Abstract
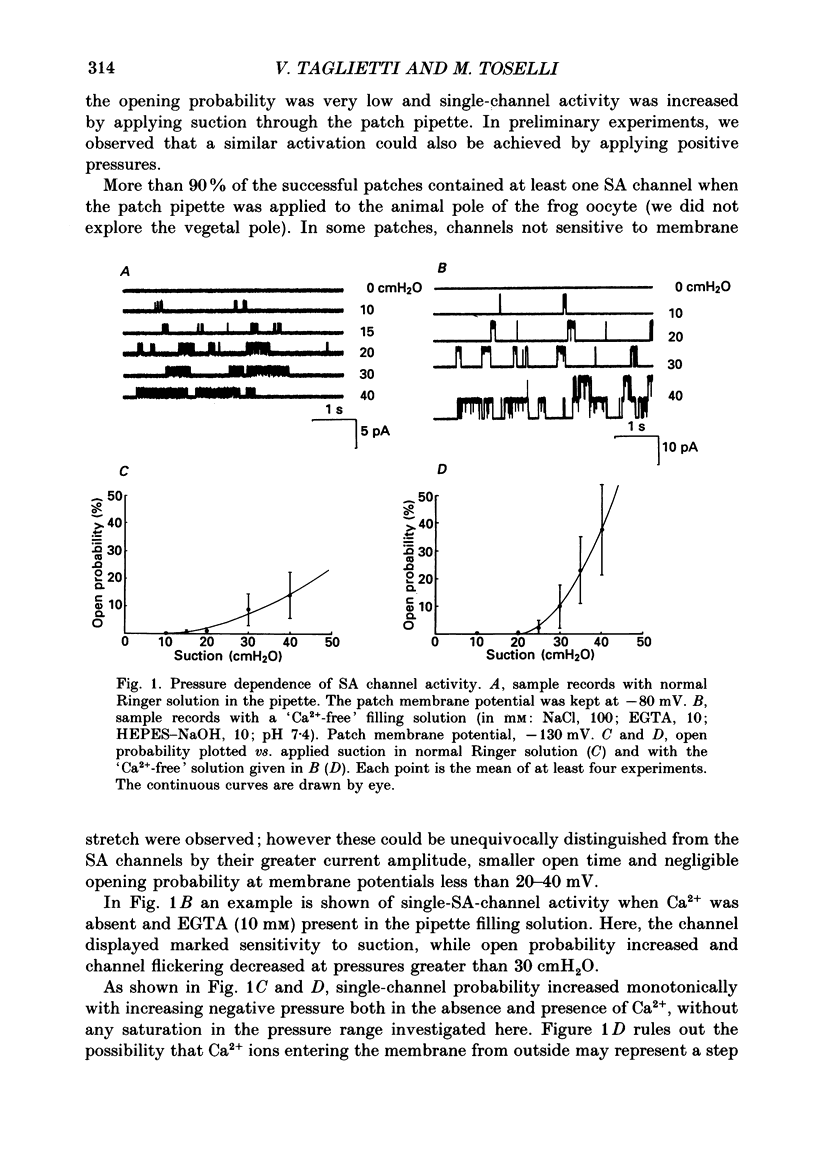
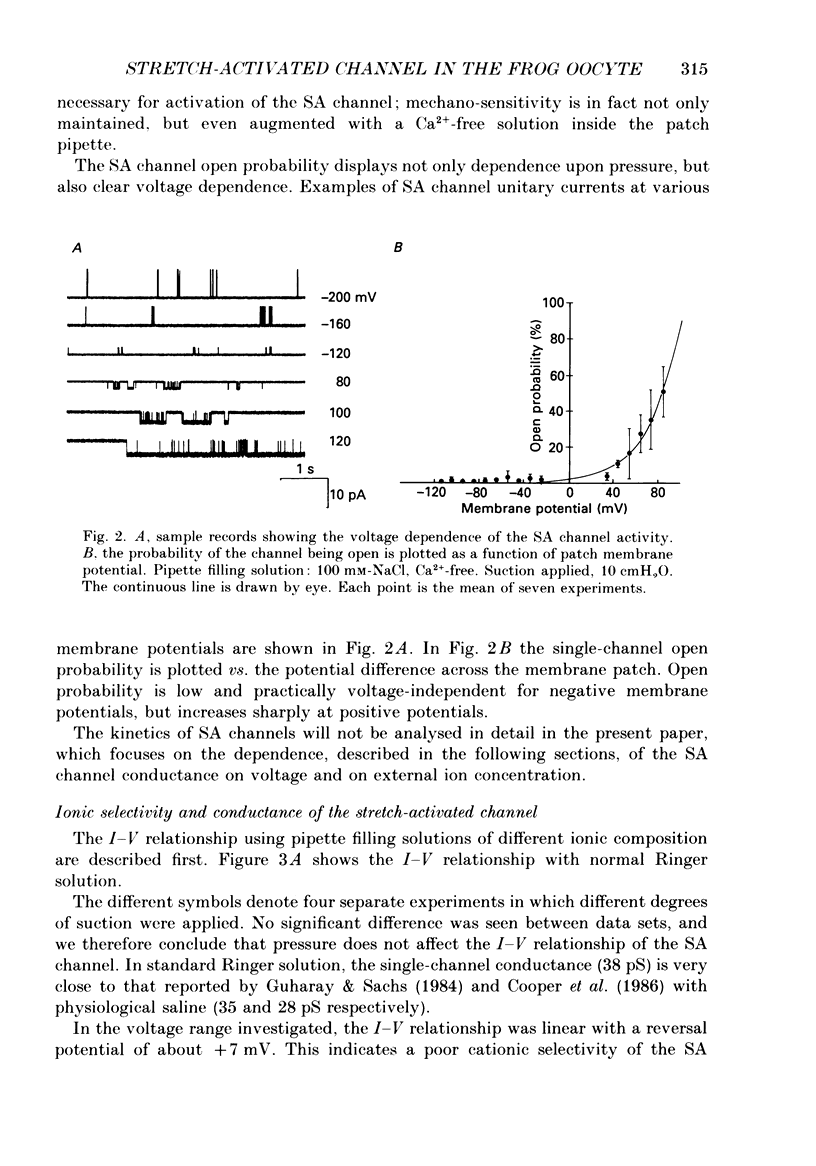
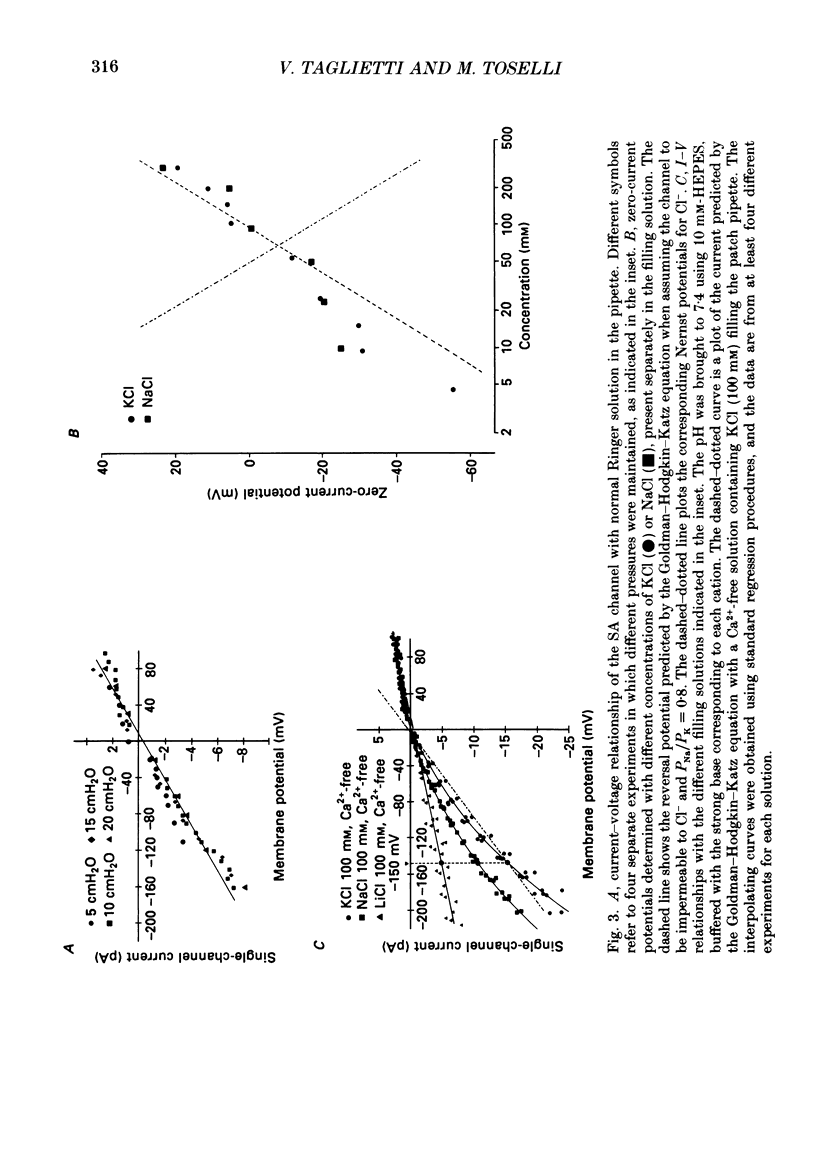
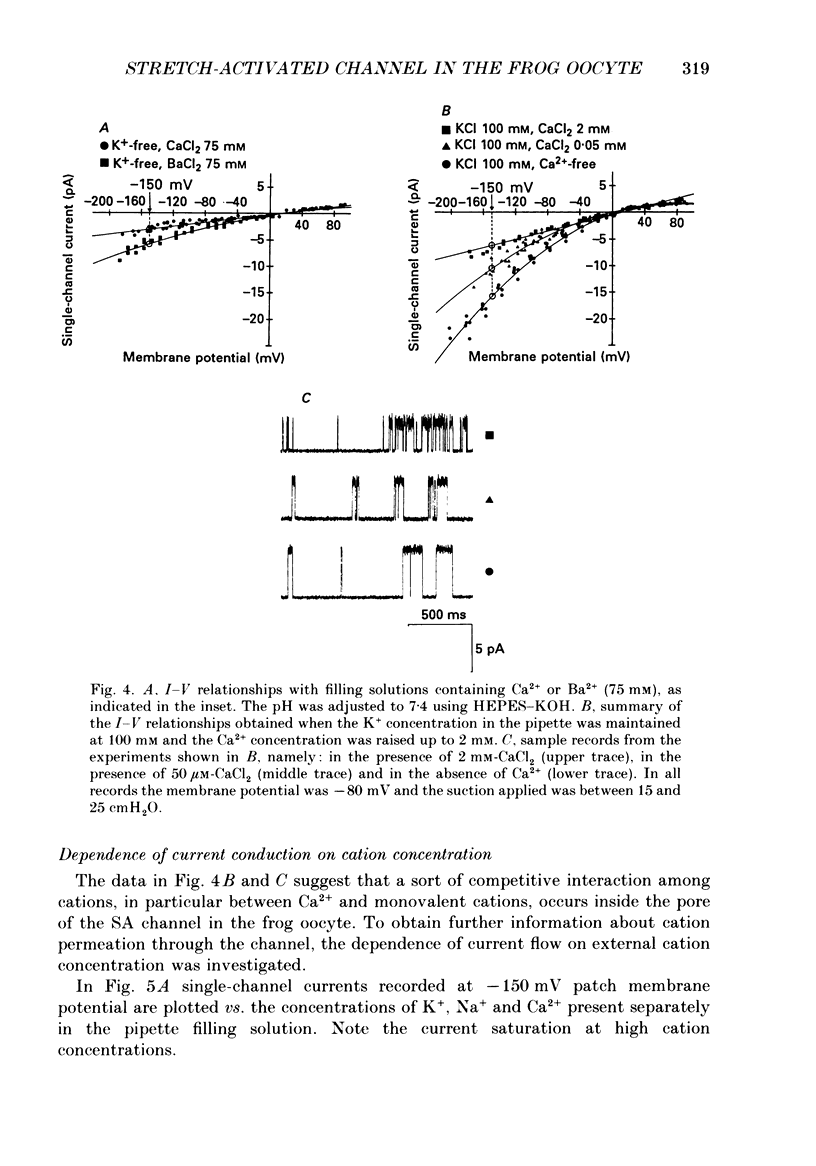
1. We have carried out patch-clamp measurements on a cationic channel in the plasma membrane of the frog oocyte, which can be specifically activated by membrane stretch. The kinetics of this channel also display a distinct dependence upon membrane potential, the probability of the channel being open increasing with membrane depolarization. 2. When the patch-clamp pipette filling solution was standard Ringer solution, the single-channel current-voltage (I-V) relationship was linear, the elementary conductance being 38 pS and the reversal potential +7 mV, suggesting very poor selectivity of the channel for the various cations. 3. The I-V relationship was highly non-linear having a strong inward-going rectification when Ca2+-free solutions were used to fill the patch pipette. These solutions also resulted in a selective, inward cationic permeability through the membrane, with K+ being more permeable than Na+ greater than Li+ greater than Ba2+ greater than Ca2+. 4. Though permeant through the stretch-activated channel, Ca2+ inhibited in a concentration-dependent manner the currents carried by other cations. La3+ (0.1 mM) was also an effective channel blocker. 5. The inward current carried by individual cations at a given membrane potential increased with increasing external cation concentration up to a saturating level, this level being maximal for K+ and minimal for Ca2+. Also the half-saturating concentration was maximal for K+ and minimal for Ca2+ at all membrane potentials. 6. In the presence of a constant Ca2+ concentration (50 microM) increasing [K+] did not change the absolute level at which the current saturated; however the half-saturating K+ concentration was greatly increased, indicating competitive inhibition between Ca2+ and K+ for the same site. 7. The data are consistent with a model based on Eyring rate theory for current conduction through ionic channels, in which we assume that the ions capable of entering the channel compete for a binding site that they must first occupy before proceeding on. The possible energy profile of the stretch-activated channel was defined by optimizing the model parameters to obtain the best fit of the experimental data. Ca2+ was found to have a smaller dissociation constant and much longer occupancy time than Na+ or K+, thus accounting for its lower permeability and inhibitory effect on current conduction by other cations through the stretch-activatable channel.
Full text
PDF











Selected References
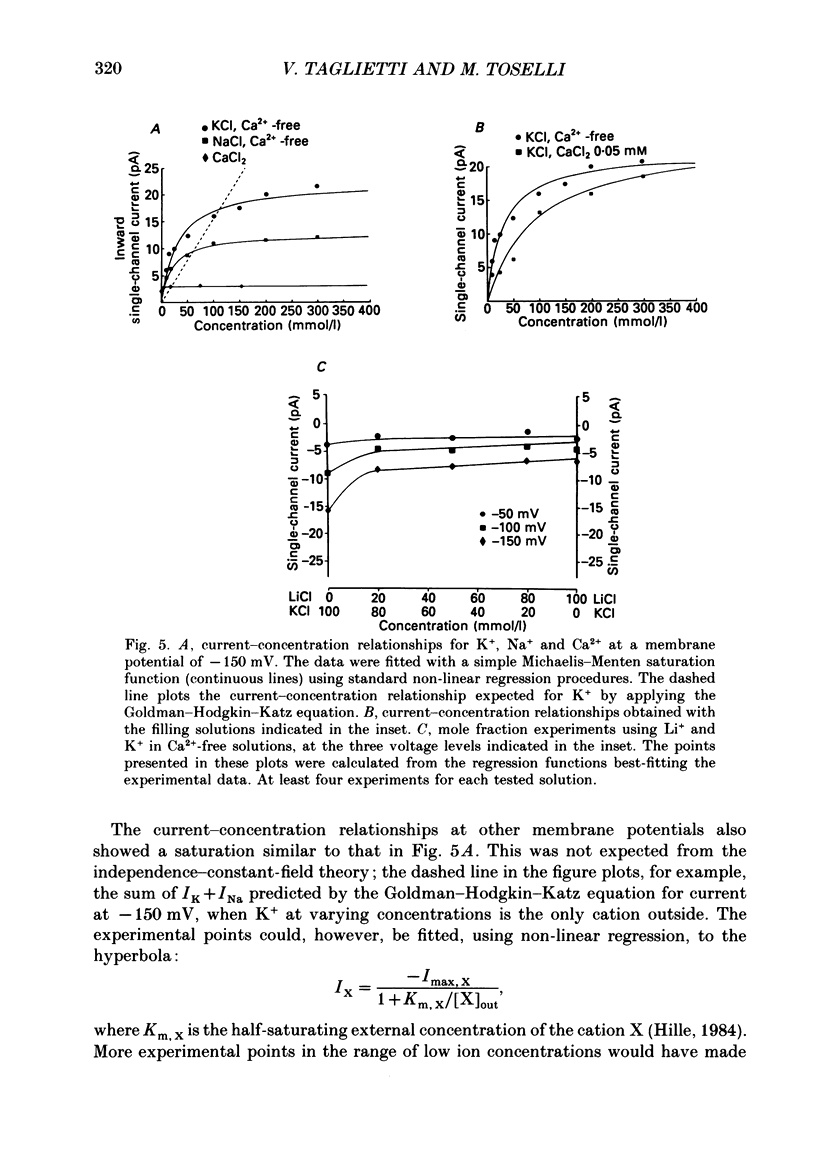
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
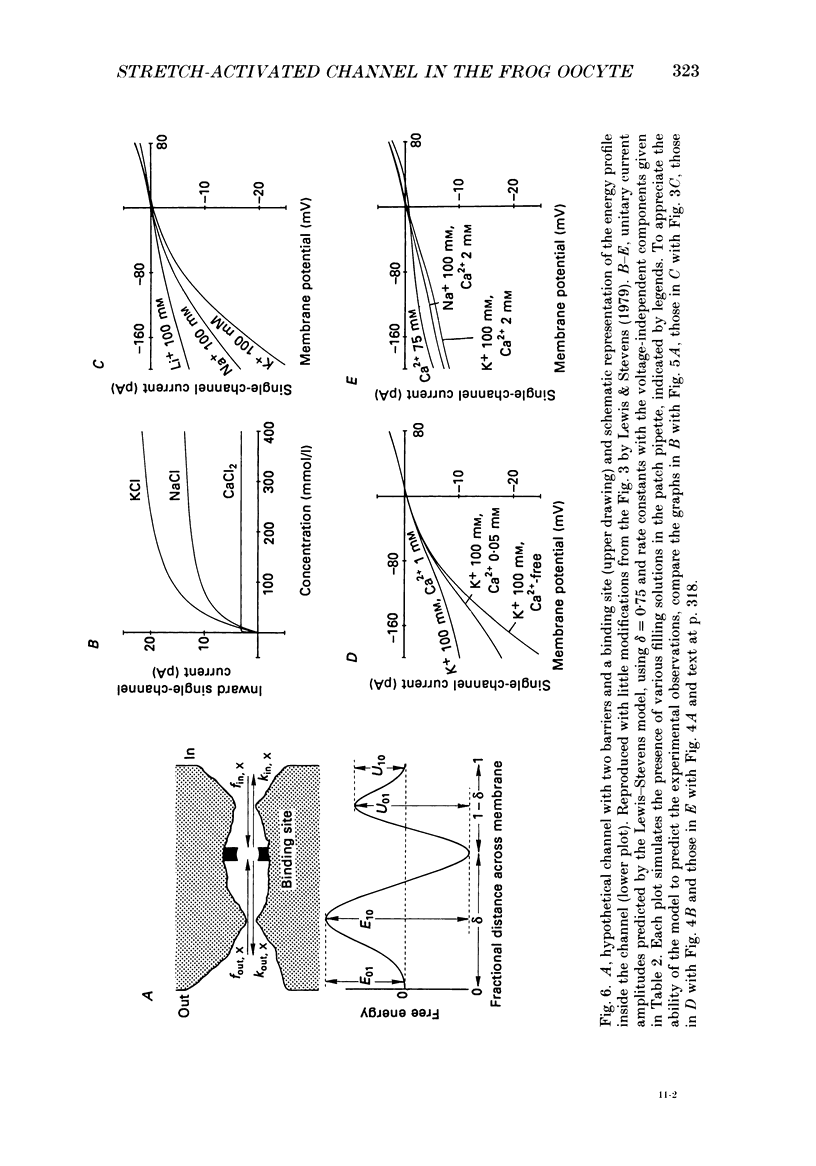
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E., Tang J. M., Rae J. L., Eisenberg R. S. A cation channel in frog lens epithelia responsive to pressure and calcium. J Membr Biol. 1986;93(3):259–269. doi: 10.1007/BF01871180. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Mechanotransducer ion channels in chick skeletal muscle: the effects of extracellular pH. J Physiol. 1985 Jun;363:119–134. doi: 10.1113/jphysiol.1985.sp015699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Taglietti V., Tanzi F., Romero R., Simoncini L. Maturation involves suppression of voltage-gated currents in the frog oocyte. J Cell Physiol. 1984 Dec;121(3):576–588. doi: 10.1002/jcp.1041210317. [DOI] [PubMed] [Google Scholar]
- Toselli M., Simoncini L., Taglietti V., Tanzi F. Acetylcholine-induced currents at plasma membrane of the frog oocyte. Neurosci Lett. 1985 Mar 15;54(2-3):179–184. doi: 10.1016/s0304-3940(85)80075-6. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B. Ion transport in the simplest single file pore. Biochim Biophys Acta. 1979 Jul 5;554(2):410–429. doi: 10.1016/0005-2736(79)90381-x. [DOI] [PubMed] [Google Scholar]


