Abstract
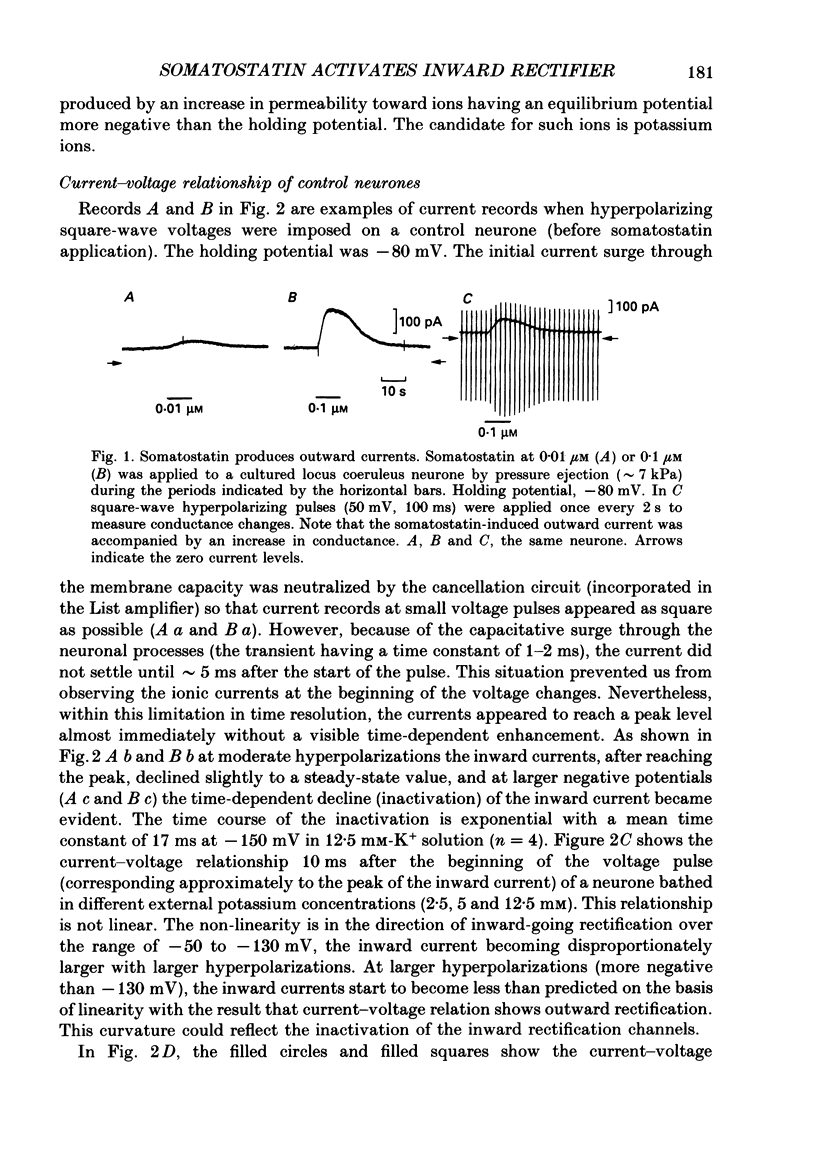
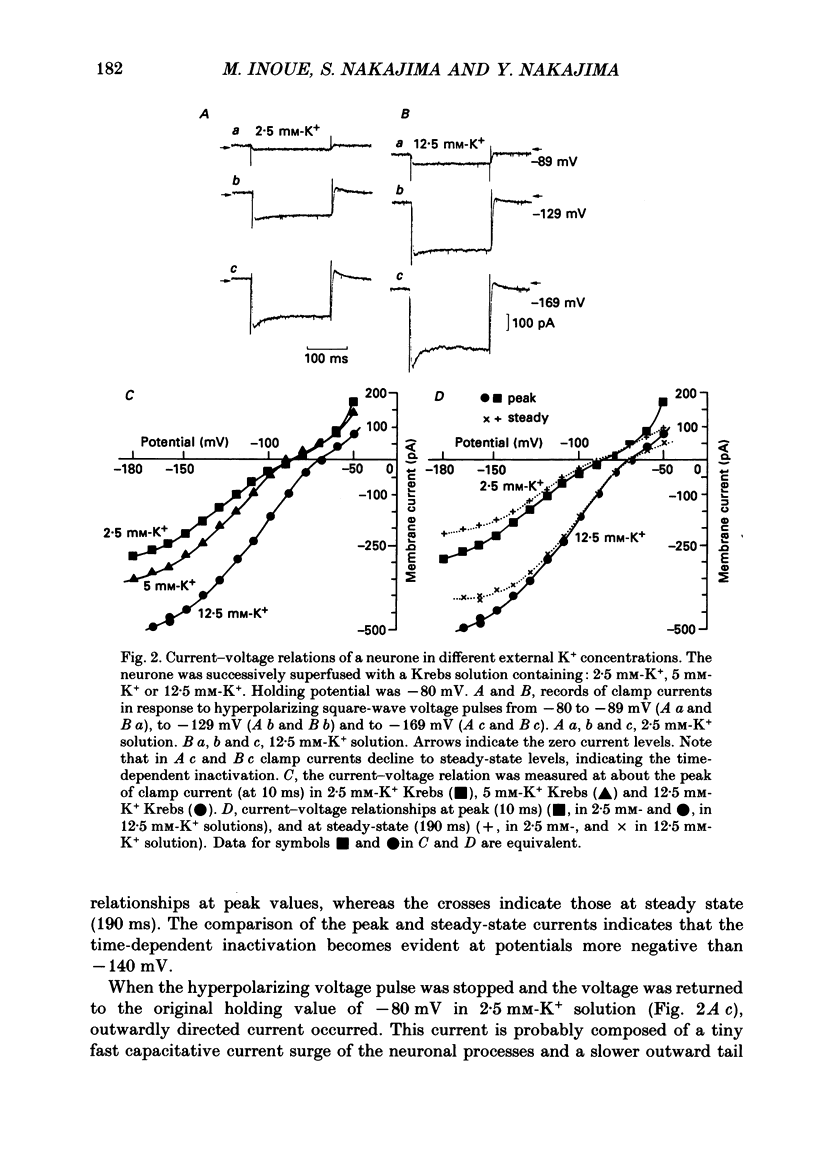
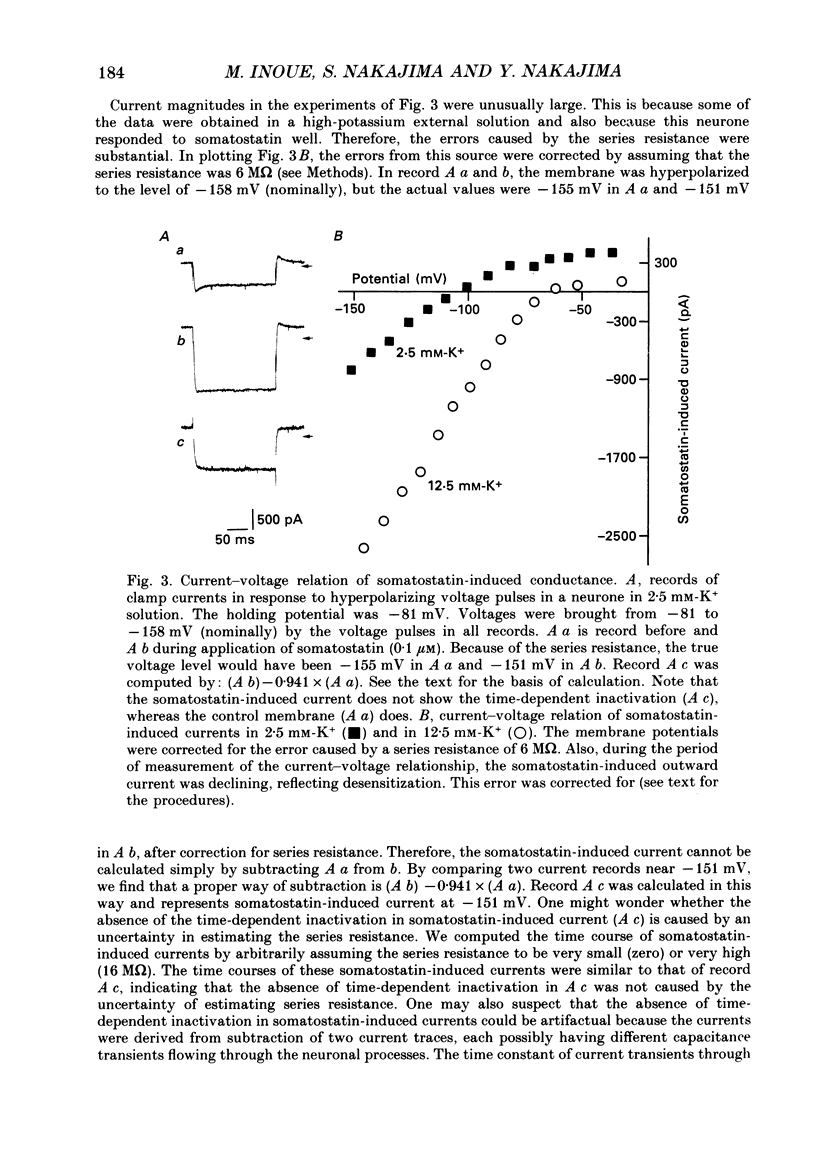
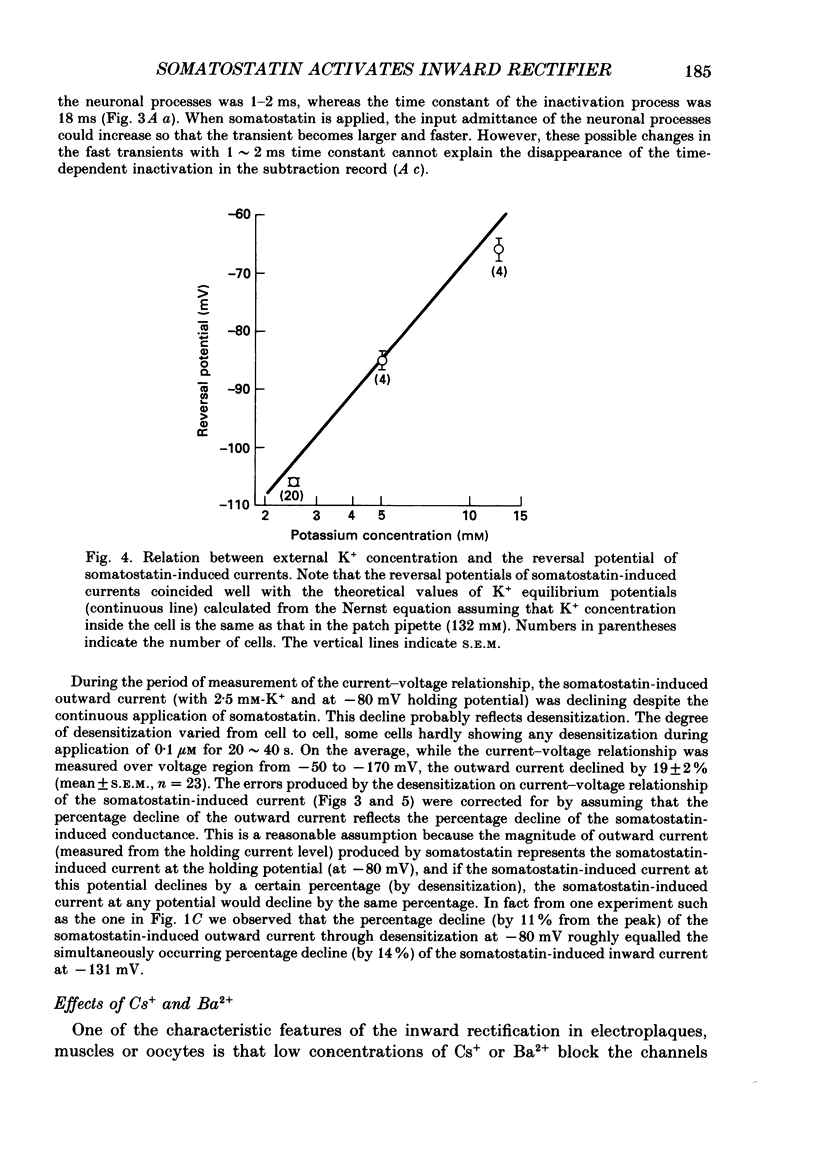
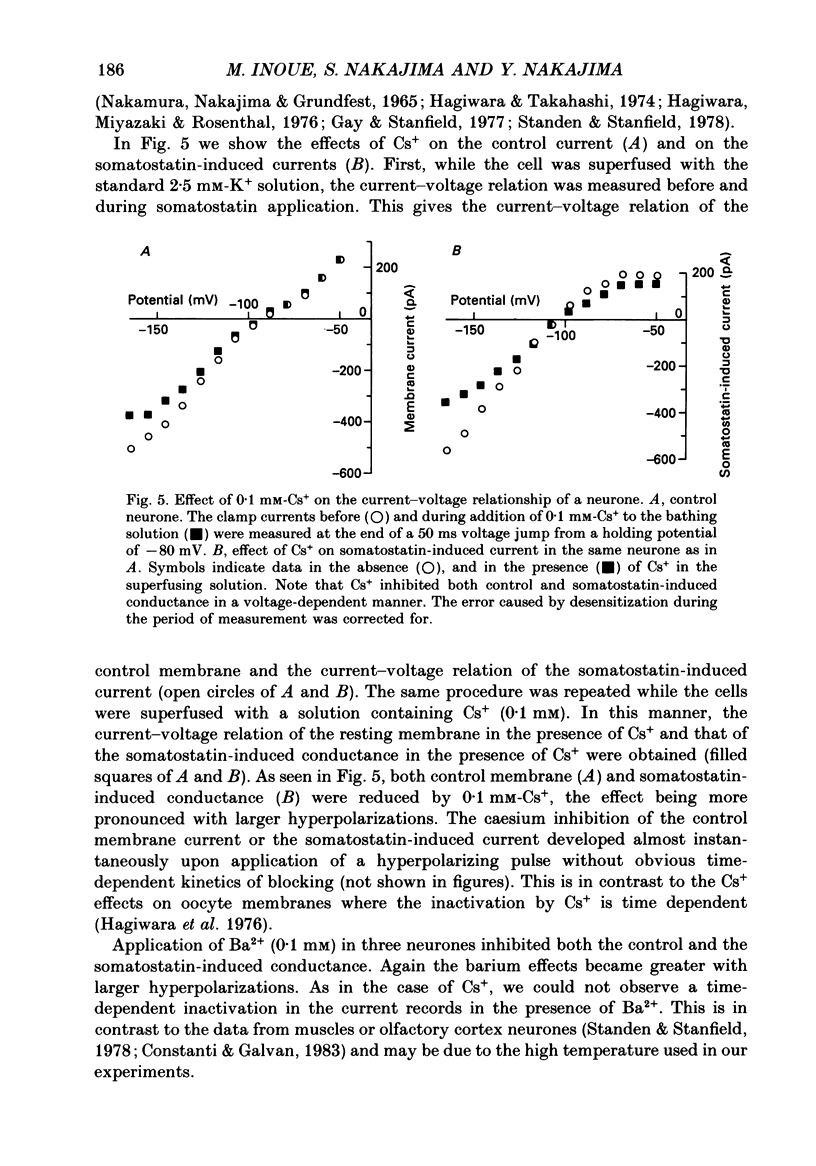
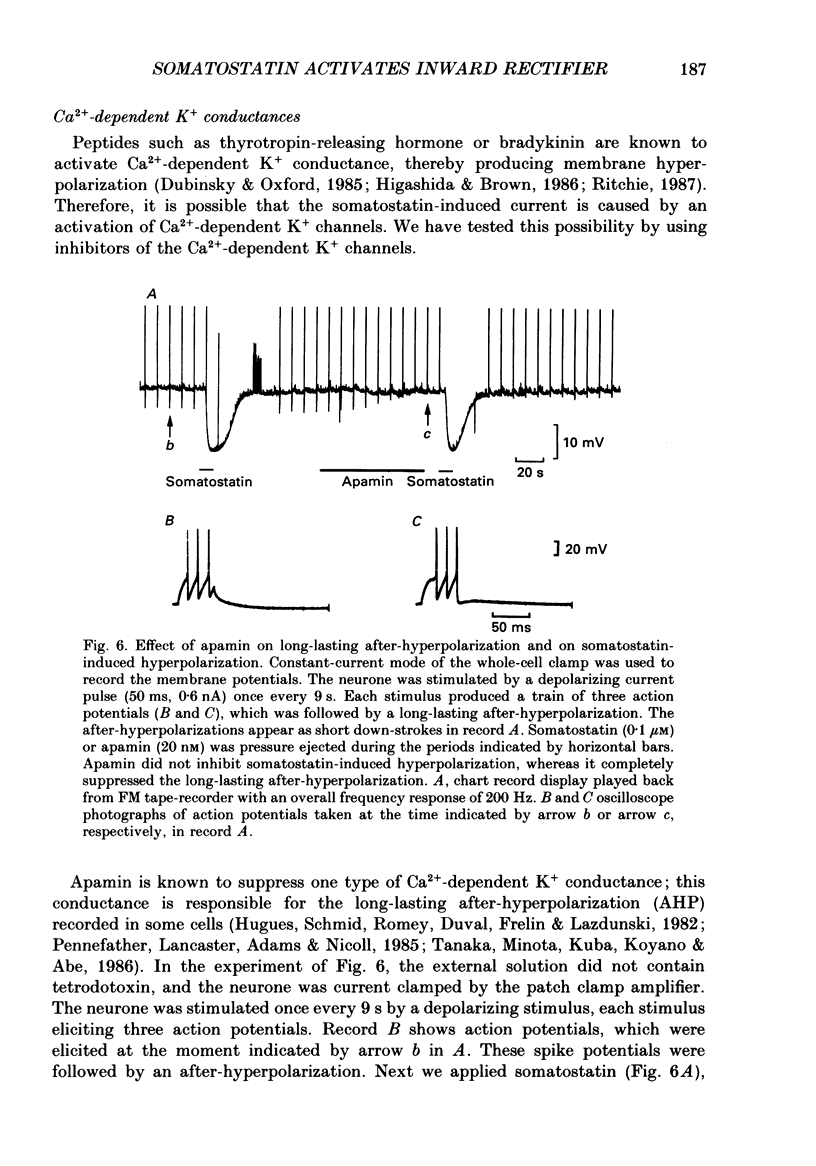
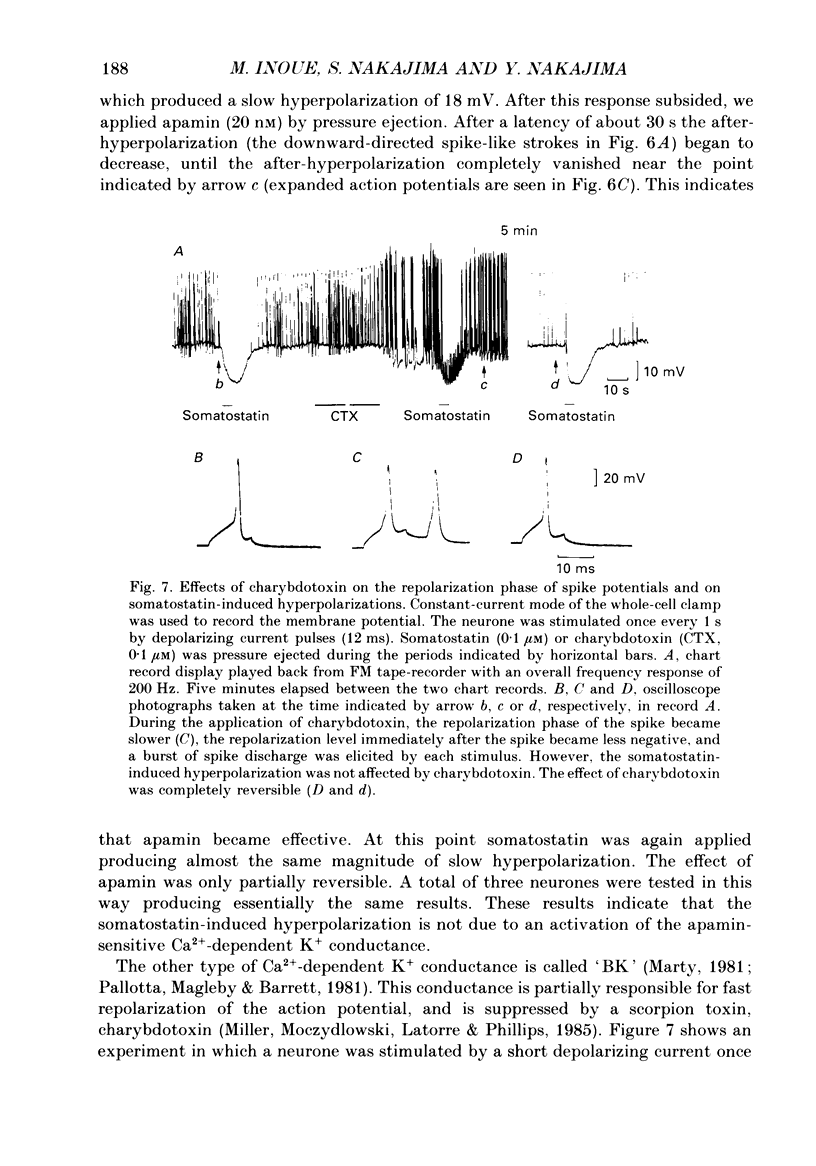
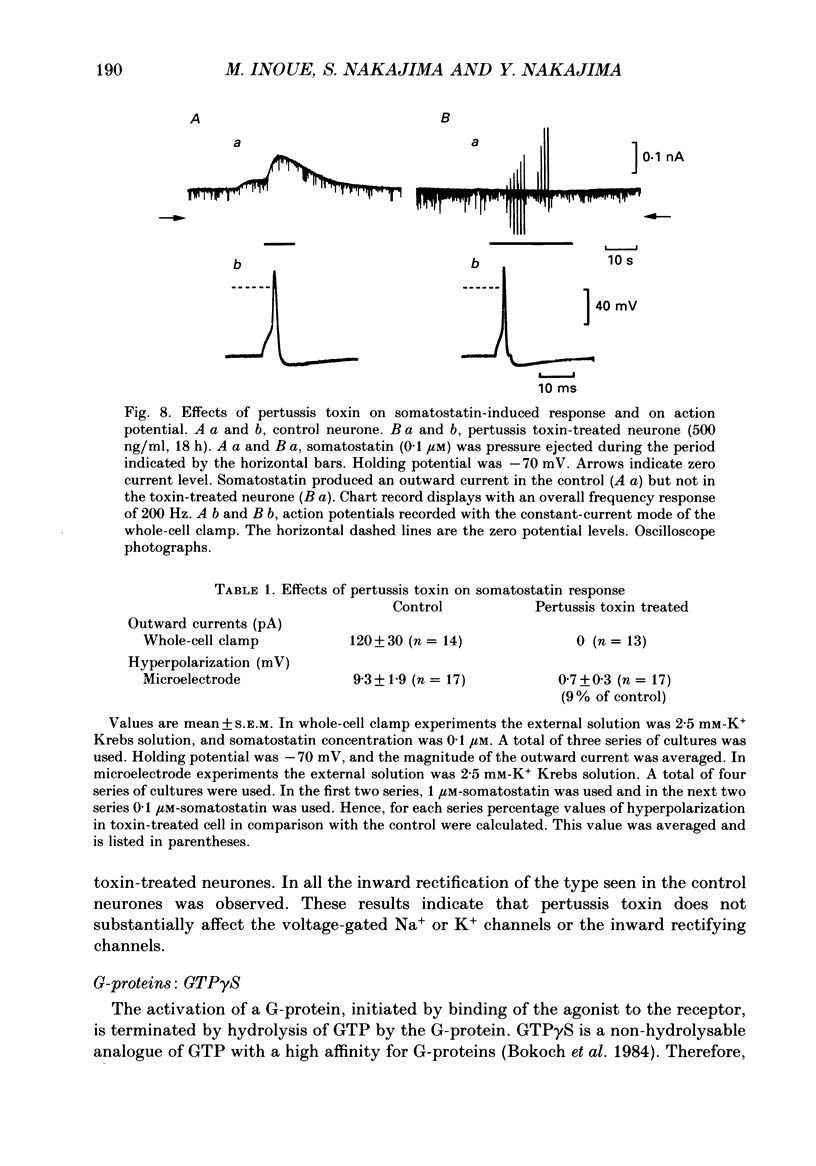
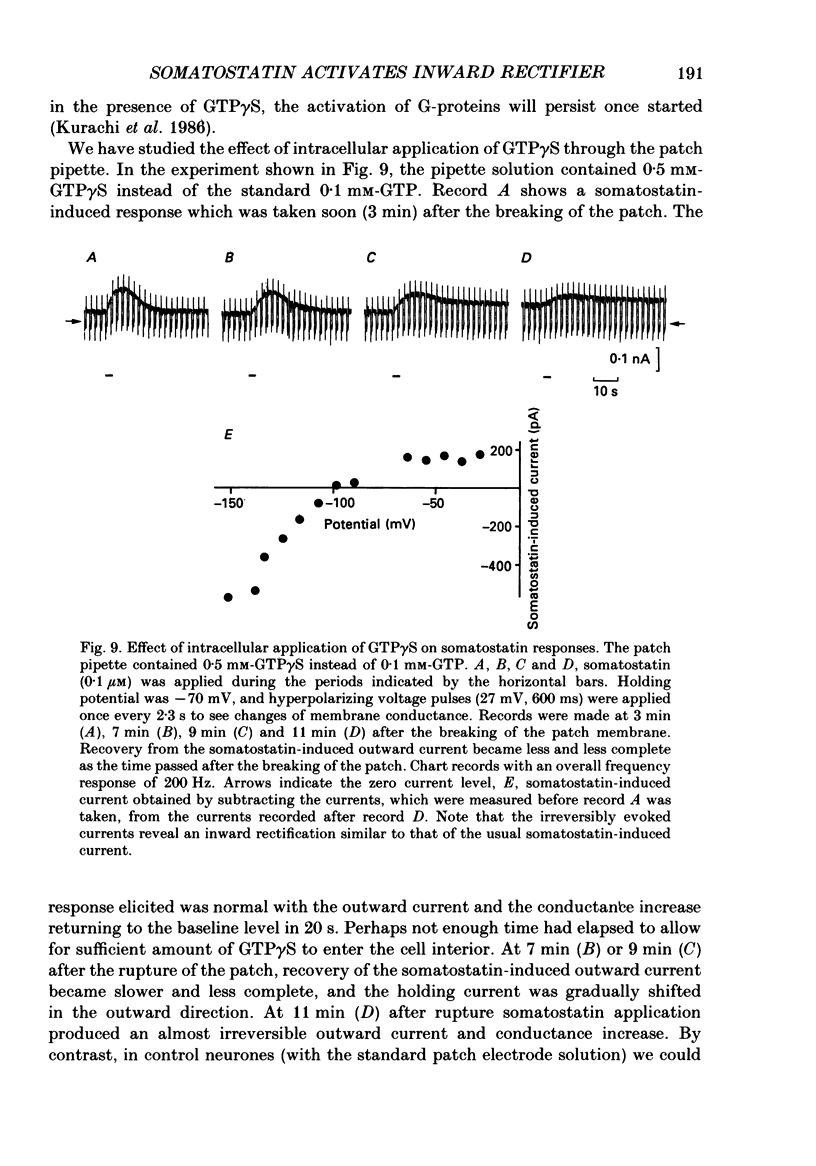
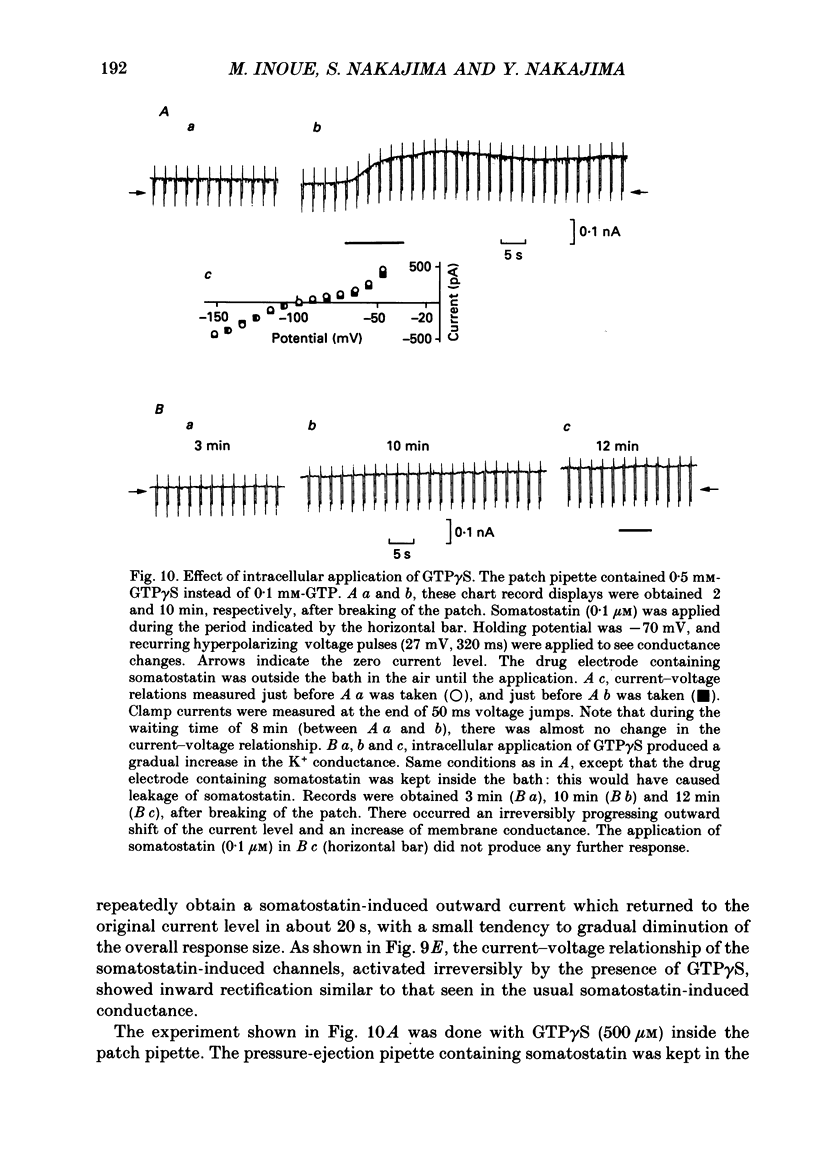
1. Membrane properties and somatostatin effects were studied in cultured locus coeruleus neurones from neonatal rats by using the whole-cell version of the patch clamp technique. 2. The current-voltage relationship of the resting cell revealed an inward-going rectification. The inward currents developed almost instantaneously upon hyperpolarizing the membrane under voltage clamp, and at large negative potentials the inward current showed a time-dependent inactivation. Extracellularly applied Cs+ or Ba2+ (0.1 mM) inhibited the inward current in a voltage-dependent manner. 3. Application of somatostatin (0.01-1 microM) produced an increase in membrane conductance. Somatostatin-induced currents were calculated by subtracting the control current from the current during the somatostatin-induced response. The somatostatin-induced current developed almost instantaneously with hyperpolarization and did not show any time-dependent inactivation. The current-voltage relationship of the somatostatin-induced current exhibited a rectification in the inward direction and showed a reversal potential. The reversal potentials were close to the K+ equilibrium potential. 4. Extracellular Cs+ or Ba2+ (0.1 mM) inhibited the somatostatin-induced currents in a voltage-dependent manner, the effectiveness increasing with hyperpolarization. The somatostatin-induced hyperpolarization was not affected by apamin (20 nM) or by charybdotoxin (100 nM). 5. These results indicate that the somatostatin-induced conductance is very similar to the inward-rectification conductance. Because the somatostatin-induced inward rectification did not exhibit a time-dependent inactivation, this rectification and the inward rectification in the control neurones may arise from two different channels. 6. Pre-treatment of neurones with pertussis toxin abolished the somatostatin-induced response, but did not affect the resting inward rectification. When GTP gamma S was applied intracellularly, somatostatin produced an irreversible activation of the inward rectification conductance. The somatostatin-induced hyperpolarization may therefore be mediated through a pertussis toxin-sensitive GTP-binding protein.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Wang Y. Y. Pertussis toxin blocks the outward currents evoked by opiate and alpha 2-agonists in locus coeruleus neurons. Brain Res. 1986 Apr 23;371(2):390–394. doi: 10.1016/0006-8993(86)90382-3. [DOI] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs J. R., Dichter M. A. Effects of somatostatin on mammalian cortical neurons in culture: physiological actions and unusual dose response characteristics. J Neurosci. 1983 Jun;3(6):1176–1188. doi: 10.1523/JNEUROSCI.03-06-01176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Kelly S. Is somatostatin an excitatory transmitter in the hippocampus? Nature. 1978 Jun 22;273(5664):674–675. doi: 10.1038/273674a0. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Dual modulation of K channels by thyrotropin-releasing hormone in clonal pituitary cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4282–4286. doi: 10.1073/pnas.82.12.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues M., Schmid H., Romey G., Duval D., Frelin C., Lazdunski M. The Ca2+-dependent slow K+ conductance in cultured rat muscle cells: characterization with apamin. EMBO J. 1982;1(9):1039–1042. doi: 10.1002/j.1460-2075.1982.tb01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Rich K. A., Herberg J. T., Grenet D., Mumby S., Codina J. Identification of a new GTP-binding protein. A Mr = 43,000 substrate for pertussis toxin. J Biol Chem. 1987 Jul 5;262(19):9239–9245. [PubMed] [Google Scholar]
- KRNJEVIC K. The distribution of Na and K in cat nerves. J Physiol. 1955 Jun 28;128(3):473–488. doi: 10.1113/jphysiol.1955.sp005319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A. The action of somatostatin on neurones of the myenteric plexus of the guinea-pig ileum. J Physiol. 1980 Jun;303:315–323. doi: 10.1113/jphysiol.1980.sp013287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux P., Pelletier G. Radioautographic localization of somatostatin-14 and somatostatin-28 binding sites in the rat brain. Peptides. 1984 May-Jun;5(3):503–506. doi: 10.1016/0196-9781(84)90078-0. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Masuko S., Nakajima Y., Nakajima S., Yamaguchi K. Noradrenergic neurons from the locus ceruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. J Neurosci. 1986 Nov;6(11):3229–3241. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Inoue M. Pertussis toxin-insensitive G protein mediates substance P-induced inhibition of potassium channels in brain neurons. Proc Natl Acad Sci U S A. 1988 May;85(10):3643–3647. doi: 10.1073/pnas.85.10.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinuma M., Katada T., Ui M. A new GTP-binding protein in differentiated human leukemic (HL-60) cells serving as the specific substrate of islet-activating protein, pertussis toxin. J Biol Chem. 1987 Jun 15;262(17):8347–8353. [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Siggins G. R. Somatostatin hyperpolarizes hippocampal pyramidal cells in vitro. Brain Res. 1981 Sep 28;221(2):402–408. doi: 10.1016/0006-8993(81)90791-5. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B., Brazeau P. Depressant action of TRH, LH-RH and somatostatin on activity of central neurones. Nature. 1975 May 15;255(5505):233–235. doi: 10.1038/255233a0. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Maurer R. Autoradiographic mapping of somatostatin receptors in the rat central nervous system and pituitary. Neuroscience. 1985 Aug;15(4):1183–1193. doi: 10.1016/0306-4522(85)90261-1. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K. Thyrotropin-releasing hormone stimulates a calcium-activated potassium current in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:611–625. doi: 10.1113/jphysiol.1987.sp016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Rubidium block and rubidium permeability of the inward rectifier of frog skeletal muscle fibres. J Physiol. 1980 Jul;304:415–435. doi: 10.1113/jphysiol.1980.sp013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Strong J. A., Fox A. P., Tsien R. W., Kaczmarek L. K. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987 Feb 19;325(6106):714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Minota S., Kuba K., Koyano K., Abe T. Differential effects of apamin on Ca2+-dependent K+ currents in bullfrog sympathetic ganglion cells. Neurosci Lett. 1986 Sep 12;69(3):233–238. doi: 10.1016/0304-3940(86)90485-4. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Yoshii M., Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N., Kojima I., Shibuya N., Ogata E. Pertussis toxin inhibits somatostatin-induced K+ conductance in human pituitary tumor cells. Am J Physiol. 1987 Jul;253(1 Pt 1):E28–E32. doi: 10.1152/ajpendo.1987.253.1.E28. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Sekura R. D., Birnbaumer L., Brown A. M. Reconstitution of somatostatin and muscarinic receptor mediated stimulation of K+ channels by isolated GK protein in clonal rat anterior pituitary cell membranes. Mol Endocrinol. 1987 Apr;1(4):283–289. doi: 10.1210/mend-1-4-283. [DOI] [PubMed] [Google Scholar]


