Abstract
Redox biocatalysis is an essential pillar of the chemical industry. Yet, the enzymes’ nature restricts most reactions to aqueous conditions, where the limited substrate solubility leads to unsustainable diluted biotranformations. Non‐aqueous media represent a strategic solution to conduct intensified biocatalytic routes. Deep eutectic solvents (DESs) are designable solvents that can be customized to meet specific application needs. Within the large design space of combining DES components (and ratios), hydrophobic DESs hold the potential to be both enzyme‐compatible – keeping the enzymes’ hydration –, and solubilizers for hydrophobic reactants. We explored two hydrophobic DESs, lidocaine/oleic acid, and lidocaine/decanoic acid, as reaction media for carbonyl reduction catalyzed by horse liver alcohol dehydrogenase, focusing on the effect of water contents and on maximizing substrate loadings. Enzymes remained highly active and stable in the DESs with 20 wt % buffer, whereas the reaction performance in DESs outperformed the pure buffer system with hydrophobic substrates (e. g., cinnamaldehyde to form the industrially relevant cinnamyl alcohol), with a 3‐fold specific activity. Notably, the cinnamaldehyde reduction was for the first time performed at 800 mM (~100 g L−1) with full conversion, which opens up new avenues to industrial applications of hydrophobic DESs for enzyme catalysis.
Keywords: Redox biocatalysis, Hydrophobic deep eutectic solvents, Alcohol dehydrogenase
Two hydrophobic DESs, lidocaine/oleic acid and lidocaine/decanoic acid, were utilized as reaction media that were enzyme‐compatible and highly substrate‐solubilizer for carbonyl reduction catalyzed by horse liver alcohol dehydrogenase, focusing on the effect of water contents and on maximizing substrate loadings, which showcases for the first time the applicability of hydrophobic DESs for industrially‐sound conditions in biocatalysis.
Introduction
Biocatalysis may be shifted from traditional aqueous media to non‐conventional media to address some issues generated by the use of aqueous conditions, such as low substrate loadings, possible reagent inhibition, and in some cases product hydrolysis. [1] In particular, the poor solubility of hydrophobic substrates in aqueous media leads to diluted reactions, causing large environmental footprints in the form of wastewater (higher reaction volume needed per product formed), as reflected by quantitative parameters (e. g., the E‐factor, the E+‐factor, and the C‐factor). [2] To reduce the environmental impact, substrate loadings of 50 g L−1, and more preferably of, or higher than 100 g L−1, are desirable for the production of fine chemicals. [8] In that respect, the concentrations of hydrophobic substrates can be substantially boosted by applying non‐aqueous media – the so‐called non‐conventional media –, which has become a useful strategy for the resource‐intensive synthesis of active pharmaceutical ingredients (APIs).[ 8 , 10 ] Moreover, using non‐conventional media offers a strategic asset to seamlessly interface biocatalysts and chemocatalysts, making them more compatible in various chemoenzymatic cascades. [12] From the use of more classic organic solvents to potentially biogenic ones (e. g., cyclopentyl methyl ether, 2‐methyltetrahydrofuran), [13] biocatalysis has also been explored in supercritical fluids (SCFs), [15] ionic liquids (ILs), [18] and more recently deep eutectic solvents. [21]
Deep eutectic solvents (DESs) have emerged as neoteric non‐aqueous solvents for biocatalysis. DESs have unique features, such as their high tunability and non‐ideal properties. The composition of a variety of hydrogen bond donors and hydrogen bond acceptors in explorable molar ratios endows DESs with a high degree of tunability of their properties (e. g., polarity, viscosity, and biodegradability). [25] Likewise, DESs can be produced from biomass‐derived compounds, leading to the coined natural deep eutectic solvents (NADES). [27] Importantly, the polarity of DESs can be fine‐tuned to (de)solubilize diverse compounds in catalytic processes. [31] As a downside, the relatively high viscosity of most DESs compared to buffer systems may hamper some of their applications.[ 25 , 32 ] To overcome this, the addition of water or elevated temperatures is effective in reducing the viscosity of most DESs, yet high temperatures can be challenging for biocatalysis unless thermostable enzymes are applied. Therefore, in most biotransformations, DESs are used in combination with buffers in specific proportions that can keep the non‐conventional nature of the media, [33] but being enzyme‐compatible at the same time. [36] The buffer content desired is different from case to case. Therefore, finding the optimal buffer content for the biotransformation in different eutectic solvents is still challenging.
Biocatalysis in DESs has been established in a variety of transformations,[ 22 , 24 , 37 ] being DESs used as solvents, co‐solvents, co‐substrates, and catalyst stabilizers. For example, many reactions engaging cofactor‐dependent oxidoreductases have been established by using DESs as solvents and cosubstrates, involving the use of smart substrates, as well as more classic cofactor regeneration systems (e. g., based on alcohol dehydrogenases and glucose dehydrogenases). [38] Most studies in biotransformations involve Type III DESs, especially the hydrophilic choline chloride (ChCl) based DESs. [24] However, the activity or stability of many biocatalysts is often reduced in these media due to the harmful effects of specific DES components, mainly by the disruption of enzyme hydration. For instance, in the case of ChCl‐glycerol, ChCl exerts a deleterious effect on enzymes. [42] On the one hand, protein engineering has been attempted to improve the catalytic performance of enzymes in DESs. [43] Moreover, solvent engineering is another powerful strategy to retain enzyme‐bound water to preserve enzyme activity. Following the established premises of biocatalysis in non‐conventional media, it should be hypothesized that some hydrophobic DESs may be advantageous over hydrophilic DESs. Therein, an ideal DES should display not only proper hydrophobicity – to enable substrate solubilization in high loadings and sufficient water‐solubilizing capacity – but also the ability to maintain enzyme hydration, thereby jointly contributing to improved catalytic efficiency. The high tunability of DESs may become an asset to define these tailored solvents.
Hydrophobic DESs have been reported for diverse applications,[ 29 , 44 ] and some have been used for chemo and biocatalysis. [46] So far, the use of hydrophobic DESs containing menthol and various fatty acids (e. g., lauric acid, decanoic acid, and dodecanoic acid) was investigated for lipase‐catalyzed esterifications for the synthesis of menthol esters, with beneficial effects on thermal stability, activity, and reusability of lipases. [47] Very recently, another hydrophobic DES, lidocaine/oleic acid (Lid‐OA), has been applied as a proof‐of‐concept to redox biocatalysis, with a main focus on the reuse of enzyme by utilizing the thermo‐switchable property of DES. [51] In particular, Lid‐OA happens to fully meet the criteria required for an ideal DES: high hydrophobicity and a relative high water capacity of 22 wt % (without forming a second phase). [52] Therefore, the promising applications of lidocaine‐based DESs in practical biocatalysis should be in‐depth explored, in particular in synthetic processes with highly water‐immiscible hydrophobic substrates, and at high loadings, to assure an economic and environmentally‐attractive biotransformation.[ 9 , 53 ]
Based on the above‐discussed rationale, this study explores the application of two lidocaine‐based DESs with two fatty acids, oleic acid (Lid‐OA), and decanoic acid (Lid‐DecA), as reaction media for the horse liver alcohol dehydrogenase (HLADH) catalyzed reduction of different carbonyl compounds for the synthesis of valuable compounds (Scheme 1). A comprehensive assessment on the impact of water contents and maximization of substrate loadings in hydrophobic environments is provided, showing that DESs can be a reaction medium for industrially‐sound conditions.
Scheme 1.
Reduction of cyclohexanone and cinnamaldehyde catalyzed by horse liver alcohol dehydrogenase (HLADH) coupled with oxidation of 1,4‐butanediol for cofactor regeneration in lidocaine‐based hydrophobic DESs.
Results and Discussion
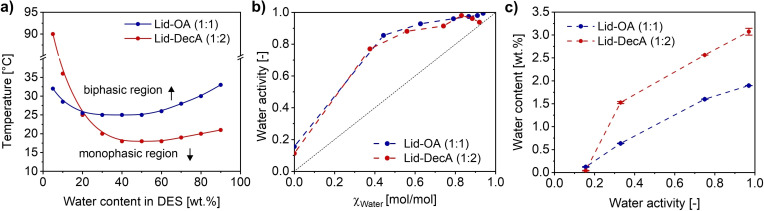
Two DESs were prepared according to the reported molar ratios, resulting in yellowish Lid‐OA (1 : 1) [52] and transparent Lid‐DecA (1 : 2) [54] (Figure S1). First, the physicochemical properties of these two DESs were experimentally determined. Lidocaine‐based DESs display thermo‐switchable hydrophobicity and may undergo phase separation upon temperature gradient, leading to lower critical solution temperatures (LCST).[ 46 , 51 , 52 ] The intensification of the molecular rotation at high temperatures and the reversal of the ionized form to its natural form jointly resulted in the phase separation.[ 46 , 52 ] Therefore, the phase behavior of DESs and DES‐water mixtures containing 5–90 wt % water was evaluated at a 0.5 °C gradient. As shown in Figure 1a, the mixtures involving Lid‐OA displayed lower variations in LCST within the range of 25–33 °C than those of Lid‐DecA. The lowest LCST of 25 °C was observed at 30–50 wt % water, in line with previous works.[ 51 , 52 ] In the case of Lid‐DecA, LCST showed a huge discrepancy at different water contents ranging from 18 °C at 40 wt % water to 90 °C at 5 wt % water. This thermal conversion property has been utilized to separate the aqueous and organic phases by switching temperatures, thus enabling the reuse of the enzyme in the aqueous medium. [51]
Figure 1.
Physicochemical properties of lidocaine‐based DESs and DES‐water mixtures. (a) LCST phase diagram, (b) water activity at room temperature, and (c) water content.
Since lidocaine‐based DESs are composed of hydrophobic constituents, their miscibility with aqueous media is limited. The water activity of both pure DESs and DES‐water mixtures containing 5–90 wt % water (equals to 0.36–0.99 mol/mol, unit conversion see Table S1) was evaluated at room temperature (Figure 1b). The water activity of freshly produced pure Lid‐OA was 0.156, while Lid‐DecA had a slightly lower value of 0.113, which was due to the absorbed water (0.074 wt % for Lid‐OA and 0.03 wt % for Lid‐DecA) from the atmosphere during the DES preparation. The water activity shows a positive deviation from its ideal behavior, which contrasts with the negative deviation of commonly used hydrophilic DESs.[ 42 , 55 ] This suggests that the DES component interacts weakly with water and even repels it, thus reducing the risk of stripping water from the enzyme surface and maintaining enzyme hydration, following the classic rules of non‐conventional solvents (based on LogP). For Lid‐DecA, the slight decrease in water activity at 30 wt % (0.82 mol/mol) indicates the phase separation at room temperature that is above the LCST, which was also observed visually. To evaluate the hydrophobicity of the DESs, the water contents of pure DESs incubated at defined water activities were quantified. Higher water contents were measured for Lid‐DecA than for Lid‐OA under the same conditions (Figure 1c), indicating Lid‐DecA has a higher water capacity and lower hydrophobicity. Due to the DESs hydrophobic nature, and for their use in enzymatic catalysis, the water content was kept ≤20 wt %, so that it can be completely dissolved in DESs forming a homogeneous system below the LCST (i. e., 20 °C). Furthermore, the viscosities of pure hydrophobic DESs at 25–45 °C, and the DES‐water mixtures with 5–20 wt % water at 20 °C were assessed. As expected, the increased temperatures resulted in lower viscosities of pure DESs, although the addition of water decreased the viscosity of Lid‐DecA but not that of Lid‐OA, which remained almost constant in the 0–20 wt % range of DES‐water mixtures (Figure S2). In any case, the observed viscosities remain all less than 280 mPa⋅s, values that are higher than that of water (0.89 mPa⋅s) but lower compared to typical ChCl‐based DESs (e. g., 343 mPa⋅s for ChCl‐Gly, 1 : 2), hence making them readily applicable in biocatalysis. [42]
To assess the potential of lidocaine‐based hydrophobic DESs in biocatalysis, the reduction of cyclohexanone catalyzed by the purified horse liver alcohol dehydrogenase (HLADH) (SDS‐PAGE see Figure S3) was first applied as a model reaction (analytic details see Table S2, Figures S4 and S5). The reaction was conducted below the LCST at 20 °C to ensure a homogeneous DES‐water system. The reduction was initially performed by directly adding equimolar cofactor NADH in DESs with 20 wt % buffer and comparing it to the pure buffer system. In both DESs, the initial reaction rates and the yields (~65 %) were comparable to those of pure buffer (Figure S6), contrary to that observed for hydrophilic DESs, where enzymes are deactivated at such less water contents (e. g., ≤20 wt %). [36] Driven by these promising results, the model reaction was carried out in combination with a “smart cosubstrate” 1,4‐butanediol for NADH regeneration to shift reaction equilibria to the product formation (Scheme 1), [56] in the DES systems containing 5–20 wt % buffer. For both DES systems, adding 20 wt % buffer led to the highest reaction rates (compared to 10 and 5 wt %), although it was still slightly slower than the observed in pure buffer system (Figure 2a–c). The enzyme displayed higher activity in Lid‐OA than in Lid‐DecA, with faster initial reaction rates in all cases. For instance, in the case of 5 wt % buffer a full conversion was achieved after 24 hours for Lid‐OA, while the Lid‐DecA lagged behind (Figure 2a). However, it is noteworthy that the reaction in Lid‐DecA with 5 wt % buffer still proceeded linearly, implying that the enzyme was not being inactivated, while in other hydrophilic DESs under water‐limited conditions strong deactivation was observed. [36] These results support the hypothesis that hydrophobic DESs are more amenable to sustaining enzyme hydration with minimal water.
Figure 2.
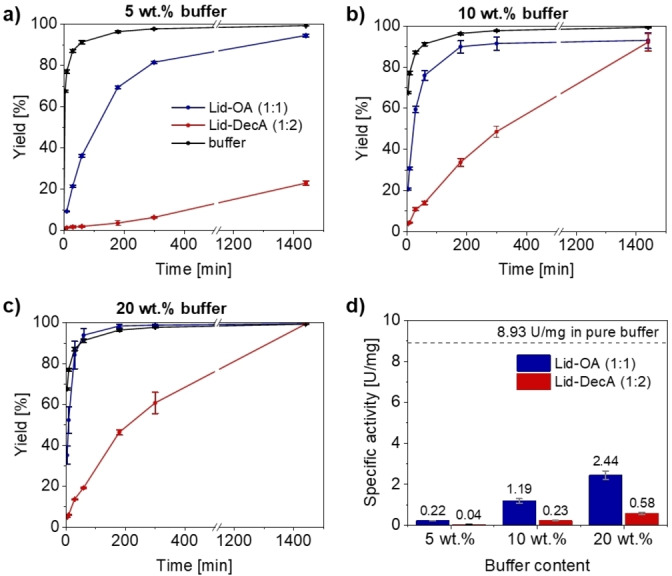
HLADH‐catalyzed reduction of cyclohexanone coupled with 1,4‐butanediol (1,4‐BD) in the DESs with 5, 10, 20 wt % buffer with reference to pure buffer. (a–c) Progressive curves, and (d) specific activities. Reaction conditions: 500 μL reaction system containing cyclohexanone (50 mM), 1,4‐butanediol (25 mM), NADH (1 mM), and HLADH (3 mg/mL for progress curves, 1 mg/mL for specific activity analysis) at 1000 rpm, 20 °C. Results are averages of biological duplicates.
To assess the enzymatic specific activity, the reaction was performed using less enzyme loadings to ensure ≤10 % conversion (i. e., ≤5 mM). The enzyme resulted most active in buffer with a specific activity of 3.6 times higher than in the best of the DES conditions (i. e., Lid‐OA with 20 wt % buffer), where the activity decreased at lower water contents (Figure 2d). The enzyme displayed higher activity in Lid‐OA than Lid‐DecA (4–5.5 times higher) for all the water contents. Compared to Lid‐OA with 20wt % (28 % relative activity), a lower specific activity (15 % relative activity) was recorded for this enzyme in choline chloride/glycerol (ChCl‐Gly, 1 : 2) with equivalent water content. [42] However, in that case the pure buffer system still outperforms the hydrophobic DESs, presumably due to the very high solubility of cyclohexanone in aqueous conditions (~850 mM at room temperature).
To shed more light on the enzymatic performance in hydrophobic DESs, the enzyme stability was next assessed, since DESs can lead to divergent effects on activity and stability of enzymes. [42] The enzyme stability was assessed in the hydrophobic DESs with 20 wt % buffer, and compared to pure buffer. The enzyme was incubated in the three systems for a specific time (up to 48 hours) and then subjected to the cyclohexanone reduction. The residual specific activities of the incubated enzyme were determined based on the initial reaction rate, while the final conversions were compared (Figure 3). As observed, high specific activities were obtained in pure buffer, but it decreased by 28 % after 48 hours of incubation. In the DES systems, the specific activity was generally lower but remained almost constant, especially for Lid‐DecA, displaying high operational stability of the enzyme (Figure 3a). At 24‐hour reaction time, the reaction still reached high yields (>90 %) for all cases, indicating that sufficient enzyme activity was still present in the reaction (Figure 3b). Overall, the enzyme showed large operational stability in the hydrophobic DESs. In combination with the promising activity observed, the lidocaine‐based hydrophobic DESs may become desirable solvents for biocatalysis.
Figure 3.

Operational stability of the purified HLADH in DESs with 20 wt % buffer with reference to pure buffer: (a) specific activity of incubated HLADH, (b) yield of cyclohexanol after 24 h. Operational conditions: HLADH (3 mg/mL) and NADH (1 mM) in 500 μL system was incubated at 1000 rpm, 20 °C for defined times at when cyclohexanone (50 mM) and 1,4‐butanediol (25 mM) were added to react at 1000 rpm, 20 °C for 24 hours. Results are averages of biological duplicates.
So far, the model reaction uses a water‐miscible substrate, which may not be the appropriate one to assess the capacity of a hydrophobic DES for industrial biocatalysis. In fact, the set‐up of enzymatic reactions in non‐conventional media is motivated by the need of using water‐immiscible highly hydrophobic substrates, for which aqueous solutions are typically suboptimal. Thus, to further validate the suitability of these hydrophobic DESs under more demanding industrially‐sound water‐immiscible substrates such as cinnamaldehyde (CinH), cinnamyl alcohol (CinOH) was synthesized (Figure 4a). Interestingly, CinH displays a solubility of 1.42 g ⋅ L−1 (10.7 mM) in buffer at 25 °C, while a considerably much higher solubility is observed in hydrophobic DESs (>800 mM). Moreover, CinOH is a useful intermediate for the synthesis of diverse valuable chemicals (e. g., cinnamyl esters, and flunarizine) for food, cosmetic, and pharma industries. [57] First experiments were performed with 50 mM CinH in buffer or in Lid‐OA and Lid‐DecA containing 20 wt % buffer (Figure 4, see also Figure S9). Comparable reaction trends were observed for the reactions performed in pure buffer and in Lid‐DecA, although the full conversion was only accomplished in Lid‐DecA, compared to that of 66 % in the buffer after 24 hours. Surprisingly, the reaction proceeded much faster in Lid‐OA and led to the plateau of 90 % conversion after 3 hours (Figure 4b). Furthermore, for this highly hydrophobic substrate, the specific activity of the enzyme was 3‐fold higher in Lid‐OA than in pure buffer (Figure 4c). The outstanding performance observed for Lid‐OA may be ascribed to its higher hydrophobicity due to the longer alkyl chain of oleic acid (C18) compared to decanoic acid (C10), thus enhancing the beneficial effect regarding water activity and enzyme hydration.
Figure 4.

(a) HLADH‐catalyzed reduction of cinnamaldehyde (CinH) coupled with oxidation of 1,4‐butanediol (1,4‐BD) in DESs with 20 wt % buffer with reference to pure buffer. (b) Progressive curves, c) specific activities. Reaction conditions: 500 μL reaction system containing CinH (50 mM), 1,4‐BD (25 mM), HLADH (3 mg/mL), and NADH (1 mM) at 1000 rpm, 20 °C. Analytic details see Table S3, Figures S7 and S8. Results are averages of biological duplicates.
As stated above, one important motivation of applying DESs is to operate biotransformations under high substrate loadings, especially in the cases where industrially‐needed water‐immiscible hydrophobic substrates are used. Thus, encouraged by the promising results at 50 mM, increasingly higher substrate loadings (200–800 mM) were applied in both DESs containing 20 wt % buffer, and compared with a pure buffer system. Surprisingly, in a pure buffer system, the reaction at 200 mM hardly proceeded and resulted in a yield of only 1.5 % after 48 hours (Figure 5a). This result could be attributed to large amounts of cinnamaldehyde surrounding the enzyme, leading to visible precipitation of the enzyme. Conversely, the reaction proceeded much faster in Lid‐OA followed by Lid‐DecA, leading to the yields of 90 % and 74 % after 24 hours, respectively (200 mM substrate loading, Figure 5a). This significant improvement in the DES systems is mainly due to the much higher solubility of cinnamaldehyde (>800 mM), which may decrease the inactivation of the enzyme by the substrate and may solve mass‐transfer limitations. At higher concentrations of cinnamaldehyde in hydrophobic DESs, the yields in all cases reached 72–85 % after 24 hours and further increased to 88–94 % after 48 hours (Figure 5b). Compared to Lid‐OA, despite the general lower yields of around 55 % for Lid‐DecA after 24 hours, the reaction was extended to 48 hours with a significant increase in yields to 85 % (Figure 5c).
Figure 5.

HLADH‐catalyzed reduction of cinnamaldehyde (CinH) coupled with 1,4‐butanediol (1,4‐BD) in DESs with 20 wt % buffer with reference to pure buffer. (a) Progressive curve of 200 mM CinH, (b) Yields in Lid‐OA, (c) Yields in Lid‐DecA. Reaction conditions: 500 μL reaction system containing CinH (50–800 mM), 1,4‐BD (25–400 mM), HLADH (2 mg/mL), and NADH (1 mM) at 1000 rpm, 20 °C. Results are averages of biological duplicates.
Furthermore, a higher concentration of 800 mM cinnamaldehyde was assessed, which corresponds to ~100 g substrate per L reaction medium. Such high substrate loading results promising for industrial conditions, and offers an appropriate resource use of solvents, leading to diminished wastes, compared to non‐intensified systems. [58] Gratifyingly, the reactions were efficient in both DESs and reached almost complete conversions after 48 hours, under the non‐optimized conditions applied. For the first time, the enzymatic reduction of cinnamaldehyde can be performed effectively under such high‐loading conditions suitable for industrial use using hydrophobic DESs as tailored reaction media. The use of hydrophobic DESs offers a working window with promising alternatives for biocatalysis in non‐conventional media.
Moreover, highly‐intensified processing conditions lead to improved environmental metrics, because resources – solvents and water – are more properly used.[ 9 , 53 , 59 ] When reactions are performed in the range of 100 g L−1, an E‐factor and PMI in the range of 10–11 should be expected, respectively (upstream part). Being an organic solvent, a C‐factor in the range of 25 kg CO2 kg Product−1 may be reached during the incineration step of the spent solvent.[ 5 , 6 , 7 , 9 , 58 ] Although environmental estimations are promising for these intensified systems and show the potential that using non‐conventional media may have (as it often triggers higher substrate loadings than aqueous conditions), results can still be improved by considering integrated processes in which the solvent can be reused several times before incineration takes place. Likewise, the design of more robust enzymes may enable processes with even higher substrate loadings, leading to more efficient and more environment‐friendly systems.
Conclusions
The applicability of hydrophobic lidocaine‐based DESs for redox biocatalysis has been explored. These hydrophobic ‘designed’ media enable efficient biotransformations under low water contents, which were here limited up to 20 wt % to sustain a homogeneous monophasic system below the LCST and to fully assess the interactions of DES‐enzyme. When water‐miscible substrates are applied (e. g., in the case of cyclohexanone reduction), Lid‐OA (1 : 1) outperforms Lid‐DecA (1 : 2), but both DESs do not result as effectively as the pure buffer system. For the DESs, the higher the water content (5–20 wt %), the higher the reaction efficiency, revealing the optimal condition of Lid‐OA (1 : 1) with 20 wt % buffer. Importantly, when the hydrophobic media were applied to water‐immiscible substrates (e. g., cinnamaldehyde), the specific activity resulted 3‐fold of the pure buffer system. Thus, cinnamaldehyde loadings were increased to 800 mM, equivalent to an industrial loading of 100 g L−1, resulting in a complete conversion. Therefore, the solubilization advantages obtained with hydrophobic DESs are presented.
This work showcases for the first time the applicability of hydrophobic DESs for industrially‐sound conditions in biocatalysis, by providing reaction media that can be enzyme‐compatible and highly substrate‐solubilizer at the same time. An important remaining step is the development of integrated downstream conditions for product isolation. Aspects related to the formation of more than one phase of these hydrophobic DESs at different temperatures may offer an opportunity window to explore these steps. Future work will focus on this, as well as on extending the technology to other enzyme systems and substrates, to untap the potential the tunable DESs for industrial (and more sustainable) biotransformations.
Conflict of Interests
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
The authors acknowledge the financial support from Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under grant no. 391127961. The project has received funding from the EU′s Horizon Europe Doctoral Network Program under the Marie Skłodowska‐Curie grant agreement no. 101072731.
Zhang N., Lahmann V., Bittner J. P., Domínguez de María P., Jakobtorweihen S., Smirnova I., Kara S., ChemSusChem 2025, 18, e202402075. 10.1002/cssc.202402075
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. van Schie M., Sporing J. D., Bocola M., Domínguez de María P., Rother D., Green Chem. 2021, 23, 3191–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ni Y., Holtmann D., Hollmann F., ChemCatChem 2014, 6, 930–943. [Google Scholar]
- 3.R. A. Sheldon, in Biocatalysis in Green Solvents (Ed.: P. Lozano), Elsevier Inc., 2022, pp. 1–22.
- 4. Tieves F., Tonin F., Fernández-Fueyo E., Robbins J. M., Bommarius B., Bommarius A. S., Alcalde M., Hollmann F., Tetrahedron 2019, 75, 1311–1314. [Google Scholar]
- 5. Domínguez de María P., Kara S., Gallou F., Molecules 2023, 28, 6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onken U., Koettgen A., Scheidat H., Schueepp P., Gallou F., Chimia 2019, 73, 730–736. [DOI] [PubMed] [Google Scholar]
- 7. Petermeier P., Domínguez de María P., Byström E., Kara S., ACS Sustainable Chem. Eng. 2024, 12, 12869–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Domínguez de María P., Hollmann F., Front. Microbiol. 2015, 6, 1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Domínguez de María P., Green Chem. 2022, 24, 9620–9628. [Google Scholar]
- 10.S. Nieto, R. Villa, A. Donaire, P. Lozano, in Biocatalysis in Green Solvents (Ed.: P. Lozano), 2022, 23–55.
- 11.A. Illanes, in White Biotechnology for Sustainable Chemistry (Eds.: M. A. Coelho, B. D. Ribeiro), 2015, 36–51.
- 12. Kourist R., González-Sabín J., ChemCatChem 2020, 12, 1903–1912. [Google Scholar]
- 13. de Gonzalo G., Alcantara A. R., Domínguez de María P., ChemSusChem 2019, 12, 2083–2097. [DOI] [PubMed] [Google Scholar]
- 14. Pace V., Hoyos P., Castoldi L., Domínguez de María P., Alcantara A. R., ChemSusChem 2012, 5, 1369–1379. [DOI] [PubMed] [Google Scholar]
- 15. Wimmer Z., Zarevúcka M., Int. J. Mol. Sci. 2010, 11, 233–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuda T., J. Biosci. Bioeng. 2013, 115, 233–241. [DOI] [PubMed] [Google Scholar]
- 17. Matsuda T., Harada T., Nakamura K., Curr. Org. Chem. 2005, 9, 299–315. [Google Scholar]
- 18. Sheldon R. A., Green Chem. 2021, 23, 8406–8427. [Google Scholar]
- 19. Fernandez-Alvaro E., Domínguez de María P., Curr. Org. Chem. 2012, 16, 2492–2507. [Google Scholar]
- 20. Itoh T., Chem. Rev. 2017, 117, 10567–10607. [DOI] [PubMed] [Google Scholar]
- 21. Xu P., Zheng G. W., Zong M. H., Li N., Lou W. Y., Bioresour. Bioprocess. 2017, 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.C. E. Paul, V. Gotor-Fernández, in Biocatalysis in Green Solvents (Ed.: P. Lozano), Elsevier Inc., 2022, pp. 467–510.
- 23. Panić M., Bubalo M. C., Redovniković I. R., J. Chem. Technol. Biotechnol. 2021, 96, 14–30. [Google Scholar]
- 24. Zhang N., Domínguez de María P., Kara S., Catalysts 2024, 14, 84. [Google Scholar]
- 25. Smith E. L., Abbott A. P., Ryder K. S., Chem. Rev. 2014, 114, 11060–11082. [DOI] [PubMed] [Google Scholar]
- 26. Hansen B. B., Spittle S., Chen B., Poe D., Zhang Y., Klein J. M., Horton A., Adhikari L., Zelovich T., Doherty B. W., Gurkan B., Maginn E. J., Ragauskas A., Dadmun M., Zawodzinski T. A., Baker G. A., Tuckerman M. E., Savinell R. F., Sangoro J. R., Chem. Rev. 2021, 121, 1232–1285. [DOI] [PubMed] [Google Scholar]
- 27. Xia N., Xiong L., Bi S., Qian F., Wang P., Bioprocess. Biosyst. Eng. 2020, 43, 1987–1997. [DOI] [PubMed] [Google Scholar]
- 28. Tian Y., Zhu M., Hu T., Liu C., Int. J. Biol. Macromol. 2023, 247, 125477. [DOI] [PubMed] [Google Scholar]
- 29. van Osch D. J. G. P., Dietz C. H. J. T., van Spronsen J., Kroon M. C., Gallucci F., van Sint Annaland M., Tuinier R., ACS Sustainable Chem. Eng. 2019, 7, 2933–2942. [Google Scholar]
- 30. Paiva A., Craveiro R., Aroso I., Martins M., Reis R. L., Duarte A. R. C., ACS Sustainable Chem. Eng. 2014, 2, 1063–1071. [Google Scholar]
- 31. Schweiger A. K., Ríos-Lombardía N., Winkler C. K., Schmidt S., Morís F., Kroutil W., González-Sabín J., Kourist R., ACS Sustainable Chem. Eng. 2019, 7, 16364–16370. [Google Scholar]
- 32. Zhang Q., De Oliveira Vigier K., Royer S., Jerome F., Chem. Soc. Rev. 2012, 41, 7108–7146. [DOI] [PubMed] [Google Scholar]
- 33. Hammond O. S., Bowron D. T., Edler K. J., Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabriele F., Chiarini M., Germani R., Tiecco M., Spreti N., J. Mol. Liq. 2019, 291, 111301. [Google Scholar]
- 35. Weng L., Toner M., Phys. Chem. Chem. Phys. 2018, 20, 22455–22462. [DOI] [PubMed] [Google Scholar]
- 36. Huang L., Bittner J. P., Domínguez de María P., Jakobtorweihen S., Kara S., ChemBioChem 2020, 21, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gotor-Fernandez V., Paul C. E., J. Biotechnol. 2019, 293, 24–35. [DOI] [PubMed] [Google Scholar]
- 38. Hassani F. Z. I., Amzazi S., Kreit J., Lavandera I., ChemCatChem 2020, 12, 832–836. [Google Scholar]
- 39. Müller C. R., Lavandera I., Gotor-Fernández V., Domínguez de María P., ChemCatChem 2015, 7, 2654–2659. [Google Scholar]
- 40. Mourelle-Insua A., Lavandera I., Gotor-Fernández V., Green Chem. 2019, 21, 2946–2951. [Google Scholar]
- 41. Maugeri Z., Domínguez de María P., ChemCatChem 2014, 6, 1535–1537. [Google Scholar]
- 42. Bittner J. P., Zhang N., Huang L., Domínguez de María P., Jakobtorweihen S., Kara S., Green Chem. 2022, 24, 1120–1131. [Google Scholar]
- 43. Wang X., Sheng Y., Cui H., Qiao J., Song Y., Li X., Huang H., Angew. Chem. Int. Ed. 2023, 63, e202315125. [DOI] [PubMed] [Google Scholar]
- 44. Cao J., Su E., J. Cleaner Prod. 2021, 314, 127965. [Google Scholar]
- 45. van Osch D. J. G. P., Dietz C. H. J. T., Warrag S. E. E., Kroon M. C., ACS Sustainable Chem. Eng. 2020, 8, 10591–10612. [Google Scholar]
- 46. Pouget F. C., Andanson J.-M., Gautier A., RSC Sustainability 2023, 1, 1826–1832. [Google Scholar]
- 47. Craveiro R., Meneses L., Durazzo L., Rocha A., Silva J. M., Reis R. L., Barreiros S., Duarte A. R. C., Paiva A., ACS Sustainable Chem. Eng. 2019, 7, 19943–19950. [Google Scholar]
- 48. Elgharbawy A., Syed S., Putra, Khan H., Azmi N., Sani M., Ab llah N., Hayyan A., Jewaratnam J., Basirun W., Processes 2023, 11, 547. [Google Scholar]
- 49. Hümmer M., Kara S., Liese A., Huth I., Schrader J., Holtmann D., Mol. Catal. 2018, 458, 67–72. [Google Scholar]
- 50. Patzold M., Burek B. O., Liese A., Bloh J. Z., Holtmann D., Bioprocess. Biosyst. Eng. 2019, 42, 1385–1389. [DOI] [PubMed] [Google Scholar]
- 51. Meyer L. E., Andersen M. B., Kara S., Angew. Chem. Int. Ed. 2022, 61, e202203823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Longeras O., Gautier A., Ballerat-Busserolles K., Andanson J.-M., ACS Sustainable Chem. Eng. 2020, 8, 12516–12520. [Google Scholar]
- 53. Domínguez de María P., Curr. Opin. Green Sustainable Chem. 2021, 31, 100514. [Google Scholar]
- 54. Griffin P. J., Cosby T., Holt A. P., Benson R. S., Sangoro J. R., J. Phys. Chem. B 2014, 118, 9378–9385. [DOI] [PubMed] [Google Scholar]
- 55. Bittner J. P., Huang L., Zhang N., Kara S., Jakobtorweihen S., J. Chem. Theory Comput. 2021, 17, 5322–5341. [DOI] [PubMed] [Google Scholar]
- 56. Kara S., Spickermann D., Schrittwieser J. H., Leggewie C., van Berkel W. J. H., Arends I. W. C. E., Hollmann F., Green Chem. 2013, 15, 330–335. [Google Scholar]
- 57. Chamouleau F., Hagedorn C., May O., Gröger H., Flavour Fragr. J. 2007, 22, 169–172. [Google Scholar]
- 58. Domínguez de María P., Kara S., RSC Sustainability 2024, 2, 608–615. [Google Scholar]
- 59.P. Domínguez de María, RSC Sustainability, 2024, Doi: 10.1039/d4su00535j. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.