Abstract
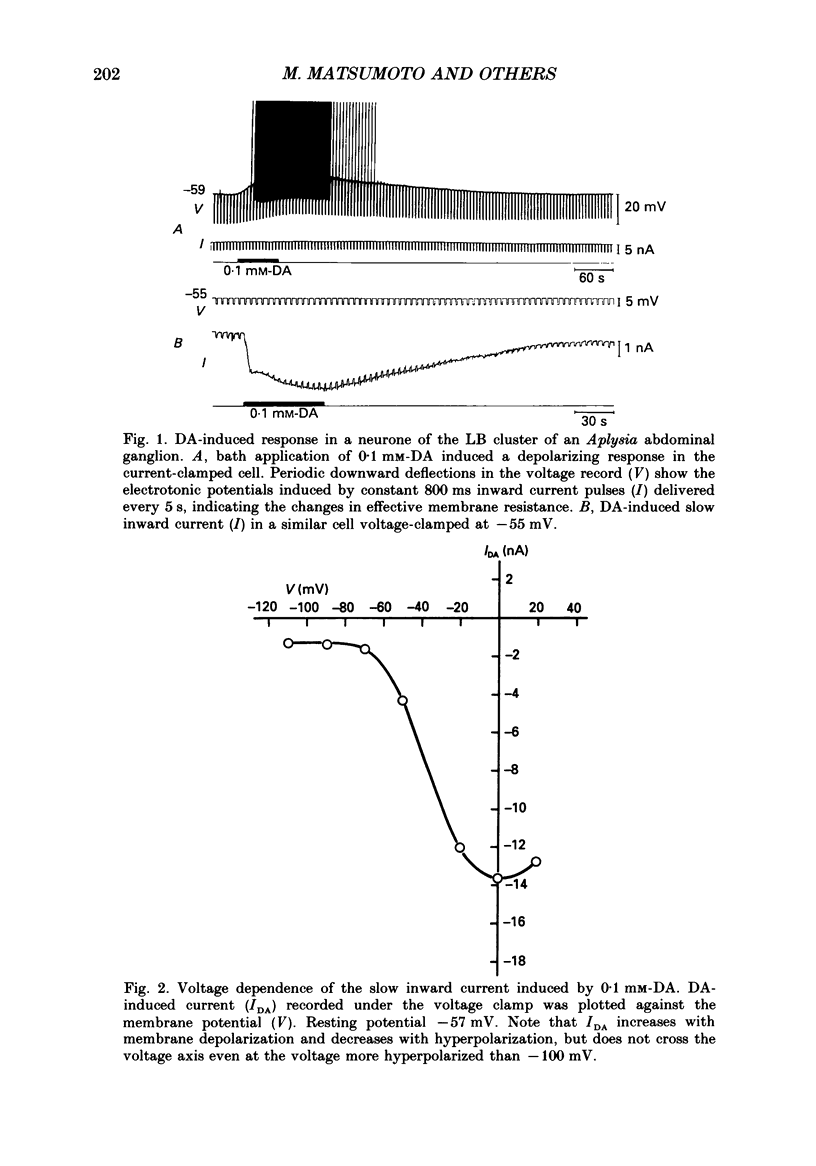
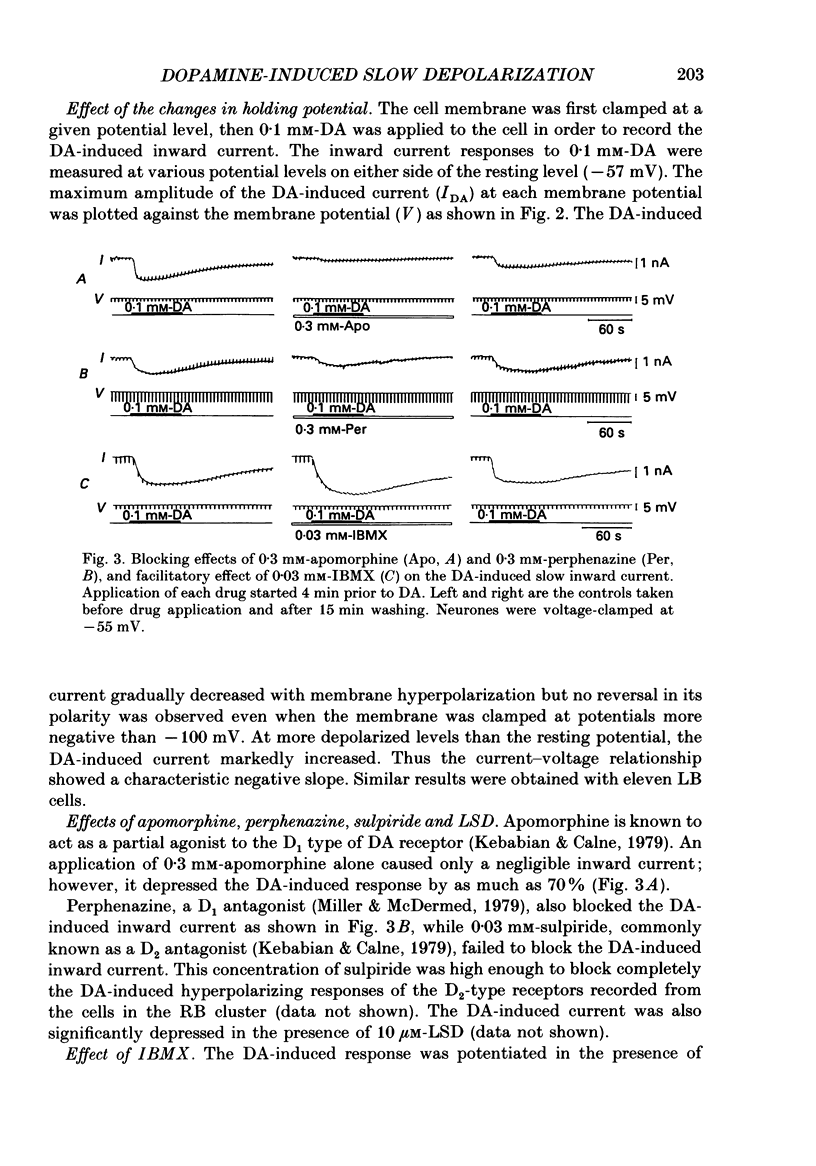
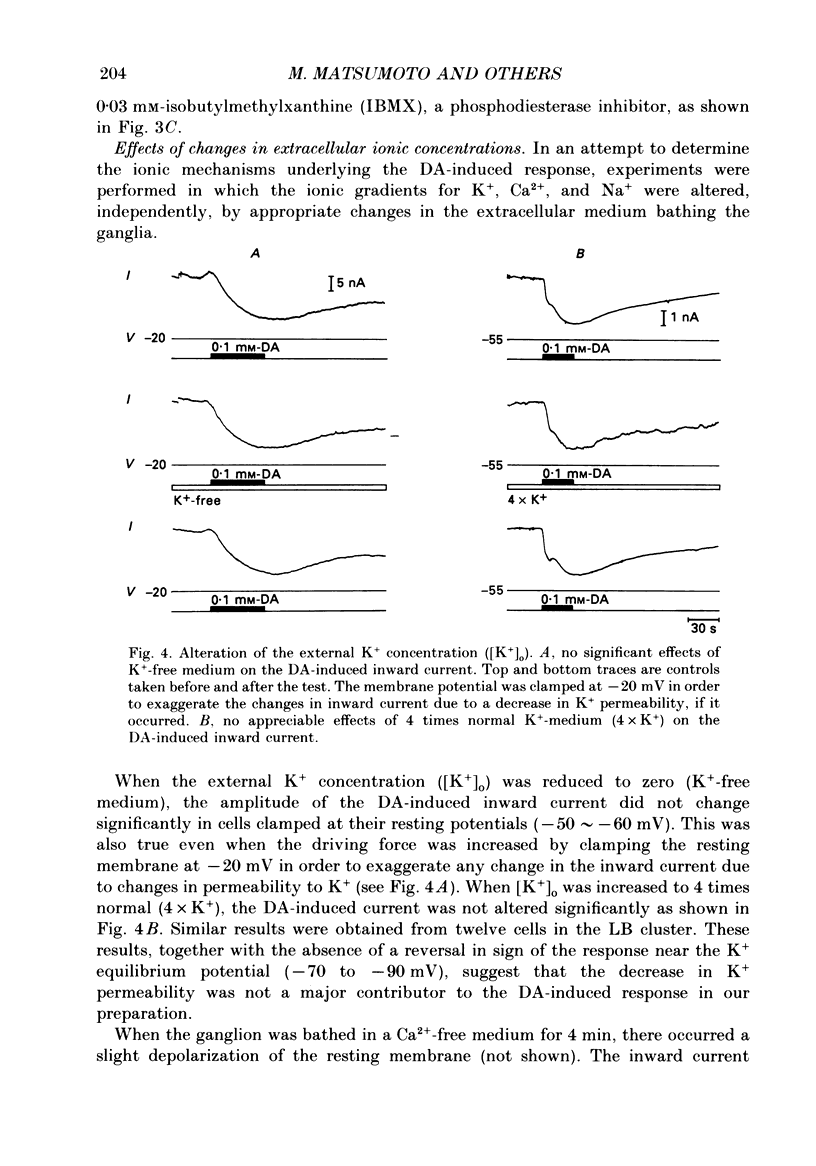
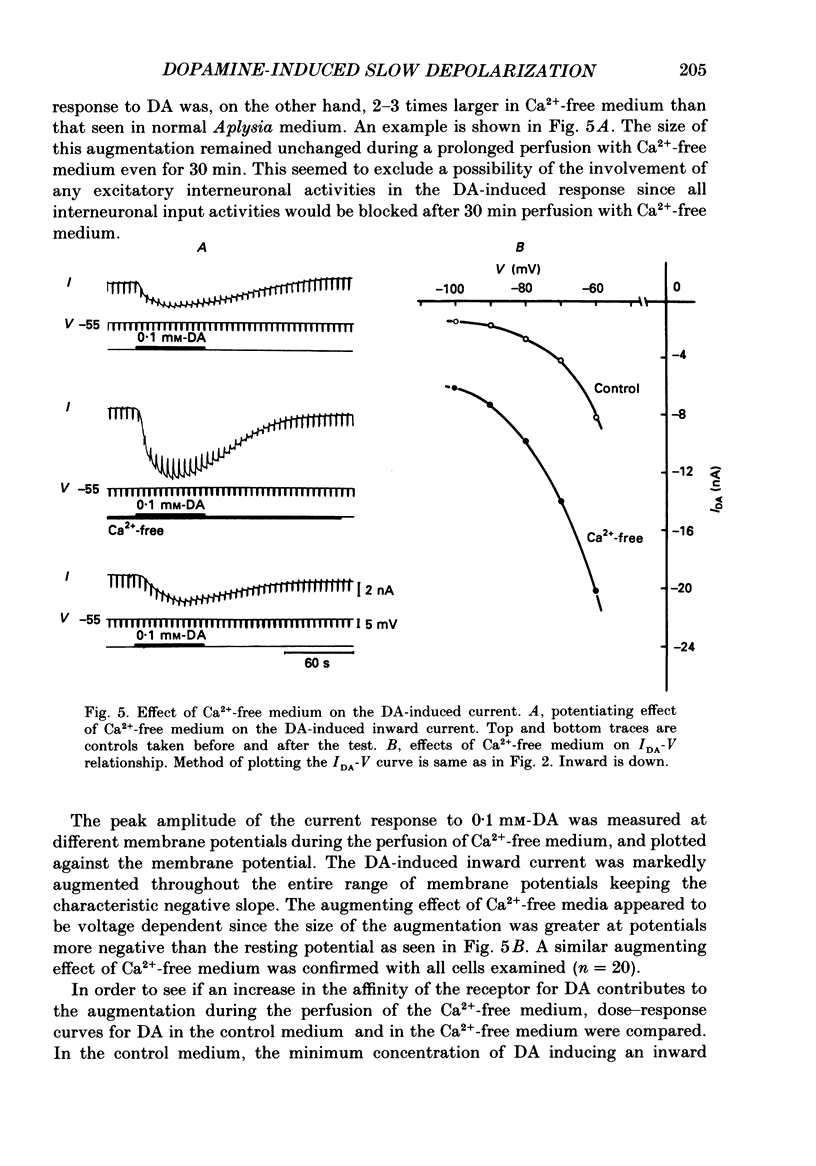
1. Current- and voltage-clamp methods were used to evaluate the intracellular and ionic mechanisms involved in dopamine-induced slow depolarizations recorded from neurones of the LB cluster in the abdominal ganglion of Aplysia kurodai. 2. In voltage-clamped cells, dopamine induced a slow inward current that, over the range studied (-40 to -110 mV), decreased in amplitude with hyperpolarization of the cell, but failed to invert when the cell was hyperpolarized beyond the reversal potential for K+,(E)K. 3. Bathing the ganglion in 3-isobutyl-1-methylxanthine (IBMX) caused a significant increase in the dopamine response. 4. Most of the responses to dopamine were markedly augmented in Ca2+-free media, but were depressed in Na+-free media. 5. An intracellular injection of cyclic adenosine 3',5'-monophosphate (cyclic AMP) into the same cell type produced an inward current which, like the response to dopamine, diminished in amplitude with hyperpolarization of the cell. 6. Like the dopamine response, the cyclic AMP response increased in the presence of IBMX, was enhanced in Ca2+-free media, was depressed in Na+-free media, and was unaffected by changes in external potassium. 7. In a few cells, although the cyclic AMP-induced responses disappeared in Na+-free media, the dopamine-induced slow inward current responses did not. However, these Na+-free resistant responses disappeared completely in Na+- and Ca2+-free media. 8. It was concluded that most of the dopamine-induced inward current responses were produced by an increase in permeability, mainly to Na+, triggered by a receptor-controlled increase in intracellular cyclic AMP.
Full text
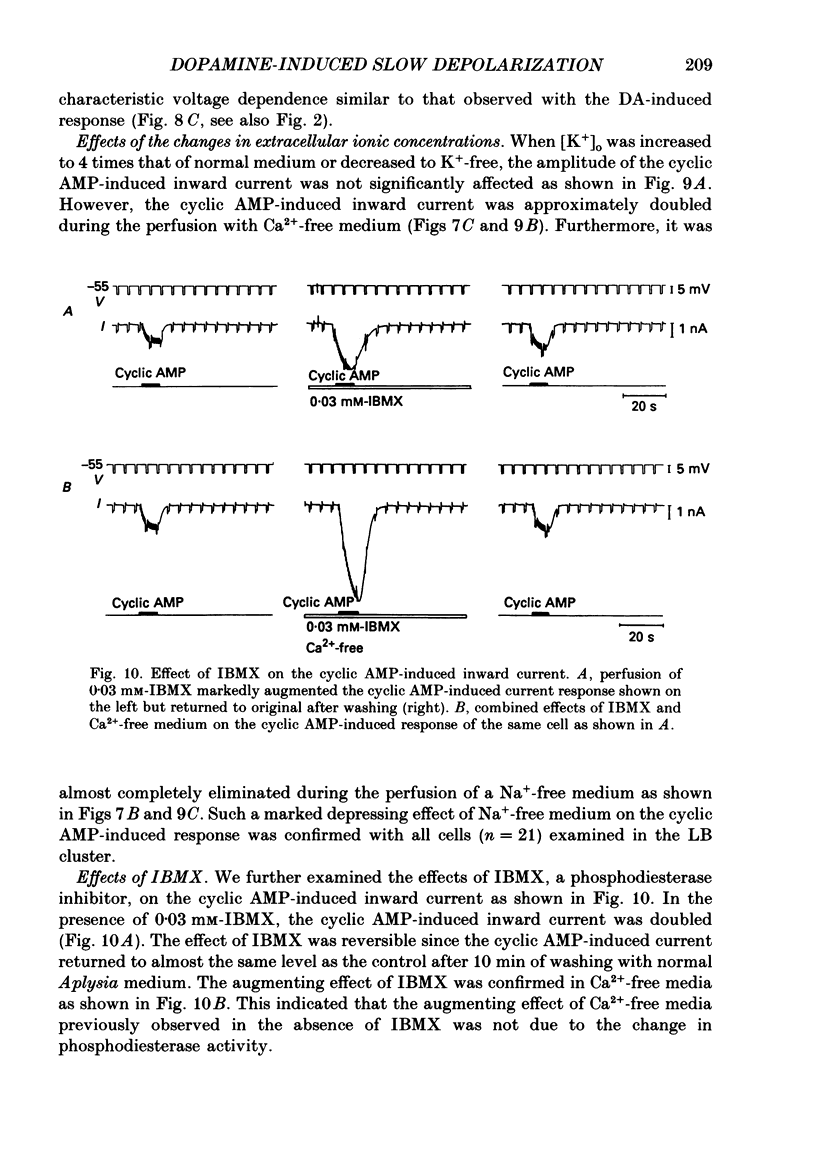
PDF


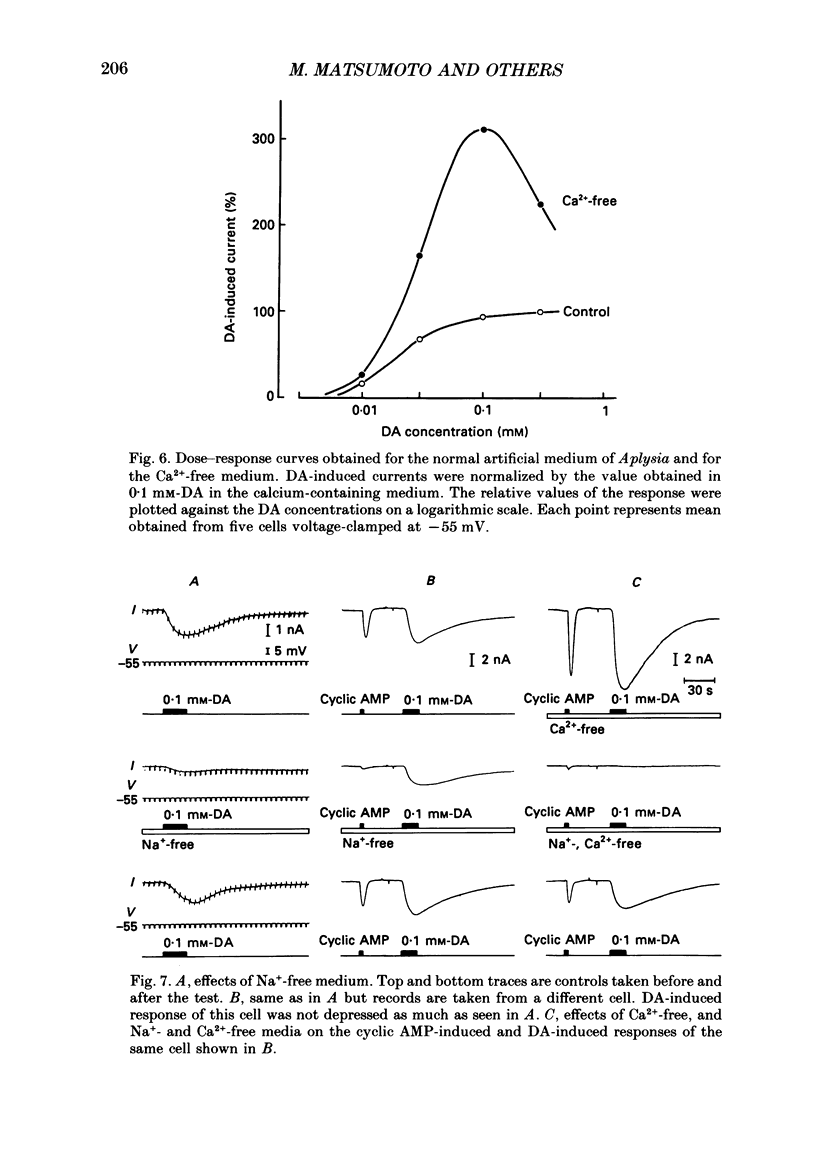
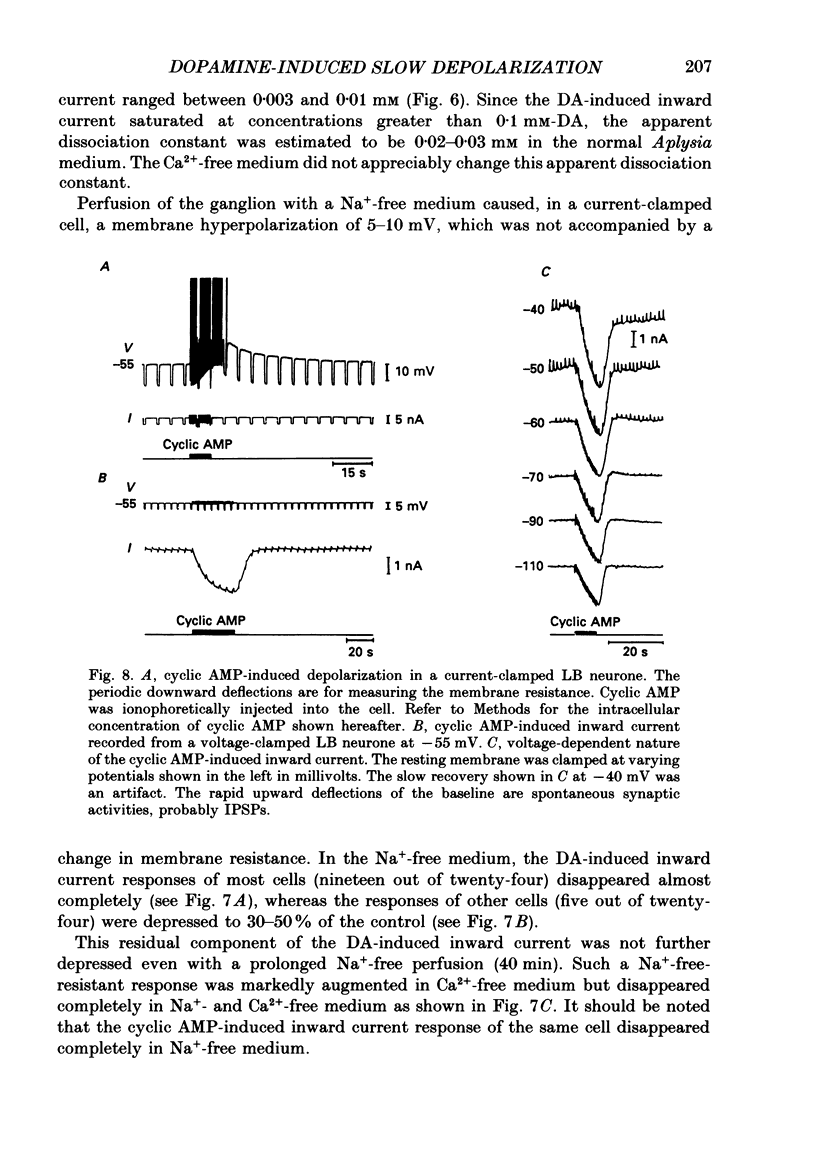




Selected References
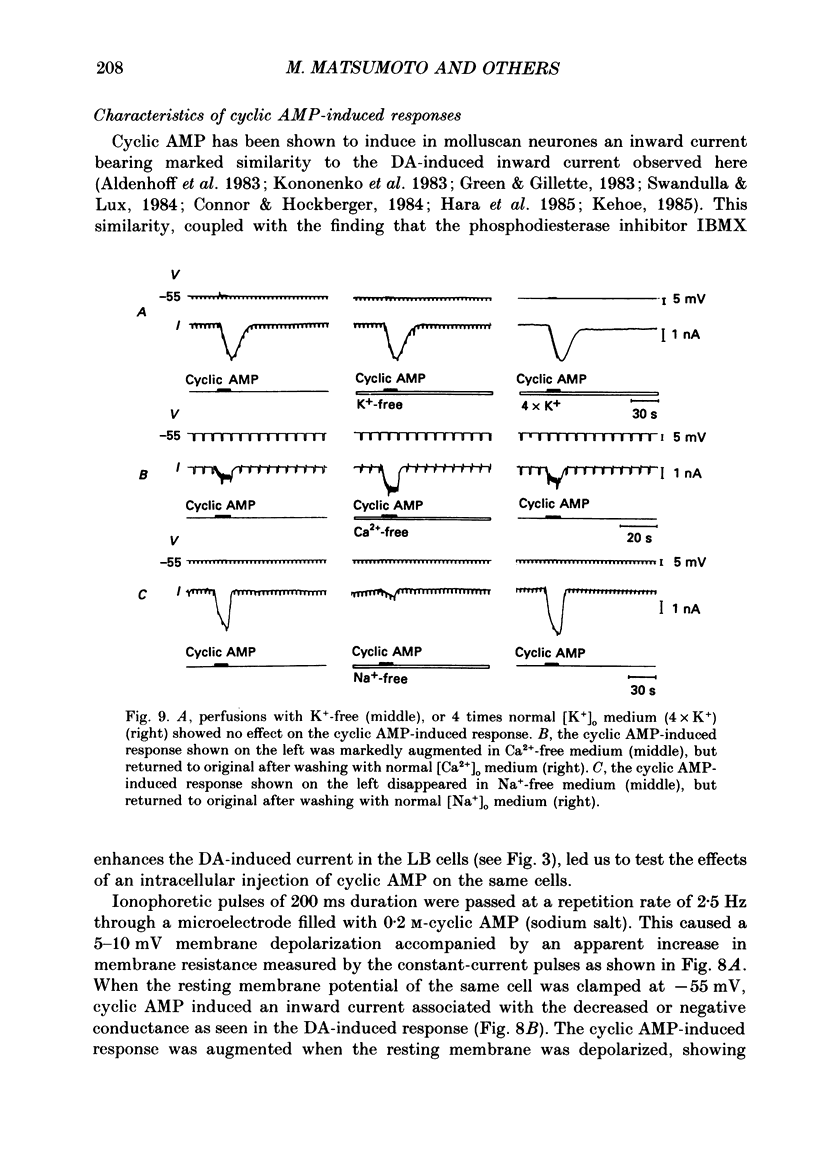
These references are in PubMed. This may not be the complete list of references from this article.
- Aldenhoff J. B., Hofmeier G., Lux H. D., Swandulla D. Stimulation of a sodium influx by cAMP in Helix neurons. Brain Res. 1983 Oct 16;276(2):289–296. doi: 10.1016/0006-8993(83)90736-9. [DOI] [PubMed] [Google Scholar]
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G., Cherubini E., Marciani M. G., Mercuri N., Stanzione P. Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Res. 1982 Aug 12;245(2):267–274. doi: 10.1016/0006-8993(82)90809-5. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Cottrell G. A. Excitatory, inhibitory and biphasic synaptic potentials mediated by an identified dopamine-containing neurone. J Physiol. 1975 Jan;244(3):589–612. doi: 10.1113/jphysiol.1975.sp010814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudier H. A., Geilen W., Cools A. R., Van Rossum J. M. Pharmacological analysis of dopamine-induced inhibition and excitation of neurones of the snail Helix aspersa. Arch Int Pharmacodyn Ther. 1974 Jun;209(2):324–331. [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Hockberger P. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones. J Physiol. 1984 Sep;354:139–162. doi: 10.1113/jphysiol.1984.sp015368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A., Davies N. W., Green K. A. Multiple actions of a molluscan cardioexcitatory neuropeptide and related peptides on identified Helix neurones. J Physiol. 1984 Nov;356:315–333. doi: 10.1113/jphysiol.1984.sp015467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Paupardin-Tritsch D., Bockaert J., Gerschenfeld H. M. Role of cyclic AMP in a serotonin-evoked slow inward current in snail neurones. Nature. 1981 Apr 30;290(5809):783–785. doi: 10.1038/290783a0. [DOI] [PubMed] [Google Scholar]
- Deterre P., Paupardin-Tritsch D., Bockaert J., Gerschenfeld H. M. cAMP-mediated decrease in K+ conductance evoked by serotonin and dopamine in the same neuron: a biochemical and physiological single-cell study. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7934–7938. doi: 10.1073/pnas.79.24.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Inokuchi H., Shinnick-Gallagher P. Dopamine depolarisation of mammalian primary afferent neurones. Nature. 1980 Feb 21;283(5749):770–772. doi: 10.1038/283770a0. [DOI] [PubMed] [Google Scholar]
- Gillette R., Green D. J. Calcium dependence of voltage sensitivity in adenosine 3',5'-cyclic phosphate-stimulated sodium current in Pleurobranchaea. J Physiol. 1987 Dec;393:233–245. doi: 10.1113/jphysiol.1987.sp016821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Patch- and voltage-clamp analysis of cyclic AMP-stimulated inward current underlying neurone bursting. Nature. 1983 Dec 22;306(5945):784–785. doi: 10.1038/306784a0. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hara N., Sawada M., Maeno T. Influences of pressure-injected cyclic AMP on the membrane current and characteristics of an identified neuron of Aplysia kurodai. Jpn J Physiol. 1985;35(6):985–1012. doi: 10.2170/jjphysiol.35.985. [DOI] [PubMed] [Google Scholar]
- Herrling P. L., Hull C. D. Iontophoretically applied dopamine depolarizes and hyperpolarizes the membrane of cat caudate neurons. Brain Res. 1980 Jun 23;192(2):441–462. doi: 10.1016/0006-8993(80)90896-3. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Synaptic block of a transmitter-induced potassium conductance in Aplysia neurones. J Physiol. 1985 Dec;369:399–437. doi: 10.1113/jphysiol.1985.sp015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. D., Scheller R. H. Egg-laying hormone of Aplysia induces a voltage-dependent slow inward current carried by Na+ in an identified motoneuron. Proc Natl Acad Sci U S A. 1986 May;83(9):3017–3021. doi: 10.1073/pnas.83.9.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai S. T., Sugimori M., Kocsis J. D. Excitatory nature of dopamine in the nigro-caudate pathway. Exp Brain Res. 1976 Feb 26;24(4):351–363. doi: 10.1007/BF00235003. [DOI] [PubMed] [Google Scholar]
- Kononenko N. I., Kostyuk P. G., Shcherbatko A. D. The effect of intracellular cAMP injections on stationary membrane conductance and voltage- and time-dependent ionic currents in identified snail neurons. Brain Res. 1983 Jun 6;268(2):321–338. doi: 10.1016/0006-8993(83)90499-7. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Berry M. S. Further identification of multiple responses mediated by dopamine in the CNS of Planorbis corneus. Can J Physiol Pharmacol. 1978 Feb;56(1):7–18. doi: 10.1139/y78-002. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Colombaioni L., Deterre P., Gerschenfeld H. M. Two different mechanisms of calcium spike modulation by dopamine. J Neurosci. 1985 Sep;5(9):2522–2532. doi: 10.1523/JNEUROSCI.05-09-02522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar T. C. Does cyclic 3' ,5'-adenosine monophosphate act as second messenger in a voltage-dependent response to 5-hydroxytryptamine in Aplysia? Br J Pharmacol. 1981 Dec;74(4):747–756. doi: 10.1111/j.1476-5381.1981.tb10707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar T. C. Voltage-dependent current evoked by dopamine and octopamine in Aplysia. Brain Res. 1981 Nov 2;223(2):448–454. doi: 10.1016/0006-8993(81)91163-x. [DOI] [PubMed] [Google Scholar]
- Sato M., Austin G., Yai H., Maruhashi J. The ionic permeability changes during acetylcholine-induced responses of Aplysia ganglion cells. J Gen Physiol. 1968 Mar;51(3):321–345. doi: 10.1085/jgp.51.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984 Dec 3;35(23):2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Lux H. D. Changes in ionic conductances induced by cAMP in Helix neurons. Brain Res. 1984 Jul 2;305(1):115–122. doi: 10.1016/0006-8993(84)91126-0. [DOI] [PubMed] [Google Scholar]
- Walsh J. P., Byrne J. H. Analysis of decreased conductance serotonergic response in Aplysia ink motor neurons. J Neurophysiol. 1985 Feb;53(2):590–602. doi: 10.1152/jn.1985.53.2.590. [DOI] [PubMed] [Google Scholar]


