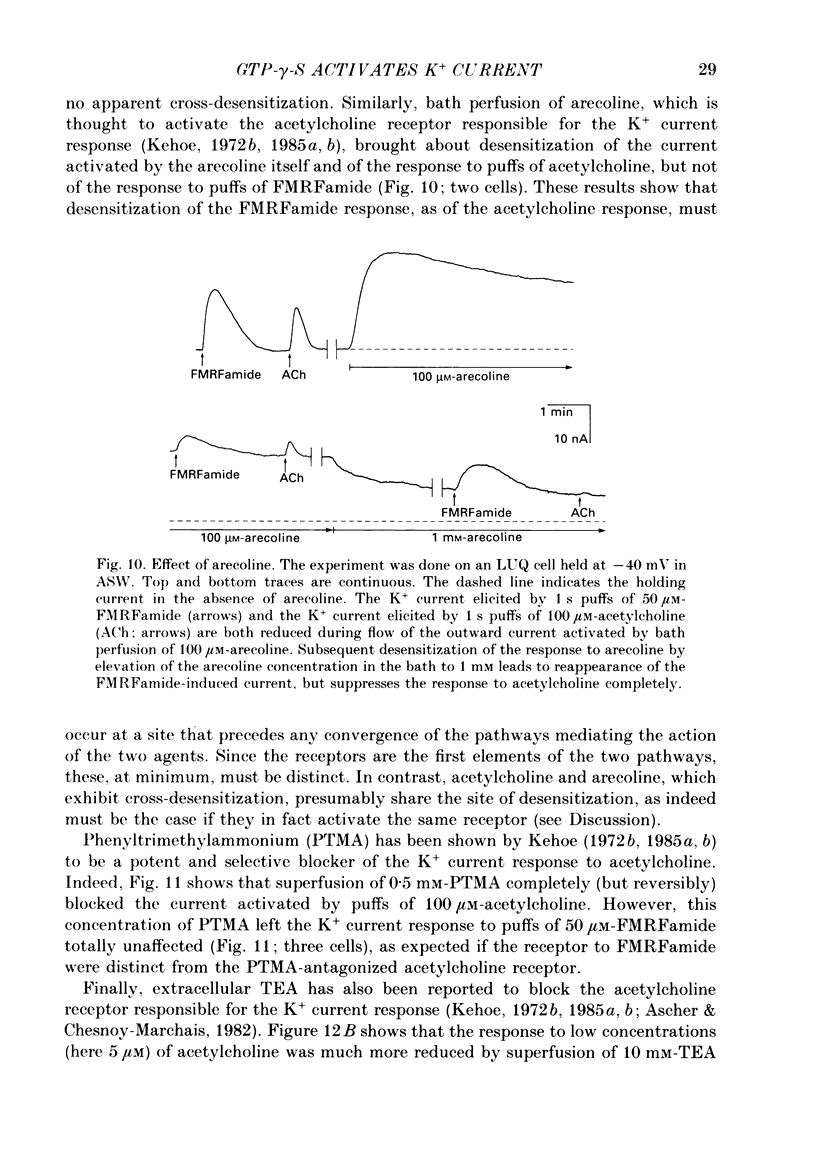
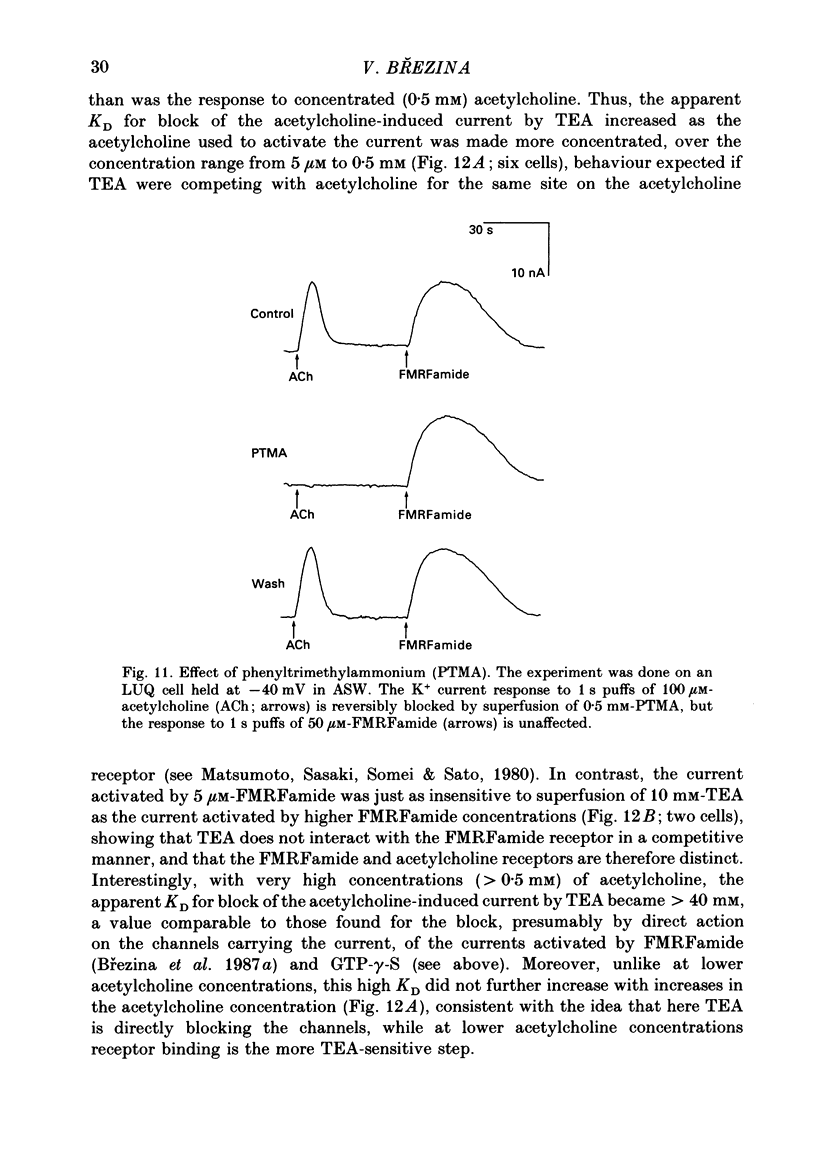
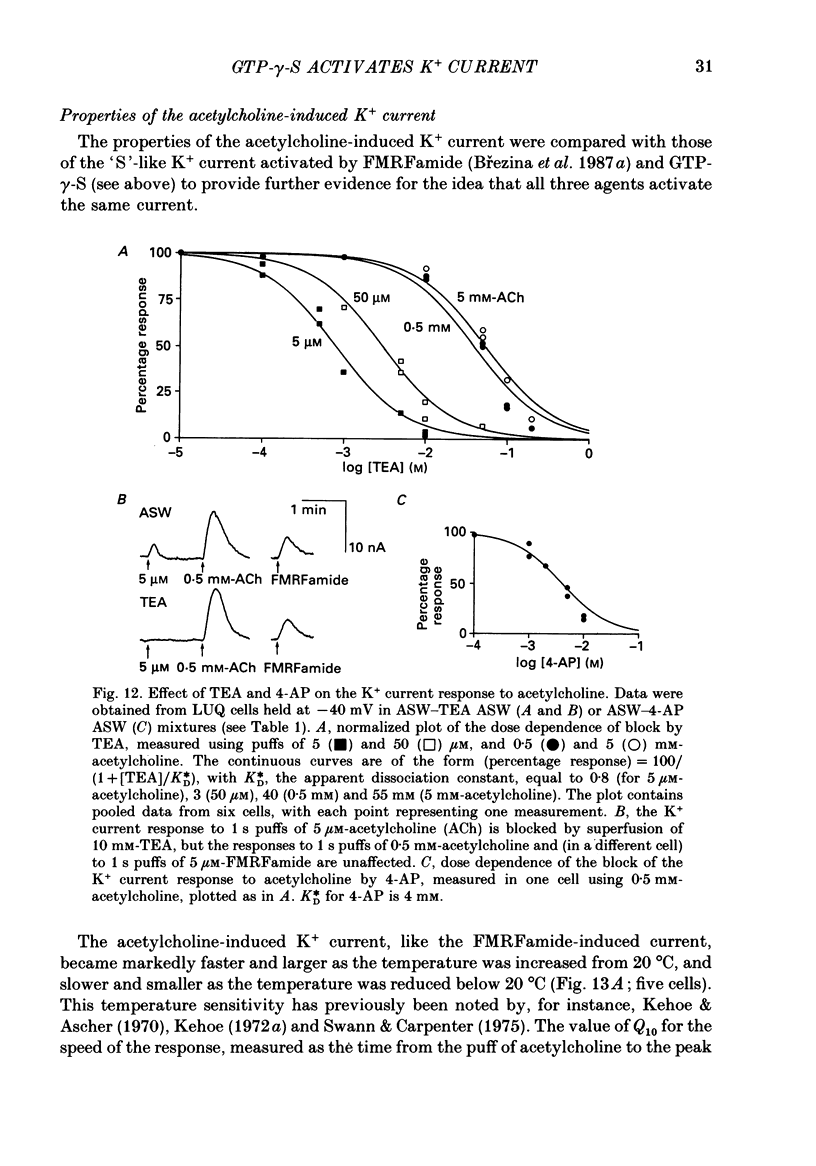
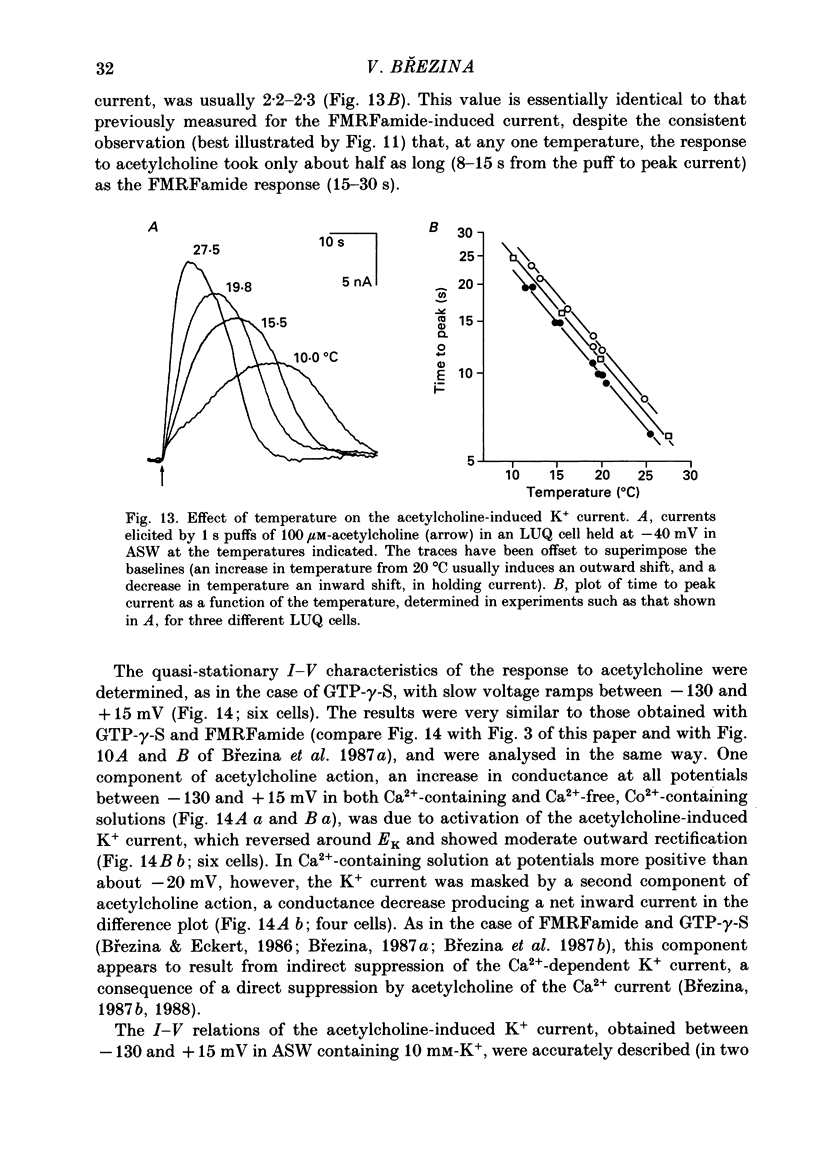
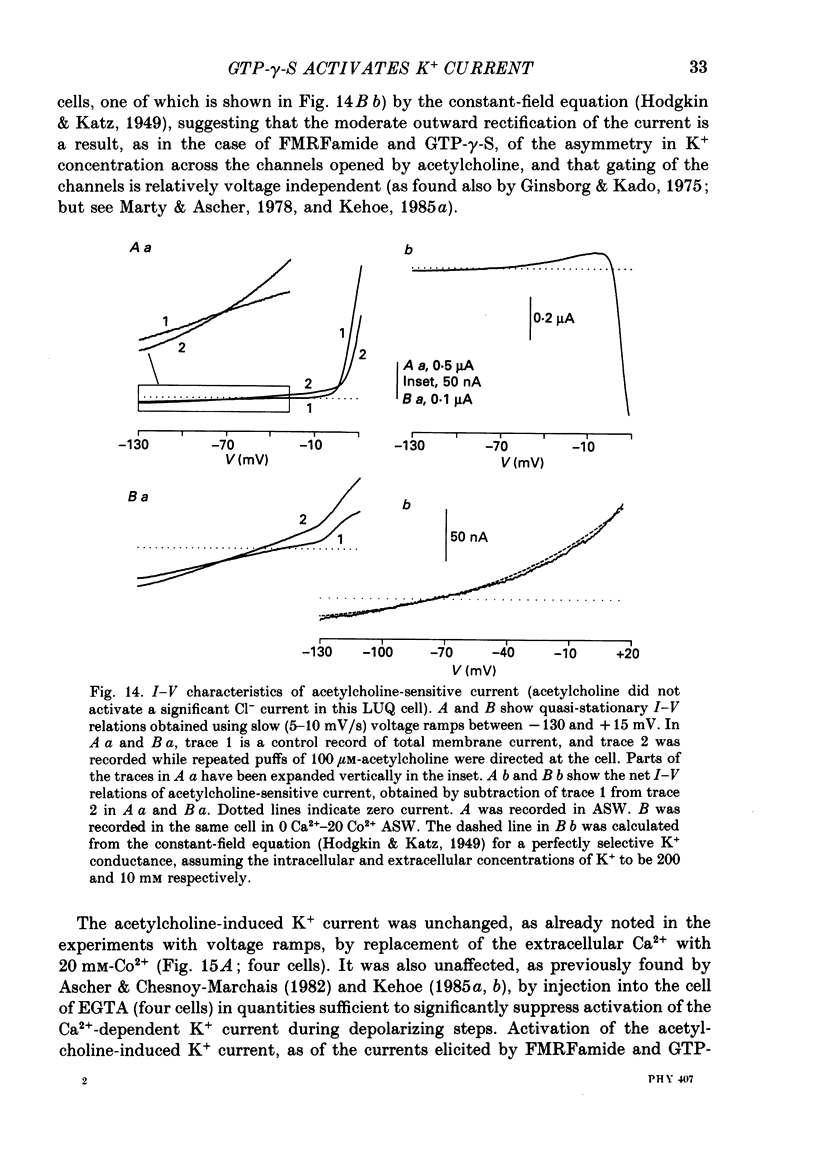
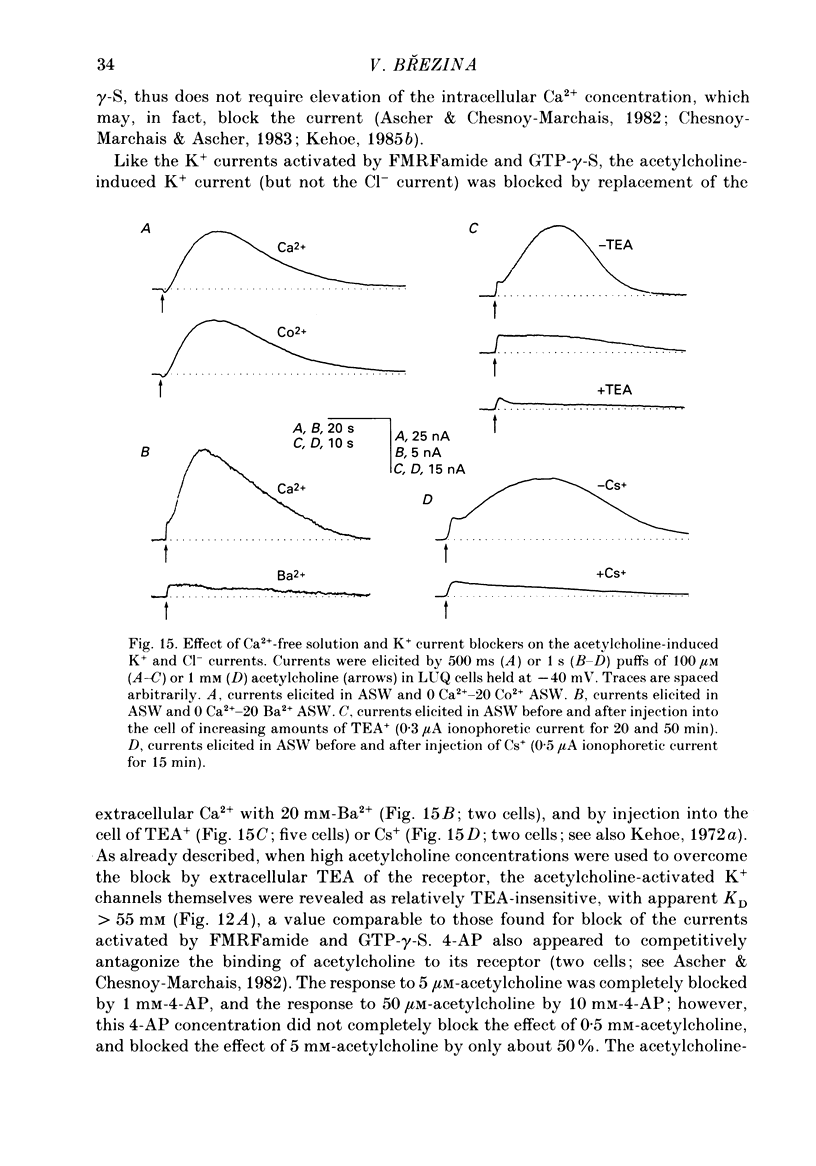





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Chesnoy-Marchais D. Interactions between three slow potassium responses controlled by three distinct receptors in Aplysia neurones. J Physiol. 1982 Mar;324:67–92. doi: 10.1113/jphysiol.1982.sp014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
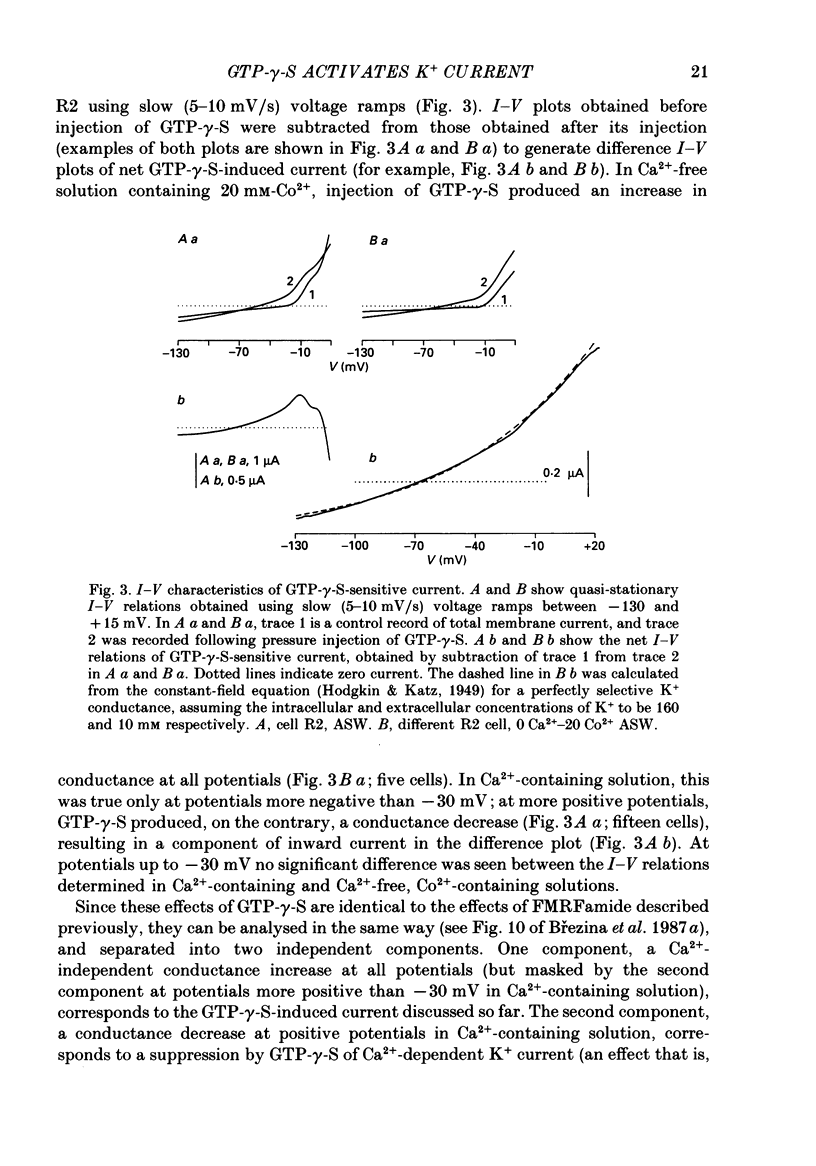
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F., Kandel E. R., Siegelbaum S. A. Neuronal inhibition by the peptide FMRFamide involves opening of S K+ channels. Nature. 1987 Jan 8;325(7000):153–156. doi: 10.1038/325153a0. [DOI] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V., Eckert R., Erxleben C. Modulation of potassium conductances by an endogenous neuropeptide in neurones of Aplysia californica. J Physiol. 1987 Jan;382:267–290. doi: 10.1113/jphysiol.1987.sp016367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V., Eckert R., Erxleben C. Suppression of calcium current by an endogenous neuropeptide in neurones of Aplysia californica. J Physiol. 1987 Jul;388:565–595. doi: 10.1113/jphysiol.1987.sp016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. O., Gusman D., Basbaum A. I., Mayeri E. Identification of Aplysia neurons containing immunoreactive FMRFamide. Neuropeptides. 1985 Dec;6(6):517–526. doi: 10.1016/0143-4179(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O., Swann J. W., Yarowsky P. J. Effect of curare on responses to different putative neurotransmitters in Aplysia neurons. J Neurobiol. 1977 Mar;8(2):119–132. doi: 10.1002/neu.480080204. [DOI] [PubMed] [Google Scholar]
- Chad J., Eckert R., Ewald D. Kinetics of calcium-dependent inactivation of calcium current in voltage-clamped neurones of Aplysia californica. J Physiol. 1984 Feb;347:279–300. doi: 10.1113/jphysiol.1984.sp015066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Ascher P. Effects of various cations on the slow K+ conductance increases induced by carbachol, histamine and dopamine in Aplysia neurones. Brain Res. 1983 Jan 17;259(1):57–67. doi: 10.1016/0006-8993(83)91066-1. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E. A light and electron microscope study of the abdominal ganglion of Aplysia californica. J Neurophysiol. 1967 Nov;30(6):1263–1287. doi: 10.1152/jn.1967.30.6.1263. [DOI] [PubMed] [Google Scholar]
- Coombs J., Thompson S. Forskolin's effect on transient K current in nudibranch neurons is not reproduced by cAMP. J Neurosci. 1987 Feb;7(2):443–452. doi: 10.1523/JNEUROSCI.07-02-00443.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critz S. D., Harper J. F., Byrne J. H. Evidence for the inhibitory subunit of adenylate cyclase (Ni) in nervous and heart tissue of Aplysia. Neurosci Lett. 1986 Feb 28;64(2):145–150. doi: 10.1016/0304-3940(86)90090-x. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Eckert R. Two components of Ca-dependent potassium current in identified neurons of Aplysia californica. Pflugers Arch. 1985 Apr;403(4):353–359. doi: 10.1007/BF00589246. [DOI] [PubMed] [Google Scholar]
- Eaton D. C. Potassium ion accumulation near a pace-making cell of Aplysia. J Physiol. 1972 Jul;224(2):421–440. doi: 10.1113/jphysiol.1972.sp009903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Kado R. T. Voltage-current relationship of a carbachol-induced potassium-ion pathway in Aplysia neurones. J Physiol. 1975 Mar;245(3):713–725. doi: 10.1113/jphysiol.1975.sp010870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D. L., Weinreich D. Two pharmacologically distinct histamine receptors mediating membrane hyperpolarization on identified neurons of Aplysia californica. Brain Res. 1979 Feb 23;162(2):281–301. doi: 10.1016/0006-8993(79)90290-7. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Kehoe J. S., Ascher P. Re-evaluation of the synaptic activation of an electrogenic sodium pump. Nature. 1970 Feb 28;225(5235):820–823. doi: 10.1038/225820a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Synaptic block of a calcium-activated potassium conductance in Aplysia neurones. J Physiol. 1985 Dec;369:439–474. doi: 10.1113/jphysiol.1985.sp015910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Synaptic block of a transmitter-induced potassium conductance in Aplysia neurones. J Physiol. 1985 Dec;369:399–437. doi: 10.1113/jphysiol.1985.sp015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Bailey C. H., Chen M., Kandel E. R. Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. II. Presynaptic conductance changes caused by histamine. J Neurophysiol. 1986 Jan;55(1):131–146. doi: 10.1152/jn.1986.55.1.131. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Brown A. M. Internal potassium and chloride activities and the effects of acetylcholine on identifiable Aplysia neurones. Nat New Biol. 1971 Feb 24;229(8):229–231. doi: 10.1038/newbio229229a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Levitan I. B. Intracellular injection of guanyl nucleotides alters the serotonin-induced increase in potassium conductance in Aplysia neuron R15. J Gen Physiol. 1984 Feb;83(2):269–285. doi: 10.1085/jgp.83.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Ascher P. Slow relaxations of acetylcholine-induced potassium currents in Aplysia neurones. Nature. 1978 Aug 3;274(5670):494–497. doi: 10.1038/274494a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Sasaki K., Somei K., Sato M. Dose-inhibition curve and its application to the analysis of ACh-receptor activity. Jpn J Physiol. 1980;30(5):743–750. doi: 10.2170/jjphysiol.30.743. [DOI] [PubMed] [Google Scholar]
- McCaman R. E., Weinreich D. On the nature of histamine-mediated slow hyperpolarizing synaptic potentials in identified molluscan neurones. J Physiol. 1982 Jul;328:485–506. doi: 10.1113/jphysiol.1982.sp014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben P., Johnson J. W., Thompson S. Analysis of FMRF-amide effects on Aplysia bursting neurons. J Neurosci. 1986 Jan;6(1):252–259. doi: 10.1523/JNEUROSCI.06-01-00252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Brown A. M. Active transport of potassium by the giant neuron of the aplysia abdominal ganglion. J Gen Physiol. 1972 Nov;60(5):519–533. doi: 10.1085/jgp.60.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Sato M. A single GTP-binding protein regulates K+-channels coupled with dopamine, histamine and acetylcholine receptors. Nature. 1987 Jan 15;325(6101):259–262. doi: 10.1038/325259a0. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Picciotto M. R., Kreiner T., Kaldany R. R., Taussig R., Scheller R. H. Aplysia neurons express a gene encoding multiple FMRFamide neuropeptides. Cell. 1985 Jun;41(2):457–467. doi: 10.1016/s0092-8674(85)80019-2. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Bernier L., Castellucci V. F., Palazzolo M., Saitoh T., Stapleton A., Kandel E. R. What molecular steps determine the time course of the memory for short-term sensitization in Aplysia? Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):811–819. doi: 10.1101/sqb.1983.048.01.084. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Swann J. W., Carpenter D. O. Organisation of receptors for neurotransmitters on Aplysia neurones. Nature. 1975 Dec 25;258(5537):751–754. doi: 10.1038/258751a0. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Levitan I. B. Intraneuronal guanylyl-imidodiphosphate injection mimics long-term synaptic hyperpolarization in Aplysia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4689–4692. doi: 10.1073/pnas.73.12.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Gola M. Forskolin interaction with voltage-dependent K channels in Helix is not mediated by cyclic nucleotides. Neurosci Lett. 1987 Jul 22;78(2):211–216. doi: 10.1016/0304-3940(87)90635-5. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Clark M. T., Pellmar T. C. Tris buffer attenuates acetylcholine responses in Aplysia neurons. Science. 1977 Apr 22;196(4288):440–441. doi: 10.1126/science.15317. [DOI] [PubMed] [Google Scholar]
- Yarowsky J., Carpenter D. O. A comparison of similar ionic responses to gamma-aminobutyric acid and acetylcholine. J Neurophysiol. 1978 May;41(3):531–541. doi: 10.1152/jn.1978.41.3.531. [DOI] [PubMed] [Google Scholar]


