Abstract
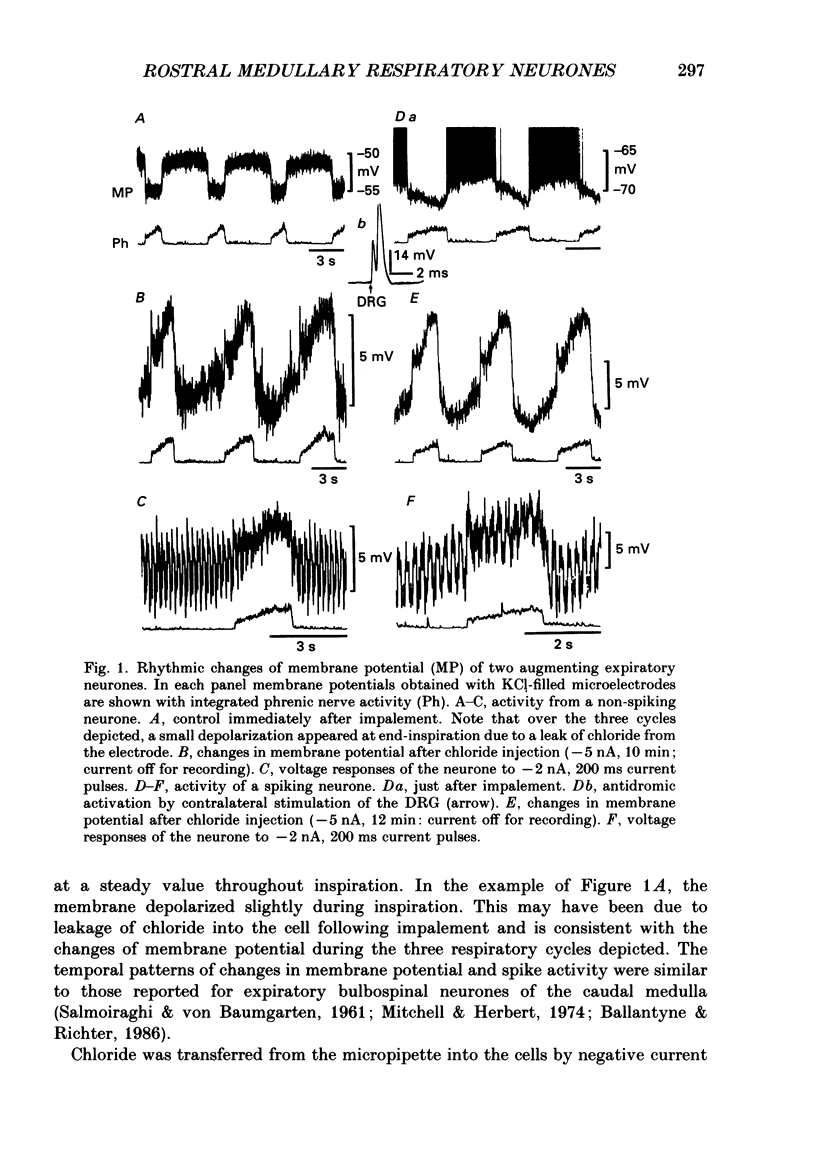
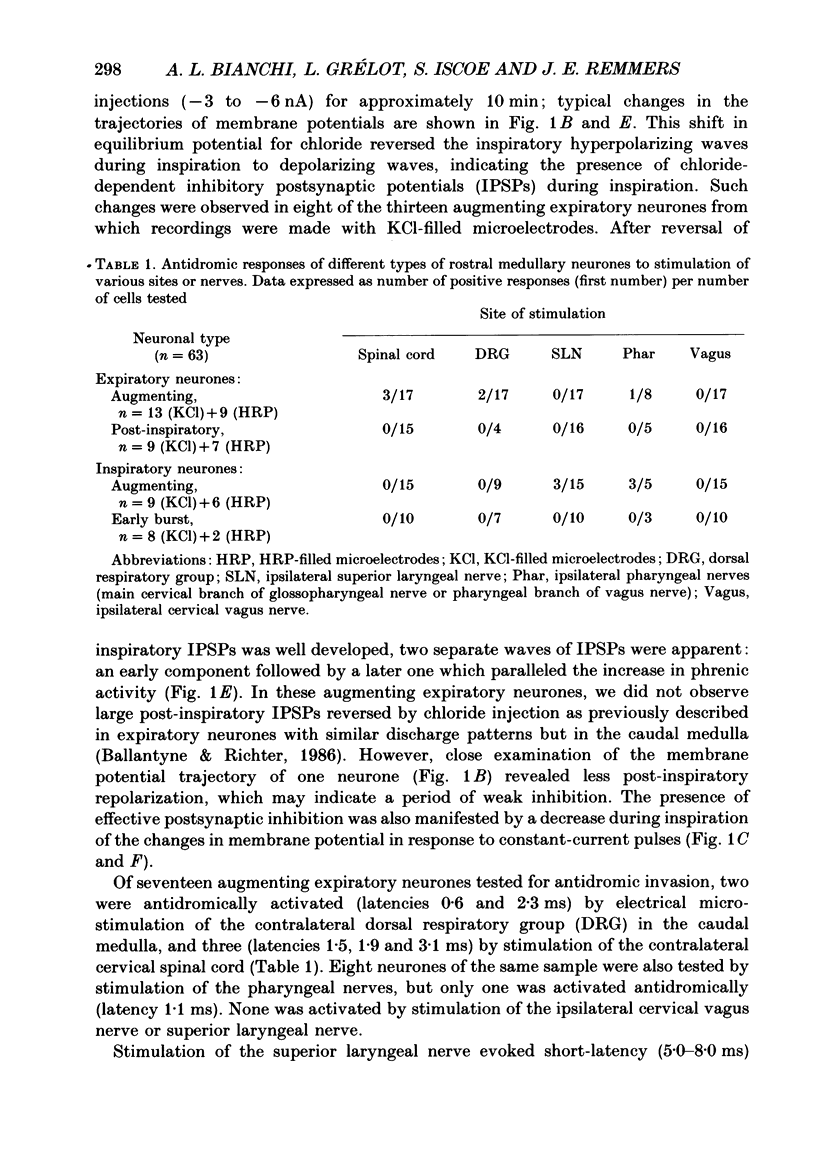
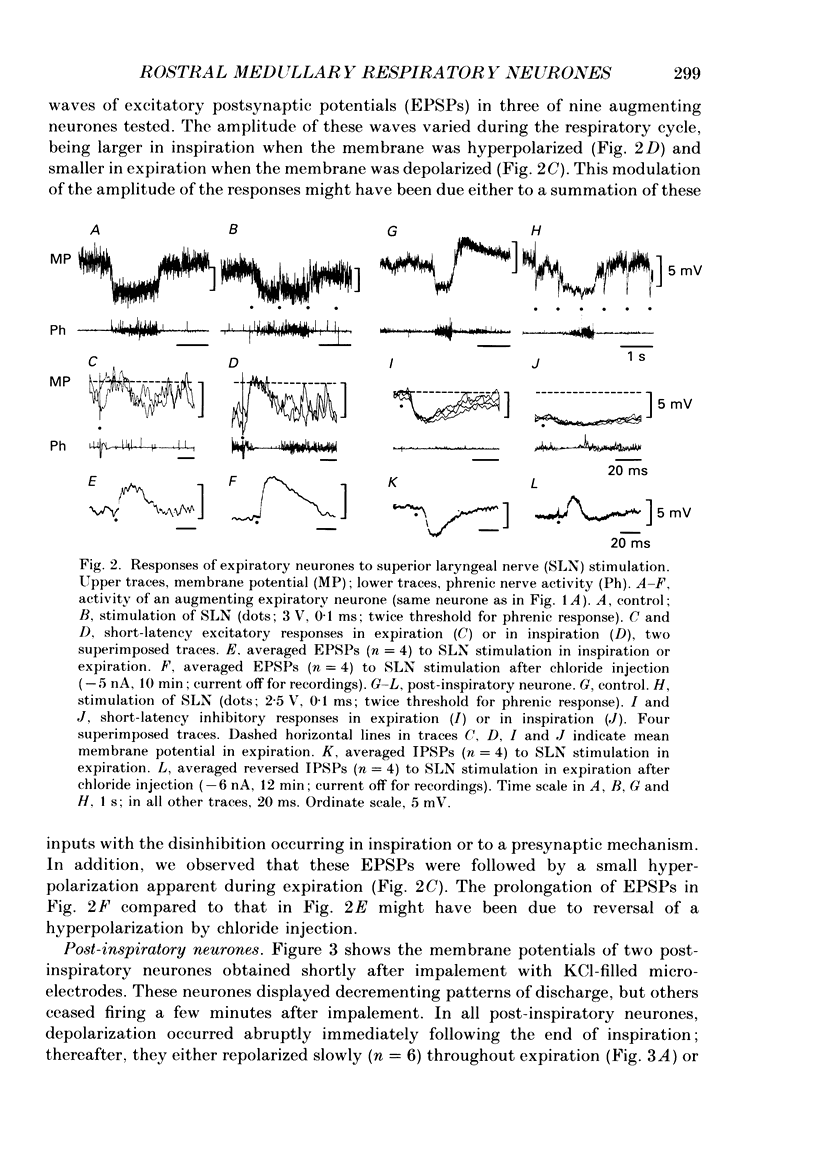
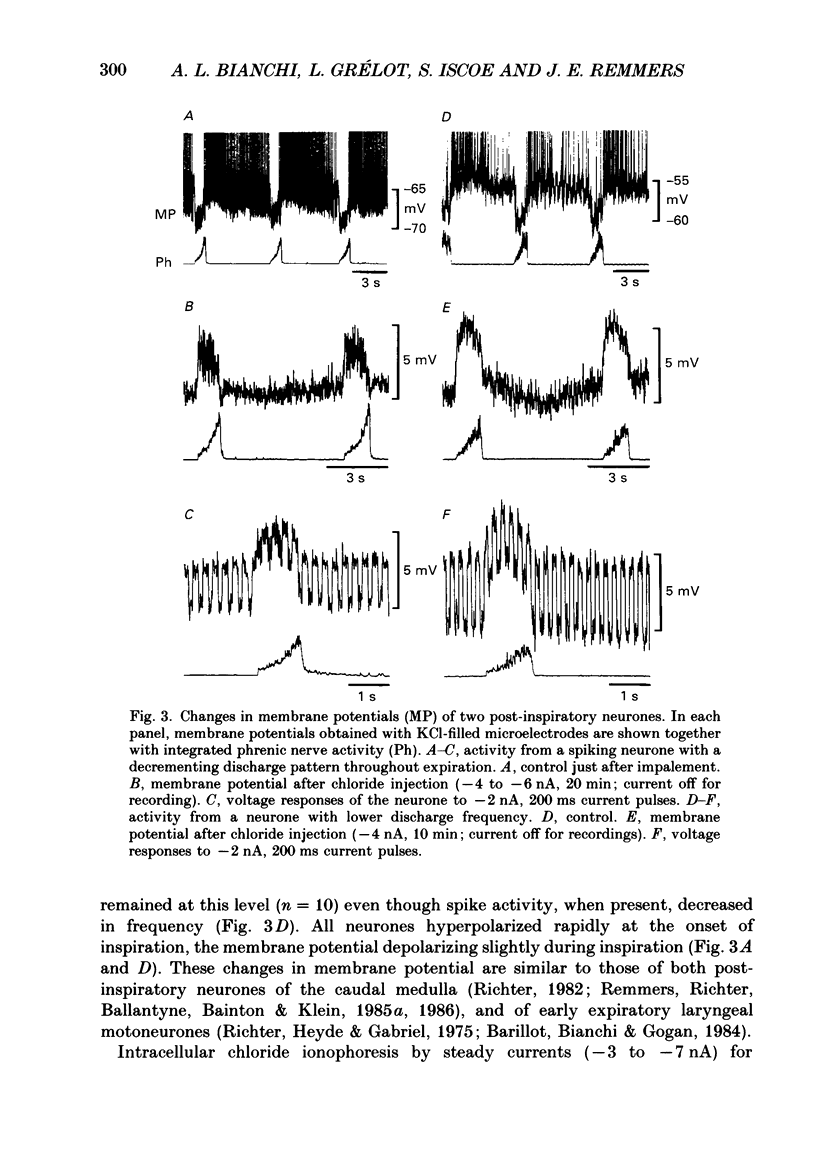
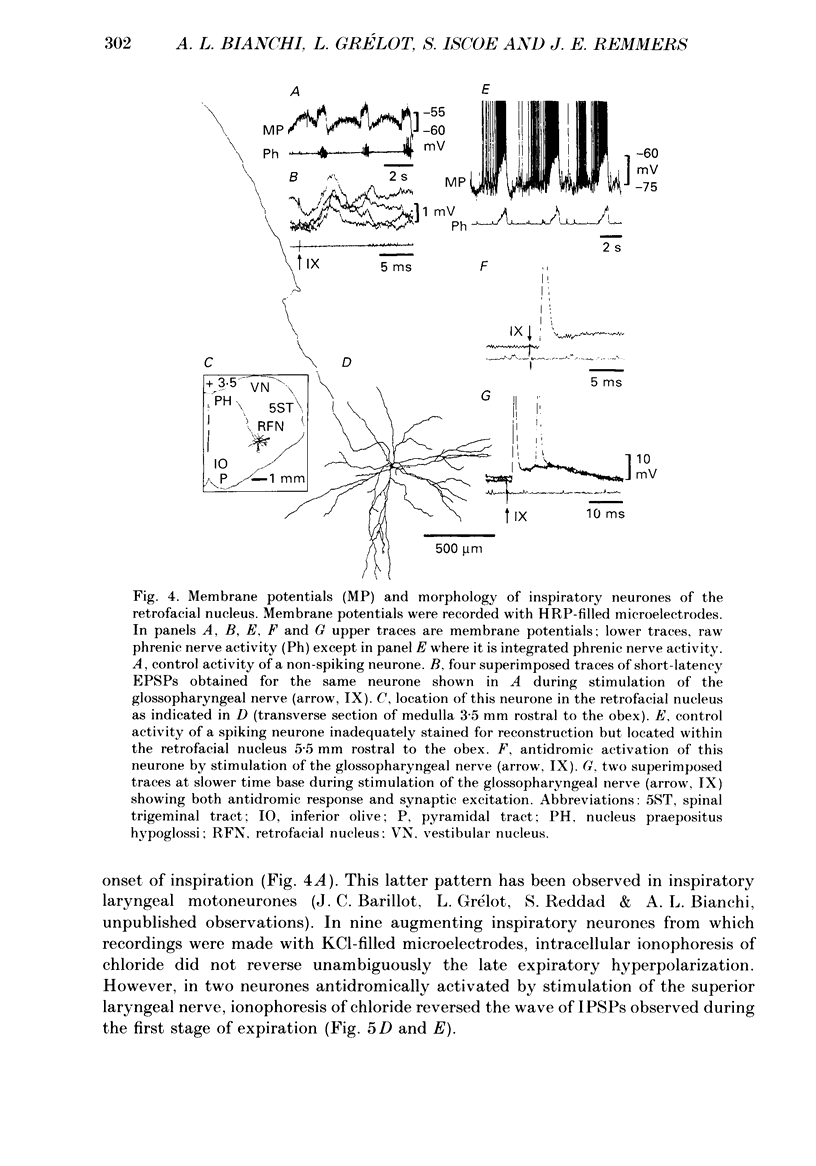
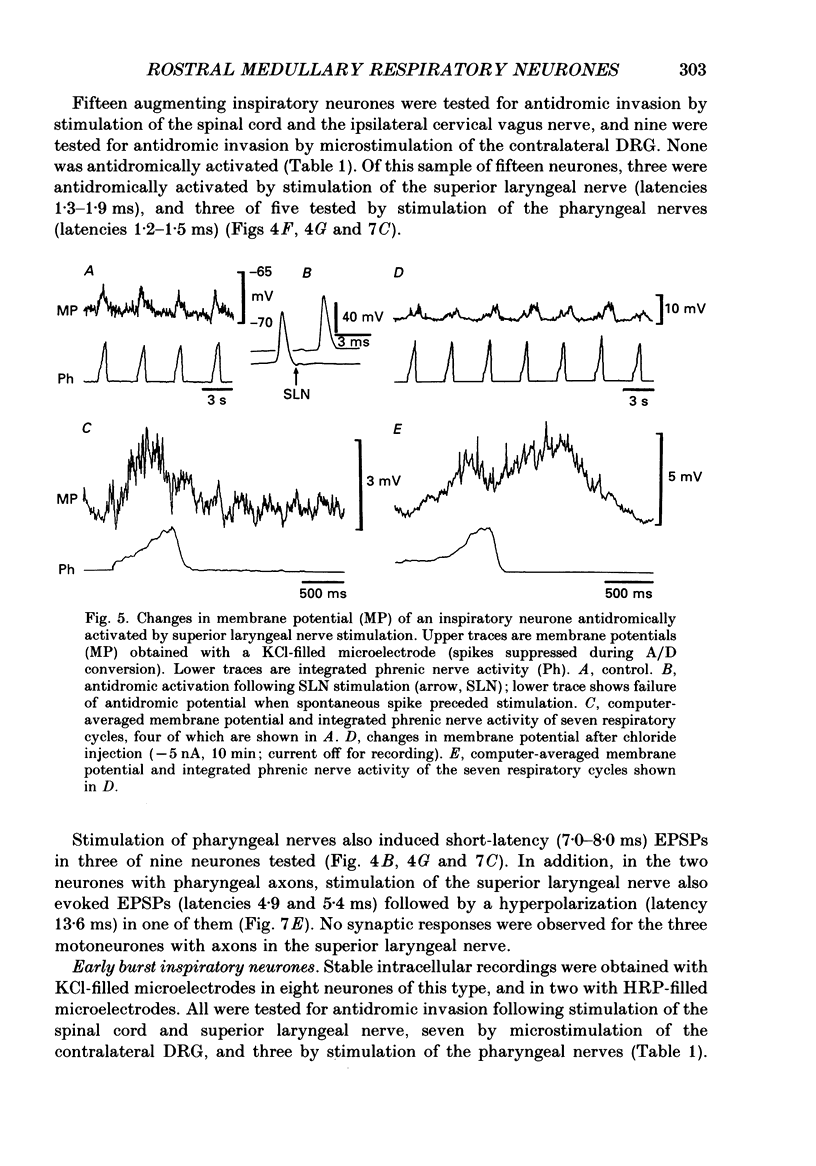
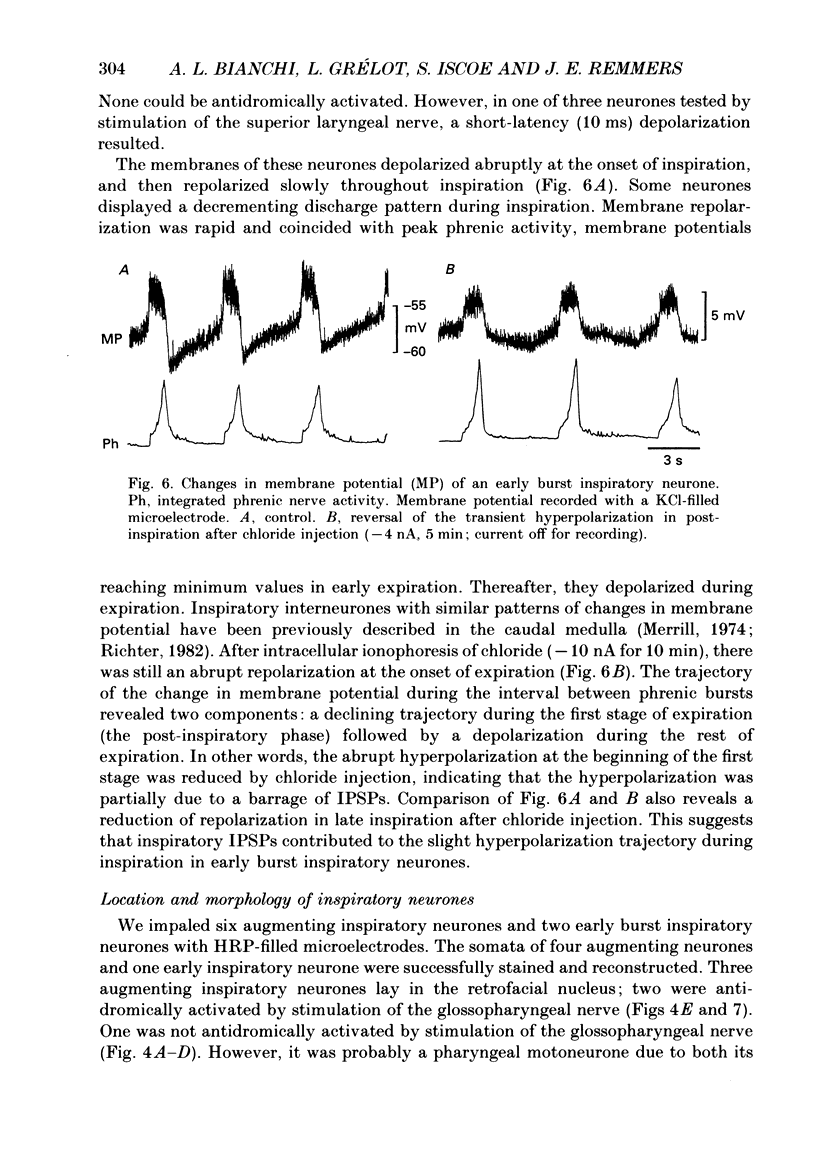
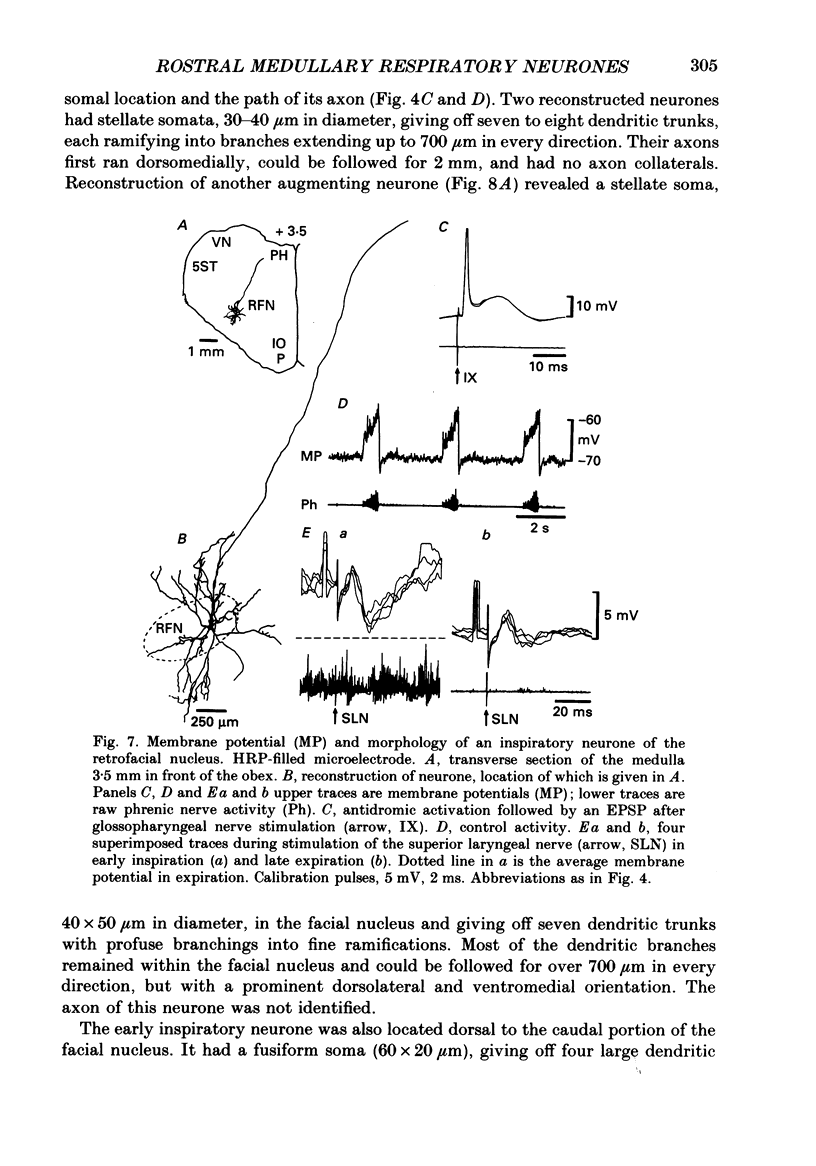
1. We recorded the membrane potentials of sixty-three respiratory neurones in the rostral, ventral medulla of decerebrate vagotomized cats. Stable recordings were obtained in thirty-eight expiratory and twenty-five inspiratory neurones. Axonal projections were identified by antidromic invasion after electrical stimulation of the region of the dorsal respiratory group (DRG), spinal cord, and the cervical vagus, superior laryngeal and pharyngeal nerves. 2. Two types of expiratory neurones were encountered: those in which the membrane potential progressively depolarized (augmenting neurons, n = 22) and those in which the membrane potential repolarized (decrementing or post-inspiratory neurones, n = 16) during the interval between phrenic bursts. Both types were hyperpolarized during inspiration by chloride-dependent, inhibitory postsynaptic potentials (IPSPs) which decreased membrane resistance. In augmenting neurones two waves of IPSPs appeared, one early and one late in inspiration. 3. Five out of seventeen augmenting expiratory neurones tested were antidromically activated by contralateral stimulation of the spinal cord (n = 3) or the DRG (n = 2). Spinal axons were not detected in any of the sixteen decrementing expiratory neurones tested. Of thirteen expiratory neurones tested with pharyngeal nerve stimulation, one (an augmenting type) was antidromically activated. Superior laryngeal or vagal axons could not be demonstrated for any expiratory neurones. 4. Two types of inspiratory neurones were also encountered: those displaying progressive depolarization throughout inspiration (n = 5) and those which gradually repolarized after maximal depolarization at the onset of inspiration (n = 10). None of the former had identifiable spinal or medullary axons, but superior laryngeal axons were demonstrated in three and pharyngeal axons were found in three. None of the latter was antidromically activated from any of the sites stimulated. 5. Stimulation of the superior laryngeal or pharyngeal nerves evoked excitatory postsynaptic potentials (EPSPs) in all neurones except in post-inspiratory neurones. In these, stimulation of the superior laryngeal or pharyngeal nerves evoked IPSPs in five of twelve neurones tested. 6. We conclude that a spectrum of respiratory neurones lie within or ventral to the retrofacial nucleus. These neurones may control upper-airway muscles or may play a role in chemoreception.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrezik J. A., Chan-Palay V., Palay S. L. The nucleus paragigantocellularis lateralis in the rat. Demonstration of afferents by the retrograde transport of horseradish peroxidase. Anat Embryol (Berl) 1981;161(4):373–390. doi: 10.1007/BF00316049. [DOI] [PubMed] [Google Scholar]
- Arita H., Kogo N., Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987 Jan 20;401(2):258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984 Mar;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillot J. C., Bianchi A. L., Gogan P. Laryngeal respiratory motoneurones: morphology and electrophysiological evidence of separate sites for excitatory and inhibitory synaptic inputs. Neurosci Lett. 1984 Jun 15;47(2):107–112. doi: 10.1016/0304-3940(84)90414-2. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., Barillot J. C. Respiratory neurons in the region of the retrofacial nucleus: pontile, medullary, spinal and vagal projections. Neurosci Lett. 1982 Aug 31;31(3):277–282. doi: 10.1016/0304-3940(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L. Localisation et étude des neurones respiratoires bulbaires. Mise en jeu antidromique par stimulation spinale ou vagale. J Physiol (Paris) 1971 Jan-Feb;63(1):5–40. [PubMed] [Google Scholar]
- Bianchi A. L. Modalités de décharge et propriétés anatomo-fonctionnelles des neurones respiratoires bulbaires. J Physiol (Paris) 1974;68(5):555–587. [PubMed] [Google Scholar]
- Budzińska K., von Euler C., Kao F. F., Pantaleo T., Yamamoto Y. Effects of graded focal cold block in rostral areas of the medulla. Acta Physiol Scand. 1985 Jul;124(3):329–340. doi: 10.1111/j.1748-1716.1985.tb07668.x. [DOI] [PubMed] [Google Scholar]
- Bystrzycka E. K. Afferent projections to the dorsal and ventral respiratory nuclei in the medulla oblongata of the cat studied by the horseradish peroxidase technique. Brain Res. 1980 Mar 3;185(1):59–66. doi: 10.1016/0006-8993(80)90670-8. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G. Axonal projections from the rostral expiratory neurones of the Bötzinger complex to medulla and spinal cord in the cat. J Physiol. 1984 May;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelot L., Bianchi A. L. Differential effects of halothane anesthesia on the pattern of discharge of inspiratory and expiratory neurons in the region of the retrofacial nucleus. Brain Res. 1987 Feb 24;404(1-2):335–338. doi: 10.1016/0006-8993(87)91390-4. [DOI] [PubMed] [Google Scholar]
- Grélot L., Bianchi A. L., Iscoe S., Remmers J. E. Expiratory neurones of the rostral medulla: anatomical and functional correlates. Neurosci Lett. 1988 Jun 29;89(2):140–145. doi: 10.1016/0304-3940(88)90370-9. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., St John W. M., Bartlett D., Jr Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):785–792. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- Kalia M., Feldman J. L., Cohen M. I. Afferent projections to the inspiratory neuronal region of the ventrolateral nucleus of the tractus solitarius in the cat. Brain Res. 1979 Jul 27;171(1):135–141. doi: 10.1016/0006-8993(79)90739-x. [DOI] [PubMed] [Google Scholar]
- Lawn A. M. The nucleus ambiguus of the rabbit. J Comp Neurol. 1966 Jun;127(2):307–320. doi: 10.1002/cne.901270211. [DOI] [PubMed] [Google Scholar]
- Lipski J., Merrill E. G. Electrophysiological demonstration of the projection from expiratory neurones in rostral medulla to contralateral dorsal respiratory group. Brain Res. 1980 Sep 22;197(2):521–524. doi: 10.1016/0006-8993(80)91140-3. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H., De Lattre J., Schläfke M. E., Trouth C. O. Effects on respiration and circulation of electrically stimulating the ventral surface of the medulla oblongata. Respir Physiol. 1970 Sep;10(2):184–197. doi: 10.1016/0034-5687(70)90082-4. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Long S., Duffin J. The neuronal determinants of respiratory rhythm. Prog Neurobiol. 1986;27(2):101–182. doi: 10.1016/0301-0082(86)90007-9. [DOI] [PubMed] [Google Scholar]
- McAllen R. M. Central respiratory modulation of subretrofacial bulbospinal neurones in the cat. J Physiol. 1987 Jul;388:533–545. doi: 10.1113/jphysiol.1987.sp016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M. Identification and properties of sub-retrofacial bulbospinal neurones: a descending cardiovascular pathway in the cat. J Auton Nerv Syst. 1986 Oct;17(2):151–164. doi: 10.1016/0165-1838(86)90090-1. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970 Nov 11;24(1):11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. The effect of carbon dioxide on the membrane potential of medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):345–349. doi: 10.1016/0006-8993(74)90759-8. [DOI] [PubMed] [Google Scholar]
- Otake K., Sasaki H., Mannen H., Ezure K. Morphology of expiratory neurons of the Bötzinger complex: an HRP study in the cat. J Comp Neurol. 1987 Apr 22;258(4):565–579. doi: 10.1002/cne.902580407. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., Richter D. W., Ballantyne D., Bainton C. R., Klein J. P. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986 Aug;407(2):190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Heyde F., Gabriel M. Intracellular recordings from different types of medullary respiratory neurons of the cat. J Neurophysiol. 1975 Sep;38(5):1162–1171. doi: 10.1152/jn.1975.38.5.1162. [DOI] [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., von BAUMGARTEN Intracellular potentials from respiratory neurones in brain-stem of cat and mechanism of rhythmic respiration. J Neurophysiol. 1961 Mar;24:203–218. doi: 10.1152/jn.1961.24.2.203. [DOI] [PubMed] [Google Scholar]
- VON BAUMGARTEN R., VON BAUMGARTEN A., SCHAEFER K. P. Beitrag zur Lokalisationsfrage bulboreticulärer respiratorischer Neurone der Katze. Pflugers Arch. 1957;264(3):217–227. doi: 10.1007/BF00369943. [DOI] [PubMed] [Google Scholar]
- von Euler C., Hayward J. N., Marttila I., Wyman R. J. Respiratory neurones of the ventrolateral nucleus of the solitary tract of cat: vagal input, spinal connections and morphological identification. Brain Res. 1973 Oct 26;61:1–22. doi: 10.1016/0006-8993(73)90512-x. [DOI] [PubMed] [Google Scholar]


