Abstract
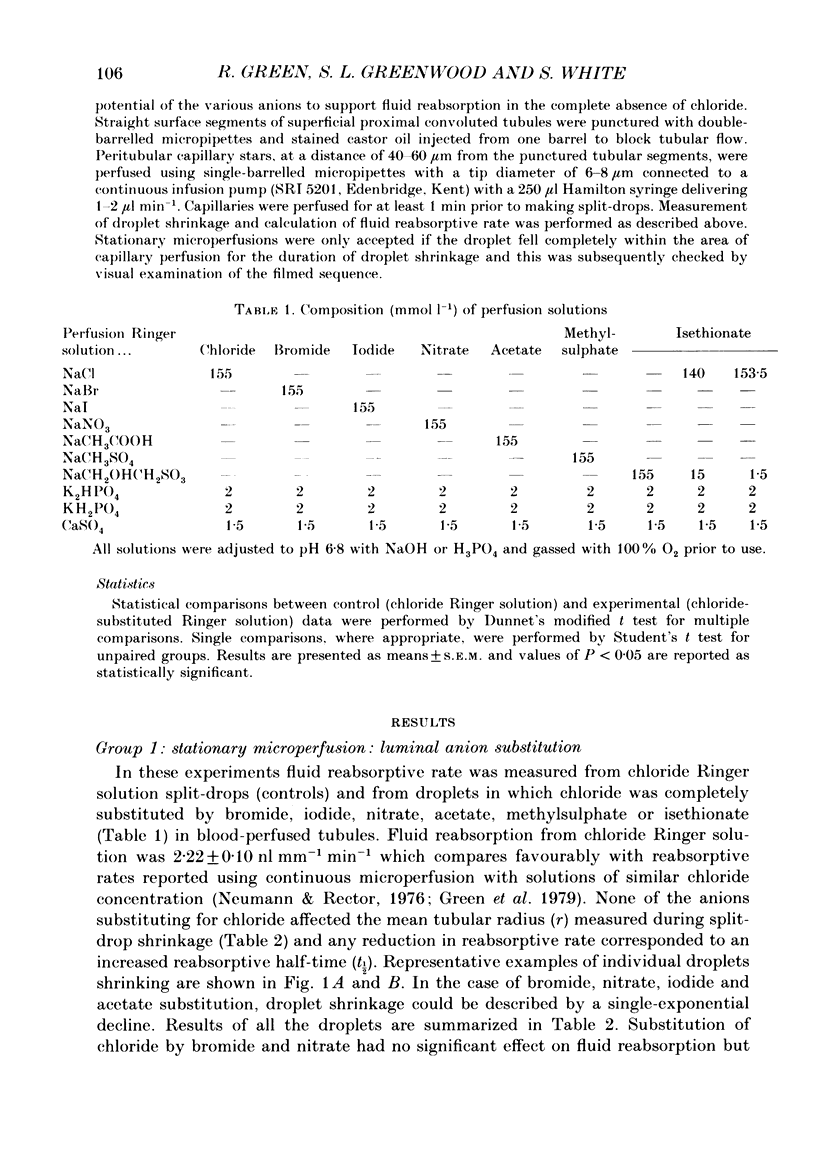
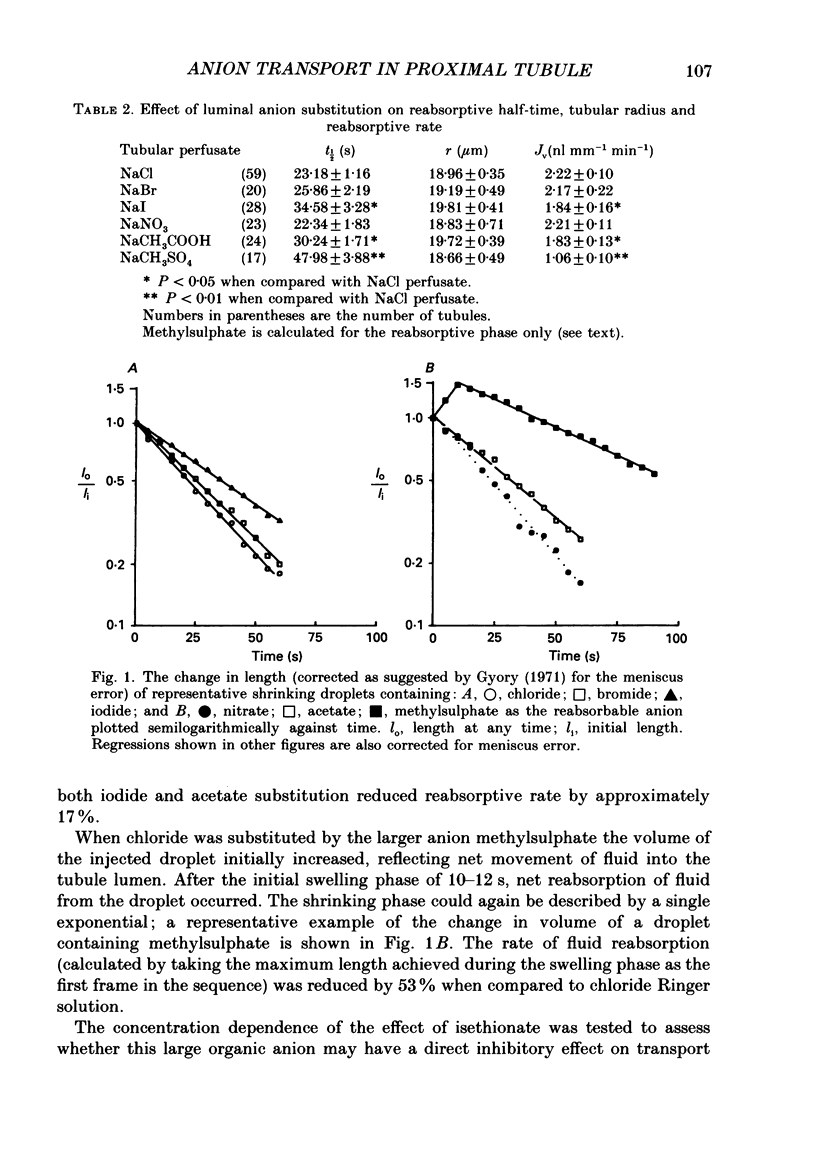
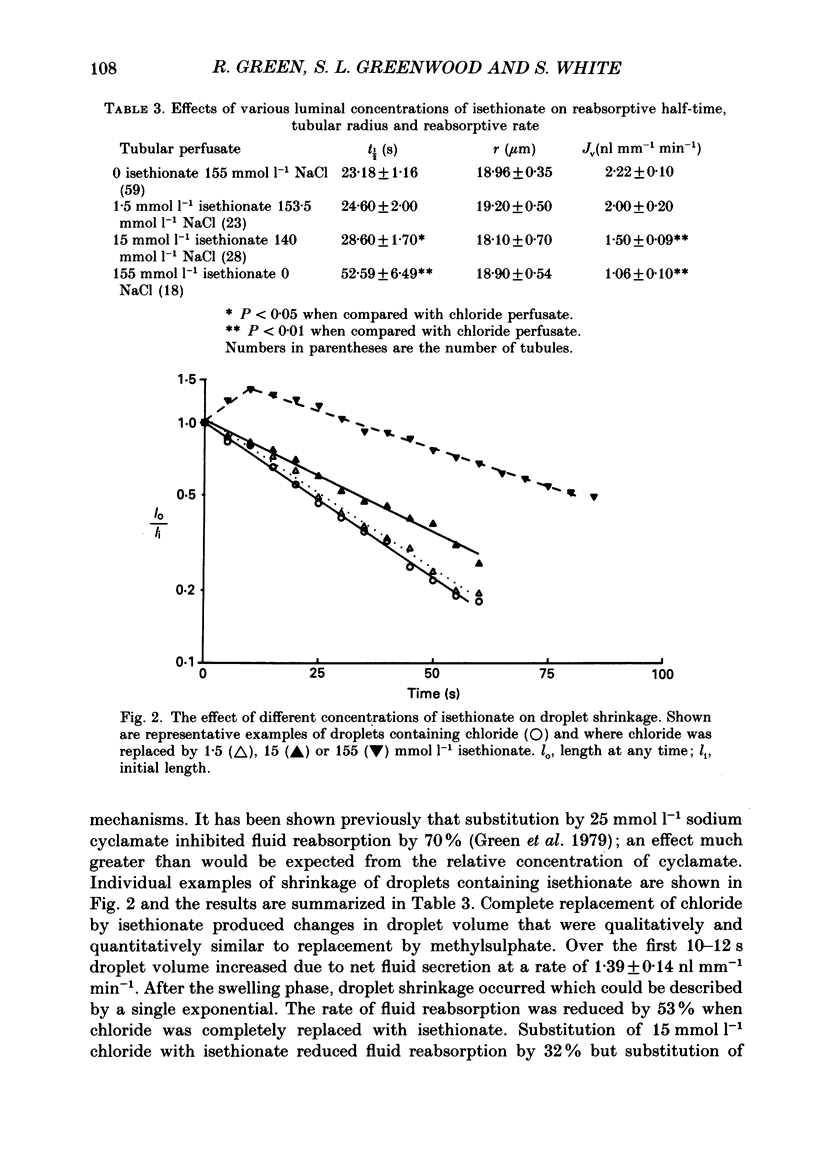
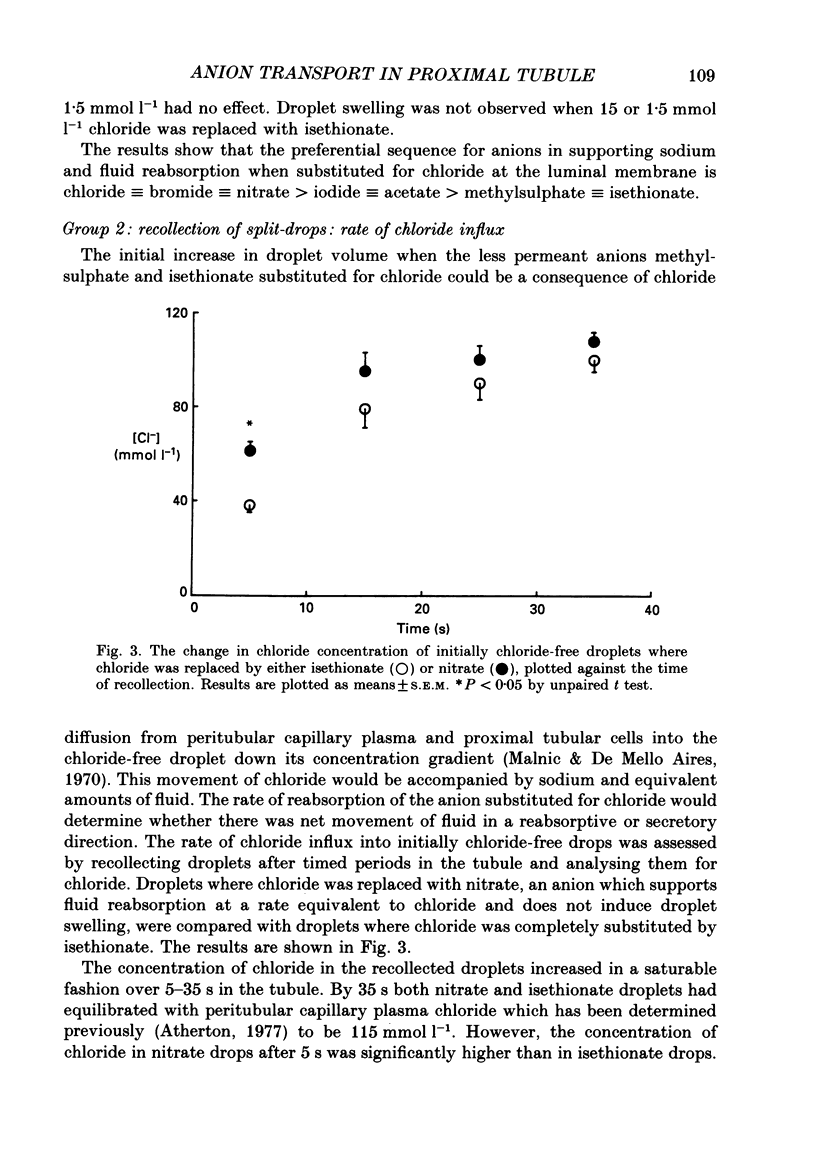
1. Fluid reabsorption from surface proximal tubules of the rat was measured in vivo using stationary microperfusion techniques. Reabsorptive rate (Jv) was measured from droplets containing chloride as the main reabsorbable anion and when chloride was substituted by bromide, iodide, nitrate, acetate, isethionate or methylsulphate in either the tubular lumen alone or in both lumen and peritubular capillaries. 2. In tubules with an intact blood supply, droplet volume decreased in a manner best described by a single exponential and substitution of chloride by nitrate or bromide had no effect on Jv. Substitution by iodide or acetate inhibited Jv by approximately 17% but substitution by methylsulphate or isethionate caused droplets to transiently increase in volume before shrinkage which was itself inhibited by approximately 50%. The inhibitory action of isethionate was found to be concentration dependent. 3. Recollection and analysis of droplets which were initially free of chloride, containing either nitrate or isethionate, showed that chloride entered these droplets, but that the initial rate of chloride entry was greater for nitrate than isethionate droplets. 4. When tubules and capillaries were perfused with chloride solutions containing no bicarbonate, Jv was reduced to about 20% of the value when peritubular capillary blood flow was intact. Substituting chloride in the tubular and capillary perfusion revealed a sequence for supporting fluid reabsorption that was identical to that when chloride was substituted in tubule fluid alone: bromide = nitrate greater than iodide = acetate greater than isethionate. Addition of 2.0 mmol l-1 NaCN reduced the reabsorptive flux to zero. 5. The results of this study are consistent with transcellular transport of anions across the proximal tubular epithelium. The pathways for anion transport are likely to involve a series of non-selective mechanisms such as anion exchangers.
Full text
PDF



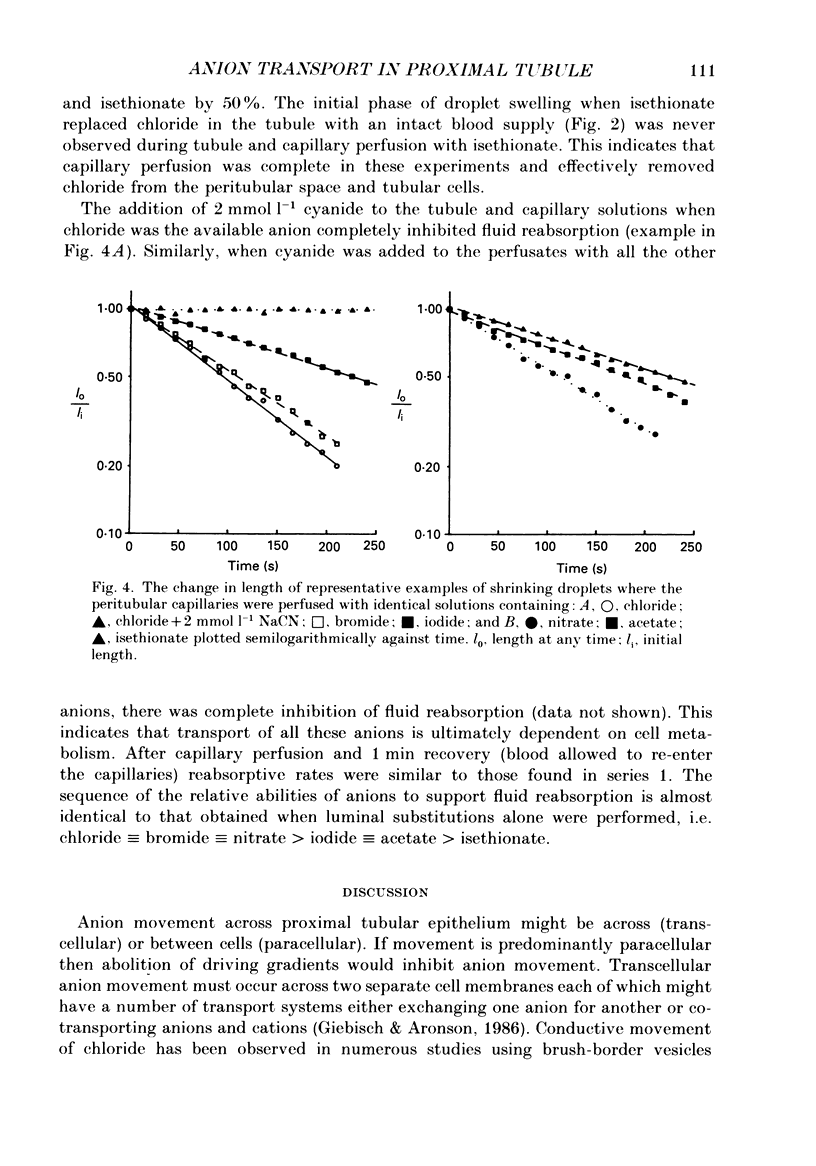





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Brading A. F. The effects of bicarbonate and foreign anions on chloride transport in smooth muscle of the guinea-pig vas deferens. J Physiol. 1985 Sep;366:267–280. doi: 10.1113/jphysiol.1985.sp015796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Howlin K. J., Preisig P. A. Active and passive components of chloride transport in the rat proximal convoluted tubule. J Clin Invest. 1985 Oct;76(4):1360–1366. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Atherton J. C. Comparison of chloride concentration and osmolality in proximal tubular fluid, peritubular capillary plasma and systemic plasma in the rat. J Physiol. 1977 Dec;273(3):765–773. doi: 10.1113/jphysiol.1977.sp012122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M., Berry C. A. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit proximal convoluted tubule. J Clin Invest. 1984 Jul;74(1):205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. H., Green R., Thomas S. Free-flow reabsorption of glucose, sodium, osmoles and water in rat proximal convoluted tubule. J Physiol. 1979 Mar;288:331–351. [PMC free article] [PubMed] [Google Scholar]
- Burnham C., Munzesheimer C., Rabon E., Sachs G. Ion pathways in renal brush border membranes. Biochim Biophys Acta. 1982 Mar 8;685(3):260–272. doi: 10.1016/0005-2736(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Case R. M., Hotz J., Hutson D., Scratcherd T., Wynne R. D. Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol. 1979 Jan;286:563–576. doi: 10.1113/jphysiol.1979.sp012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantrelle B. M., Cogan M. G., Rector F. C., Jr Active and passive components of NaCl absorption in the proximal convoluted tubule of the rat kidney. Miner Electrolyte Metab. 1985;11(4):209–214. [PubMed] [Google Scholar]
- Chipperfield A. R. An effect of chloride on (Na+K) co-transport in human red blood cells. Nature. 1980 Jul 17;286(5770):281–282. doi: 10.1038/286281a0. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., HARRIS E. J., NISHIE K. The exchange of frog muscle Na+ and K+ in the presence of the anions Br-, NO3-, I- and CNS-. J Physiol. 1957 Mar 11;135(3):560–566. doi: 10.1113/jphysiol.1957.sp005730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. The selectivity of ion channels in nerve and muscle. Neuroscience. 1982 Jun;7(6):1335–1366. doi: 10.1016/0306-4522(82)90249-4. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Effects of some monovalent anions on fluxes of Na and K, and on glucose metabolism of ouabain treated human red cells. Acta Physiol Scand. 1967 Oct-Nov;71(2):168–185. doi: 10.1111/j.1748-1716.1967.tb03723.x. [DOI] [PubMed] [Google Scholar]
- GERTZ K. H. [Transtubular sodium chloride transport and permeability for nonelectrolytes in the proximal and distal convolution of the rat kidney]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;276:336–356. [PubMed] [Google Scholar]
- Garland H. O., Brunt J. N., Taylor C. J., Green R. Computerised image analysis of split-drop micropuncture data. Pflugers Arch. 1979 Jul;381(1):11–14. doi: 10.1007/BF00582325. [DOI] [PubMed] [Google Scholar]
- Green R., Bishop J. H., Giebisch G. Ionic requirements of proximal tubular sodium transport. III. Selective luminal anion substitution. Am J Physiol. 1979 Mar;236(3):F268–F277. doi: 10.1152/ajprenal.1979.236.3.F268. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Ionic requirements of proximal tubular sodium transport. I. Bicarbonate and chloride. Am J Physiol. 1975 Nov;229(5):1205–1215. doi: 10.1152/ajplegacy.1975.229.5.1205. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Luminal hypotonicity: a driving force for fluid absorption from the proximal tubule. Am J Physiol. 1984 Feb;246(2 Pt 2):F167–F174. doi: 10.1152/ajprenal.1984.246.2.F167. [DOI] [PubMed] [Google Scholar]
- Green R., Windhager E. E., Giebisch G. Protein oncotic pressure effects on proximal tubular fluid movement in the rat. Am J Physiol. 1974 Feb;226(2):265–276. doi: 10.1152/ajplegacy.1974.226.2.265. [DOI] [PubMed] [Google Scholar]
- Györy A. Z. Reexamination of the split oil droplet method as applied to kidney tubules. Pflugers Arch. 1971;324(4):328–343. doi: 10.1007/BF00592461. [DOI] [PubMed] [Google Scholar]
- Howlin K. J., Alpern R. J., Berry C. A., Rector F. C., Jr Evidence for electroneutral sodium chloride transport in rat proximal convoluted tubule. Am J Physiol. 1986 Apr;250(4 Pt 2):F644–F648. doi: 10.1152/ajprenal.1986.250.4.F644. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Anion exchange pathways for Cl- transport in rabbit renal microvillus membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F513–F521. doi: 10.1152/ajprenal.1987.253.3.F513. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Chloride/formate exchange with formic acid recycling: a mechanism of active chloride transport across epithelial membranes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6362–6365. doi: 10.1073/pnas.82.18.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., McManus T. J., Haas M., Forbush B., 3rd, Duhm J., Flatman P. W., Saier M. H., Jr, Russell J. M. Physiology and biophysics of chloride and cation cotransport across cell membranes. Fed Proc. 1987 May 15;46(7):2377–2394. [PubMed] [Google Scholar]
- Lucci M. S., Warnock D. G. Effects of anion-transport inhibitors on NaCl reabsorption in the rat superficial proximal convoluted tubule. J Clin Invest. 1979 Aug;64(2):570–579. doi: 10.1172/JCI109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw I., Friedrich T., Burckhardt G. Properties of an anion exchanger in rat renal basolateral membrane vesicles. Am J Physiol. 1984 Mar;246(3 Pt 2):F334–F342. doi: 10.1152/ajprenal.1984.246.3.F334. [DOI] [PubMed] [Google Scholar]
- Malnic G., Mello Aires M. Microperfusion study of anion transfer in proximal tubules of rat kidney. Am J Physiol. 1970 Jan;218(1):27–32. doi: 10.1152/ajplegacy.1970.218.1.27. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Rayburn C. S., Liddell N. E. Inhibition by anions of human red cell carbonic anhydrase B: physiological and biochemical implications. Science. 1976 Feb 6;191(4226):469–472. doi: 10.1126/science.813299. [DOI] [PubMed] [Google Scholar]
- Neumann K. H., Rector F. C., Jr Mechanism of NaCl and water reabsorption in the proximal convoluted tubule of rat kidney. J Clin Invest. 1976 Nov;58(5):1110–1118. doi: 10.1172/JCI108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Proton pathways in rat renal brush-border and basolateral membranes. Biochim Biophys Acta. 1983 Oct 12;734(2):210–220. doi: 10.1016/0005-2736(83)90119-0. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Boyden D. A. Mechanism, regulation and physiological significance of the loop diuretic-sensitive NaCl/KCl symport system in animal cells. Mol Cell Biochem. 1984;59(1-2):11–32. doi: 10.1007/BF00231303. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Watkins M. L., Andreoli T. E. Volume absorption in the pars recta. I. "Simple" active Na+ transport. Am J Physiol. 1978 Apr;234(4):F332–F339. doi: 10.1152/ajprenal.1978.234.4.F332. [DOI] [PubMed] [Google Scholar]
- Stein W. D. Intrinsic, apparent, and effective affinities of co- and countertransport systems. Am J Physiol. 1986 Apr;250(4 Pt 1):C523–C533. doi: 10.1152/ajpcell.1986.250.4.C523. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Capasso G., Rumrich G., Papavassiliou F., Klöss S. Coupling between proximal tubular transport processes. Studies with ouabain, SITS and HCO3-free solutions. Pflugers Arch. 1977 Apr 25;368(3):245–252. doi: 10.1007/BF00585203. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Radtke H. W., Rumrich G. The role of bicarbonate and other buffers on isotonic fluid absorption in the proximal convolution of the rat kidney. Pflugers Arch. 1971;330(2):149–161. doi: 10.1007/BF00643031. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Greger R., Dunham P. B., Benjamin M. A., Frizzell R. A., Field M., Spring K. R., Ives H. E., Aronson P. S., Seifter J. Ion transport processes in apical membranes of epithelia. Fed Proc. 1984 Jul;43(10):2473–2487. [PubMed] [Google Scholar]
- Warnock D. G., Yee V. J. Chloride uptake by brush border membrane vesicles isolated from rabbit renal cortex. Coupling to proton gradients and K+ diffusion potentials. J Clin Invest. 1981 Jan;67(1):103–115. doi: 10.1172/JCI110002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. J., Greenwood S. L., Green R. The effect of DIDS on fluid reabsorption from the proximal convoluted tubule of the rat: dependence on the presence of bicarbonate. Q J Exp Physiol. 1988 Nov;73(6):951–958. doi: 10.1113/expphysiol.1988.sp003229. [DOI] [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Anion transport by isolated rabbit gall bladders. Am J Physiol. 1967 Nov;213(5):1199–1204. doi: 10.1152/ajplegacy.1967.213.5.1199. [DOI] [PubMed] [Google Scholar]
- Wieth J. O. Bicarbonate exchange through the human red cell membrane determined with [14C] bicarbonate. J Physiol. 1979 Sep;294:521–539. doi: 10.1113/jphysiol.1979.sp012944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]


